Heterogeneity in familial dominant Paget disease of bone and muscular dystrophy
Abstract
The combination of autosomal dominant, early onset Paget disease of bone (PDB) and muscular dystrophy is an unusual disorder. We recently mapped the disorder in a large family from central Illinois with PDB and proximal limb-girdle type of muscular dystrophy (LGMD), and in 3 additional families with hereditary inclusion body myopathy (HIBM), Paget disease of bone and frontotemporal dementia, to a unique locus on chromosome 9p21.1-q12. The present study describes an unrelated 10-member family with autosomal dominant PDB and a scapuloperoneal type of muscular dystrophy. Clinical, biochemical, and radiological evaluations were performed to delineate clinical features in this family. Progression of the muscular dystrophy begins with weakness in the distal muscles of the legs accompanied by foot drop. EMG and muscle biopsy are compatible with a primary dystrophy. Onset of Paget disease is early, at a mean age of 41 years, with initial distribution in the long bones and eventual infiltration of the spine and pelvis. Creatine phosphokinase (CPK) and alkaline phosphatase levels are elevated in affected individuals. Molecular analyses excluded all known loci for Paget disease of bone, scapuloperoneal muscular dystrophy (SPMD), fascioscapulohumeral muscular dystrophy (FSH), amyotrophic lateral sclerosis (ALS), Bethlem myopathy, two forms of autosomal dominant limb-girdle muscular dystrophy (LGMD), and the critical region for LGMD or HIBM/PDB on chromosome 9p21.1-q12, thus providing evidence for genetic heterogeneity among families with the unique combination of muscular dystrophy and Paget disease of bone. © 2002 Wiley-Liss, Inc.
INTRODUCTION
Paget disease of bone (PDB) is a clinically and molecularly diverse disorder characterized by an increase in bone remodeling and hypertrophy leading to abnormal bone structure, bone pain, and deformity which typically presents in the 5th–6th decades of life. There is evidence for a major genetic component to Paget disease, with up to 40% of patients having affected first-degree relatives [Morales-Piga et al., 1995]. Despite the strong familial association of PDB, mutations in the TNFRSF11A gene located on chromosome 18q have only recently been shown to cause disease [Cody and Singer, 1997; Hughes et al., 2000]. A weak association of PDB to the HLA locus on chromosome 6p has been reported [Fotino et al., 1977] and further molecular heterogeneity has been established [Nance et al., 2000]. Recently, in a linkage study of 24 French-Canadian families, Laurin et al. [2001] mapped two novel loci for autosomal dominant PDB at 5q31 and 5q35-qter.
Although the hallmark feature of all inherited muscular dystrophies is muscular weakness, clinical features such as inheritance patterns, age of onset, rate of progression, muscles affected, and biochemical analyses are widely heterogeneous in these disorders [Bushby, 1999]. In addition to the clinical heterogeneity, genetic heterogeneity has been well documented with a vast amount of genes identified for a number of dystrophies.
The association of PDB and any neuromuscular disorder is rare [Caughey et al., 1957; McBride, 1966; Tucker et al., 1982]. We previously described a family presenting with an autosomal dominant limb-girdle type of muscular dystrophy in association with Paget disease of bone [Kimonis et al., 2000]. Linkage analysis in this family excluded the candidate genes for PDB and autosomal dominant and recessive LGMD. A genome search in this family and in three additional families with hereditary inclusion body myopathy (HIBM), PDB, and frontotemporal dementia resulted in linkage to chromosome 9p22.3-q12 [Kovach et al., 2001].
Presently, we describe a new family with a scapuloperoneal type of muscular dystrophy associated with Paget disease of bone. The clinical features of the disorder show heterogeneity to those previously described and are most compatible with scapuloperoneal muscular dystrophy (SPMD), an autosomal dominant myopathy with distal weakness and predominant involvement of the scapula and peroneal muscles, with linkage of the gene to chromosome 12q13.3-q15 [Isozumi et al., 1996]. Linkage analysis excluded all known loci for Paget disease of bone, scapuloperoneal muscular dystrophy, amyotrophic lateral sclerosis, Bethlem myopathy and two forms of autosomal dominant limb-girdle muscular dystrophy (Table I). Haplotype analysis for a high density of markers excluded linkage to chromosome 9p22.3-q12. The present family provides evidence for clinical and genetic heterogeneity in muscular dystrophy with the association of Paget disease of bone.
