A phase 2 trial of mini-hyper-CVD, blinatumomab, and ponatinib in Philadelphia positive acute lymphoblastic leukemia
Wei-Ying Jen and Elias Jabbour are contributed equally to this work.
Graphical Abstract
Twenty adults with newly diagnosed (ND) or relapsed/refractory (RR) Ph-positive acute lymphoblastic leukemia (ALL), or chronic myeloid leukemia in lymphoid blast phase (CML-LBP), were treated with mini-hyperCVD, ponatinib, and blinatumomab. Complete molecular response was achieved in 78% of ND patients, while CR/CRi was achieved in 100% of RR and CML-LBP. The 3-year overall survival rate was 76% (95% CI, 47%–90%).
Resistance to the BCR::ABL1 tyrosine kinase inhibitors (TKIs) in Philadelphia chromosome (Ph)-positive acute lymphoblastic leukemia (ALL) is driven by the emergence of ABL1 kinase domain T315I mutations. Hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone, alternating with methotrexate and cytarabine (HyperCVAD), with ponatinib produces complete molecular response (CMR) rates of 86% and 6-year overall survival (OS) of 76%.1
The chemotherapy-free combination of blinatumomab and ponatinib has shown promising activity in newly diagnosed (ND) Ph-positive ALL, with a CMR rate of 89% and a 2-year OS of 89%.2 However, patients with relapsed/refractory (RR) disease or chronic myeloid leukemia in lymphoid blast phase (CML-LBP) did not fare as well. In the latter group, the CMR rate was 33%, with a 1-year event-free survival (EFS) of 50%. Furthermore, isolated CNS relapses have been observed with the chemotherapy-free approaches.
We hypothesized that combining low-intensity chemotherapy with ponatinib and blinatumomab is feasible for the treatment of ND or RR Ph-positive ALL or CML-LBP, while potentially addressing the emerging problem of isolated CNS relapse.
This was an open-label, single-arm, phase 2 trial of patients ≥18 years with ND or RR Ph-positive ALL, or CML-LBP. Prior treatment with up to two courses of chemotherapy was allowed as patients may have been treated elsewhere before transfer to our institution, or had treatment initiated before confirmation of Ph-positive status. If patients were enrolled in complete remission (CR), they were only evaluable for survival and safety. Patients were required to have adequate organ function, a performance status ≤2, and no recent history of pancreatitis or active cardiovascular disease. Full inclusion and exclusion criteria are shown in Table S1. All patients provided written informed consent according to institutional guidelines and the Declaration of Helsinki. The study was approved by our Institutional Review Board.
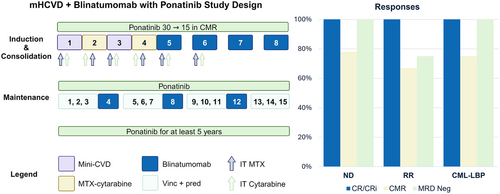
All patients received ponatinib 30 mg daily, reduced to 15 mg daily once in CMR (Figure S1). CMR was defined as the absence of detectable BCR::ABL1 transcripts by real-time quantitative polymerase chain reaction (RT-qPCR) at an assay sensitivity of ≤0.01% for p210 transcripts, or 4-log reduction from diagnosis for p190 transcripts. Ponatinib was continued uninterrupted throughout induction, consolidation, and maintenance, and then for at least 5 years thereafter.
All patients received induction with four courses of mini-hyper-CVD (mHCVD), alternating with mini-methorexate and cytarabine, the details of which have been published previously.3 Consolidation was four courses of blinatumomab 28 μg/day for 4 weeks, followed by a 2-week break. In the first blinatumomab course, the dose was 9 μg/day for the first 4 days to mitigate the risk of cytokine release syndrome (CRS) and CNS events. Fifteen courses of maintenance were administered. Courses 1–3, 5–7, 9–11, and 13–15 were with vincristine (2 mg on Day 1) and prednisone (50 mg on Days 1–5) every 4 weeks, while cycles 4, 8, and 12 were with blinatumomab 28 μg/day and lasted 6 weeks (4 weeks on, 2 weeks off, Figure S1). Twelve intrathecal injections of alternating methotrexate (12 mg) and cytarabine (100 mg) were given for CNS prophylaxis. Patients with active CNS disease were treated with twice-weekly triple (cytarabine 40 mg, methotrexate 12 mg, hydrocortisone 50 mg) intrathecal injections until disease clearance, then weekly x4, then every other week x4, and then monthly for up to 20–22 doses. Allogeneic stem cell transplant (allo-SCT) was performed at investigator discretion.