| Marker | MIM number* | Locus | Chromosome |
|---|---|---|---|
| D6S105, D6S502 | 167250 | PDB1 | 6p21.2 |
| D18S60, D18S42 | 602080 | PDB2 | 18q21–q22 |
| D9S43 | 605382 | LGMD/PDB | 9p22.3q12 |
| D12S101, D12S82 | 181430 | SPMD | 12q13.3–q15 |
| D2S1245 | 603689 | Edstrom myopathy | 2q24–q31 |
| D21S1255, D21S1903 | 105400 | ALS1 | 21q22.1 |
| D2S345, D2S2328 | 158810 | Bethlem myopathy (COL6A3) | 2q37 |
| D21S1903, D21S1897 | 158810 | Bethlem myopathy (COL6A1, COL6A2) | 21q22.3 |
| D5S500, D5S414 | 159000 | LGMD1A | 5q22.3–q31.1 |
| D1S305 | 159001 | LGMD1B | 1q11–q21 |
- * Online Mendelian Inheritance in Man (OMIM). 2001. Baltimore. John Hopkins University Center for Medical Genetics. http://www3.ntm.gov/omim/.
MATERIALS AND METHODS
Clinical Studies
Clinical studies were approved by the Springfield Committee for Research Involving Human Subjects and consent was obtained from nine members of a two-generation family (Fig. 1). Clinical, biochemical, and radiological studies and past medical records were reviewed. Diagnosis for muscular dystrophy was based on the presence of muscle weakness and elevated creatine phosphokinase (CPK) levels in the blood. Skeletal surveys including the skull, spine, hips, long bones, and hands and feet were performed to evaluate Paget disease of bone. Quantification of bone-specific alkaline phosphatase and osteocalcin levels was performed by Quest Diagnostics (San Juan Capistrano, CA) and Emory University, (Atlanta, GA), respectively, in order to detect evidence of Paget disease.
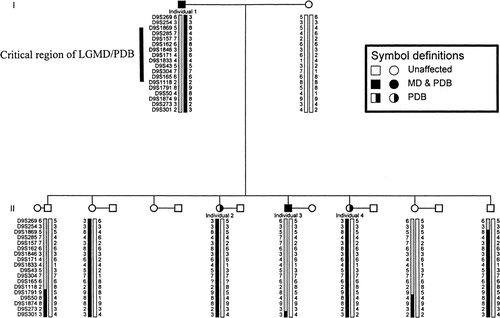
Haplotype analysis of critical region on chromosome 9 for a family affected with autosomal dominant SPMD and PDB. Circles denote females, squares denote males, slashed symbol denotes deceased individuals.
DNA Marker Analysis
Peripheral venous blood was obtained by standard venipuncture. Genomic DNA was extracted by means of the PUREGENE DNA Isolation Kit (Gentra Systems; Minneapolis, MN). In cases where blood samples could not be obtained, buccal scrapings were collected and processed using standard methods. Genotyping was performed to exclude a region previously linked to autosomal dominant limb-girdle like muscular dystrophy and PDB, in addition to known loci for Paget disease of bone, scapuloperoneal muscular dystrophy, amyotrophic lateral sclerosis, Bethlem myopathy, and limb-girdle muscular dystrophy (Research Genetics, Inc., Huntsville, AL).
Microsatellite Repeat Analysis
Genotyping was performed with markers for chromosome 9p22.3-q12, the critical region previously identified [Kovach et al., 2001]. PCR amplification using microsatellite markers was performed on the DNA samples using standard procedure. The microsatellite markers were analyzed following amplification by PCR with forward primers tagged with fluorescent dyes. Reactions consist of 15 ng genomic DNA, 0.8 μM appropriate primers, 200 μM deoxynucleotide triphosphates, and 0.5 units Taq polymerase in a buffer of 10 mM Tris-HCl (pH 8.3), 50 mM KCl, and 25 mM MgCl2. After an initial denaturing step of five min at 95°C, amplification was performed for 35 cycles (one min/95°C; one min/55°C; one min/72°C) followed by a final extension at 72°C for seven minutes. Amplification products prepared in a denaturing formamide loading solution were analyzed on a 6% denaturing polyacrylamide gel by electrophoresis for one–three hr. The results were then visualized with an FMBIO-100 fluorescent image-scanning unit (Hitachi Genetic Systems, Mirai Bio, Inc., Alaineda, CA). A PowerPlex allelic ladder (Promega, Inc., Madison, WI) was loaded onto each gel, to permit sizing of individual alleles.
RESULTS
Clinical Description
Nine members of a two-generation family from Ohio participated in the study. The family included an affected father and eight children, three of whom are affected (one male, two females). Clinical data based upon physical examination, past medical records, biochemical and radiological studies in affected individuals are summarized in Table II and laboratory data are summarized in Table III.
| Case | Age | Sex | PDB | Myopathy | Age onset of PDB | Age onset of myopathy |
|---|---|---|---|---|---|---|
| 1 | 70 | M | + | + | 43 | 44 |
| 2 | 43 | F | + | − | 41 | − |
| 3 | 41 | M | + | + | 41 | 37 |
| 4 | 40 | F | + | − | 40 | − |
| Mean | 48 | 2M/2F | 4 | 2 | 41 | 41 |
- +, affected; –, unaffected.
| Case | Alkaline phosphatase (30–130 U/L) | Osteocalcin (8–52 ng/mL) | CPK (20–220 U/L) | Pyridinoline (23.2–43.2 μmol/mol) | Deoxypyridinoline (6.4–11.0 μmol/mol) | X-ray findings of Paget |
|---|---|---|---|---|---|---|
| 1 | 530 | 140 | 40 | 941.4 | 195.4 | Widespread |
| 2 | 186 | ND | 158 | ND | ND | Widespread |
| 3 | 331 | 3 | 662 | ND | ND | R hemipelvis and proximal femur |
| 4 | 299 | 1 | 95 | 23.5 | 5.5 | Humerus |
| Mean | 336.5 | 48 | 239 |
- ND, not determined.
Individual 1, age 70 years, was diagnosed with Paget disease of the left knee at 43 years, and evidence of muscular weakness and foot drop was noted at 44 years. Progression of the disorder included weakness in the legs and shoulders causing scapular winging. An EMG at 48 years suggested a dystrophic rather than a neuropathic process. Muscle biopsy showed changes of a chronic, active, primary myopathic disorder. Presently, he has a severe, generalized, distal > proximal weakness. He has a tracheostomy and gastrostomy and is bedbound. His PDB is generalized and severe, and has caused deformity of his long bones. Previously, his creatine phosphokinase (CPK) level had been elevated to 776 U/L; however, it is currently normal at 40 U/L (normal range, 60–222 U/L). His alkaline phophatase level (530 U/L, normal range, 30–130 U/L) and osteocalcin level (140 ng/mL, normal range, 8–52 ng/ml) are markedly elevated. His Paget disease is currently treated with residronate (Actonel).
Individual 2, a female 43 years of age, had onset of pain at age 28 years in her neck, spine, and lower back followed by hip and shoulder pain. However, she was not formally diagnosed with Paget disease of the pelvis, hip, arm, and spine until age 41 years. X-rays showed abnormally increased activity in the right humeral head, scapula, lumbar and sacral spine, and pelvis. After treatment with Fosamax (alendronate), her moderately elevated alkaline phosphatase level of 186 U/L was reduced to 76 U/L, and radiological activity of PDB decreased. An EMG three years ago was reported as normal. Recently, weakness in the extensor hallucis longus, peroneus longus, and particularly the long toe flexors was noted along with “achy shoulders” and difficulty lifting her arms.
Individual 3, a male 41 years of age, noticed foot drop at age 37 years. He has noticed no progression of footdrop; however, his legs have become noticeably thinner with some bowing. He wears a bilateral brace for weakness in his lower legs. Additionally, he experiences difficulty lifting his arms. Upon recent examination, both proximal muscle weakness involving the deltoid muscles and peripheral weakness, particularly of the foot dorsiflexors, was noted. An EMG showed acute denervation potentials in several muscles. Nerve conduction studies were normal. CPK levels are markedly elevated at 662 U/L (normal range 20–220 U/L). Testing for fascioscapulohumeral muscular dystrophy by FISH (fluorescent in situ hybridization) was negative for the deletion on chromosome 4q35. Recent radiological studies revealed an increased prominence of the trabeculae of the right hemipelvis and right proximal femur with minimal cortical thickening suggestive of early Paget disease of bone. Additionally, his alkaline phosphatase level is elevated to 331 U/L.
Individual 4, a female age 40 years, was diagnosed with Paget disease in the right humerus at age 39 years. Treatment with residronate (Actonel) has been successful for her right arm pain; however, she subsequently developed pain in her left humerus. No muscular weakness was noted on examination, although there is tenderness reported in all muscle groups. Her CPK was normal and alkaline phosphatase was elevated to 299 U/L.
Laboratory Evaluation
Paget disease of bone
Four individuals had features of PDB with bone pain, elevated alkaline phosphatase levels, and radiological evidence of PDB (Table III). The mean value of total alkaline phosphatase in affected individuals prior to treatment was 336.5 U/L with a range of 186–530 IU/L. All members unaffected by PDB had normal alkaline phosphatase levels. Osteocalcin was normal in all affected members except in the oldest individual. His osteocalcin was elevated to 140 ng/ml (normal range, 8–52 ng/ml). In addition, his urine pyridinoline/deoxypyridinoline cross links were substantially elevated (Table I).
Myopathy
The two individuals with myopathy had initial foot drop. The weakness is more severe in the distal muscle groups. Progression of the disorder in individual 1 has included severe muscle weakness, particularly in the legs, feet, arms, shoulders, and pelvic girdle. CPK levels were elevated in the early stages of the myopathy to776 U/L in individual 1 and 662 U/L in individual 3.