The primary outcome measure was CMR at any point for ND patients and overall response rate (ORR, complete remission [CR] + CR with incomplete hematological recovery [CRi]) for RR patients. Major molecular response (MMR) was defined as BCR::ABL1 ≤ 0.1% for p210 transcripts and a 3-log reduction from baseline for p190 transcripts. Measurable residual disease (MRD) was assessed by multicolor flow cytometry (MFC, sensitivity 10−4) and/or next-generation sequencing (NGS, sensitivity 10−6). The NGS assay was a Food and Drug Administration-approved commercial assay (clonoSEQ®, Adaptive Biotechnologies Corporation, Seattle, WA), which utilized disease-specific rearrangements of immunoglobulin heavy and light chains. It was sent at diagnosis and at follow-up bone marrow biopsies at the discretion of the treating physician. Survival endpoints included OS (time from treatment start to death from any cause), event-free survival (EFS; time from treatment start to failure [refractory disease, relapse or death]), and relapse-free survival (RFS; time from CR to relapse or death).
Statistical analysis was done with R, version 4.3.1 (R Foundation, Austria). Survival curves were plotted with the Kaplan–Meier method.
Twenty patients were treated (Figure S2): 12 ND, 4 RR, and 4 CML-LBP. Baseline characteristics are shown in Table S2. There was no difference in baseline characteristics between ND patients enrolled in CR and treatment-naïve patients, except for a higher proportion of treatment-naïve patients having a p210 transcript (Table S3). Patients enrolled in CR were treated with HyperCVAD or mHCVD and a TKI (dasatinib, only one received ponatinib) prior to enrollment. Five ND patients (42%) with fluorescence in situ hybridization performed had BCR::ABL1 detected in myeloid cells.4 Two patients with CML-LBP progressed from underlying CML after treatment with imatinib and dasatinib respectively, one with a T315I mutation at enrollment.
Responses are presented in Table S4. In the ND cohort, CMR was achieved in seven of nine (78%) evaluable patients, with five (71%) achieving it by completion of mHCVD. All evaluable ND patients achieved MRD negativity by MFC (n = 7) and NGS (n = 5). Of the five NGS-negative patients, one had discordant BCR::ABL1 results; this patient never achieved CMR. None of the ND patients underwent allo-SCT in CR1.
In the RR and CML-LBP cohorts, four of eight (50%) were in CR at enrollment, of whom one was in CMR. All evaluable patients achieved CR, with CMR achieved in five of seven (71%). Three patients underwent allo-SCT, and two received ponatinib maintenance post-allo-SCT at the discretion of the transplant physician.
Two (17%) ND patients who achieved CMR relapsed. One had isolated CNS relapse (nonadherent to ponatinib, no CNS disease at baseline) and one had MRD relapse after 13 and 21 months. One RR patient never obtained CMR or NGS MRD negativity and relapsed after 3 months. ABL1 kinase domain mutation analysis revealed T315I, E255K, and E255V mutations.
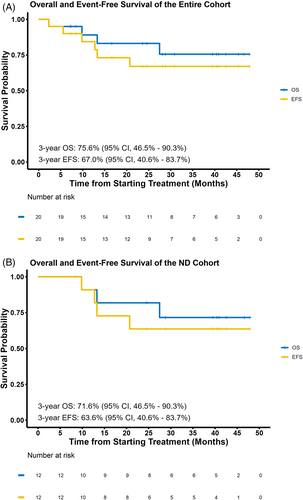
At a median follow-up of 31 months (95% confidence interval [CI], 24–41), the median OS, EFS (Figure 1), and RFS were not reached for the entire cohort. In the ND cohort, the 3-year OS, EFS, and RFS rates were 72% (95% CI, 49–100), 64% (95% CI, 41–100), and 64% (95% CI, 41–100), respectively. Treatment-naïve patients had a 3-year EFS of 100% (95% CI, 100–100). For the RR cohort, the 3-year OS, EFS, and RFS rates were 75% (95% CI, 43–100), 50% (95% CI, 19–100), and 50% (95% CI, 19–100), respectively. In CML-LBP, the 2-year OS, EFS, and RFS rates were 100% (95% CI, 100–100).
There were three (15%) deaths on-study. The 60-day mortality was 0%. Two non-neutropenic ND patients died in CR from complications of COVID-19 prior to the availability of COVID-19 vaccines. One RR patient with CNS involvement at diagnosis and arachnoiditis from prior intrathecal cytarabine died in CR from progressive necrotizing leukoencephalopathy. No leukemia-related deaths have occurred. Treatment-emergent adverse events (TEAE) are shown in Table S5. The most common grade ≥3 TEAEs were infections in 11 (55%) and febrile neutropenia in 5 (25%). Ponatinib was discontinued in one patient who had a grade 3 pulmonary embolism. One patient had grade 3 altered mental status during blinatumomab maintenance, which resolved with steroids and interruption of blinatumomab. There were no CRS events.