Muscle biopsy
A biopsy on individual 3 was obtained at age 41 years. Hematoxylin and eosin staining showed variation in myofiber size and mild interstitial fibrosis. Both hypertrophic and atrophic fibers were present. Scattered split fibers and degenerating myofibers were seen. Rare rimless vacuoles were noted. There was minimal interstitial and perivascular lymphohistiocytic infiltration and no evidence of vasculitis. Enzyme histochemistry with DPNH, ATPase at 4.6 and 9.8, Gomori trichrome, esterase, oil red O, PAS, cytochrome oxidase, and Congo red highlighted these findings. ATPase at 4.6 and 9.8 showed atrophic fibers to be type I and II. Congo red did not show amyloid deposits in the vacuoles. Overall findings were consistent with a myopathy with a significant denervation component. Immunohistochemical stains for dystrophin 1, 2, and 3 and alpha-sacroglycan were normal.
Muscle biopsy on individual 1 obtained 16 years ago showed prominent changes compatible with an active primary myopathy with a neurogenic component. Immunocytochemistry was not performed on this sample. Because of the advanced stage of his disease, a muscle biopsy has not been repeated.
Electromyographic (EMG) studies
EMGs performed on the two individuals with myopathy were suggestive of a dystrophic process. There was no suggestion of a motor neuron disease. Nerve conduction velocity studies were normal.
Genotype analysis
Individuals in the family were genotyped with molecular markers for candidate disease loci. Genetic recombinations observed between disease and these markers in affected individuals excluded all known loci for PDB, SPMD, ALS, LGMD1A, and LGMD1B (Table I). Figure 1 presents haplotype analysis of markers spanning the critical region for chromosome 9p22.3-q12 in autosomal dominant limb-girdle muscular dystrophy and Paget disease of bone (Kovach et al., submitted for publication), flanked by markers D9S1869 and D9S1118. Affected individuals 2 and 4 share a common haplotype that differs from affected individual 3. The father (individual 1) is homozygous for markers D9S43 through D9S1118; therefore, it is possible that these individuals share a common disease allele within this interval. However, this is unlikely since the genotype of flanking markers is consistent with a haplotype without recombinations.
DISCUSSION
Although neurological complications of PDB are frequently encountered because of the intimate relationship of the central nervous system and skeletal structure, the association of Paget disease with a neuromuscular disorder is rare. The four previous reports of an association of PDB with a neuromuscular disorder have been clinically diverse. Caughey et al. [1957] described a family diagnosed with myotonic dystrophy, Tucker et al. [1982] reported a family with amyotrophic lateral sclerosis, and McBride [1966] and Kimonis et al. [2000] described a myopathy with a limb-girdle distribution. In the report by Kimonis et al., individuals develop proximal weakness at a mean age of 45.6 years. PDB onset is at a mean age of 41.5 years and affects primarily the pelvis and hips with sparing of the long bones. Kovach et al. [2001] mapped the gene for autosomal dominant limb-girdle myopathy/inclusion body myopathy and Paget disease of bone in this family to 9p22.3-q12.
This report provides evidence for clinical and molecular heterogeneity within this unique disorder. In contrast to the distal > proximal weakness, heralded by foot drop pattern in the legs, a proximal > distal pattern is evident in the upper extremities. This family's myopathy is suggestive of scapuloperoneal muscular dystrophy (SPMD) rather than LGMD. The myopathy in this family appears non-specific on muscle histology with rare rimless vacuoles and normal immunocytochemistry. The present family has elevated CPK levels during active forms of the disorder; this had previously been noted in only one individual—in the family reported by Kimonis et al. [2000]—and in no previous reports. In the Kimonis et al. [2000] report, PDB preceded the myopathy, which is progressive, therefore the lack of penetrance of the myopathy in two individuals in this family may be age-related.
The distribution of the PDB in this family is also different. Early distribution of Paget disease in the present family occurs primarily in the long bones associated with deformity and eventually progresses to the spine and other bones. Kimonis et al. [2000] reported PDB primarily in the spine and pelvis.
Exclusion of the chromosome 9p22.3-q12 region, in addition to the known loci for autosomal dominant PDB, SPMD, ALS, Bethlem myopathy, LGMD1A, and LGMD1B, reveals that this disorder is indeed unique. Although LGMD1C and LGMD1D were not excluded, the reported clinical features are very different from these cases. Studies in additional families with a similar phenotype are essential for identification of the genes in this rare combination of disorders, thus permitting understanding of the common biochemical defects of both bone and muscle.
Acknowledgements
We thank the members of the family and their physicians for their participation in our study.