The combination of mHCVD with ponatinib and blinatumomab is effective and safe in Ph-positive ALL and CML-LBP. In ND patients, the CMR rate was 78% with a 3-year OS rate of 72%, despite no patients undergoing allo-SCT. All patients with RR Ph-positive ALL and CML-LBP with active disease at the time of enrollment achieved CR.
Chemotherapy-free regimens have shown excellent outcomes in Ph-positive ALL.2, 5 However, they may not be a good option in RR Ph-positive ALL, or CML-LBP. In the ponatinib and blinatumomab study, 6 of 60 (10%) had CML-LBP; only 2 (33%) achieved CMR, compared with a CMR rate of 87% in de novo Ph-positive ALL.2 In our study, 12 of 20 (60%) patients had a predominant p210 BCR::ABL1 transcript, typically associated with CML, with BCR::ABL1 detected in myeloid cells. Despite this, we observed a high CMR rate of 75%. In the five ND patients with BCR::ABL1 in myeloid cells, the CR and CMR rates were 100%, suggesting promising activity.
Another issue with the chemotherapy-free regimens is the lack of CNS penetration. There were four (6%) isolated CNS relapses in each of the ponatinib-blinatimomab and dasatinib-blinatumomab studies.2, 5 Six of eight of these occurred after 20 months of CR, suggesting that long follow-up is necessary to detect CNS relapses. With our strategy incorporating cytotoxic CNS-directed chemotherapy, we have observed one isolated CNS relapse after 31 months of follow-up. However, given the small sample size of this trial, larger numbers are required before any conclusions about the risk of CNS relapse with this regimen can be made. Presenting leukocytosis >70 × 109/L is associated with an increased risk of CNS relapse (article in press), and whether mHCVD can adequately address this remains to be defined. For Ph-positive ALL, we have increased the number of intrathecal chemotherapy injections to 15. However, chemotherapy with high-dose methotrexate and cytarabine may be required if isolated CNS relapses persist.
Consolidation allo-SCT has been recommended for Ph-positive ALL. In a report of ponatinib with intensive chemotherapy, 26 of 30 ND patients underwent allo-SCT in CR1.6 In our study, the rate of allo-SCT was low due to the high rate of CMR and NGS MRD negativity. Our practice is to defer allo-SCT in patients achieving 3-month CMR, as we have previously shown that it does not improve OS or RFS. In addition, although not all patients achieved CMR, recent data suggest that persistent low-level BCR::ABL1 may reflect a CML-like chronic phase, rather than true residual ALL. All seven patients (5 ND, 2 RR/CML-LBP) who achieved undetectable MRD by NGS remain alive and relapse-free, despite one never achieving CMR. In contrast, two patients in CMR relapsed, suggesting that NGS may be a better discriminator of relapse risk, and that patients achieving NGS MRD negativity could possibly be cured without allo-SCT. Accordingly, we now use NGS to guide allo-SCT decisions, in preference to RT-qPCR, and will report on these outcomes.
The main limitation of the study is the small sample size. In light of the favorable results of this combination regimen, we are currently expanding accrual to confirm these findings and identify patients who may benefit from this strategy. We postulate that this regimen will be of value in select patients, particularly in CML-LBP, where the addition of chemotherapy may be necessary for optimal disease control.
AUTHOR CONTRIBUTIONS
EJ and HMK designed the study. RG and WYJ collected and analyzed the data. EJ and WYJ wrote the manuscript. HMK revised the manuscript. NJS, GCI, FGH, NJ, NP, NGD, LM, GB, and KC recruited and treated patients in the study. All authors reviewed and approved the final version of the manuscript.
FUNDING INFORMATION
Ponatinib was provided free of charge by Takeda Oncology, which also provided study funding. Blinatumomab was provided free of charge by Amgen. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. This research is supported in part by the MD Anderson Cancer Center Leukemia SPORE CA100632.
CONFLICT OF INTEREST STATEMENT
EJ has received consulting fees and research funding from Abbvie, Pfizer Inc., BMS, Amgen, Takeda Oncology, and Adaptive Biotechnologies. NJS has received consulting fees from Pfizer Inc., GlaxoSmithKline, NKARTA, Autolus and Sanofi, research funding from Takeda Oncology, Astellas Pharma Inc., Xencor, Stemline Therapeutics and NextCure and honoraria from Adaptive Biotechnologies, Novartis, Amgen, Takeda Oncology, Pfizer Inc., Astellas Pharma Inc., Sanofi, and BeiGene. GCI received consultancy or advisory role fees from Novartis, Kura Oncology, Syndax Pharmaceuticals, AbbVie, and NuProbe and received research funding from Celgene, Novartis, Kura Oncology, Syndax Pharmaceuticals, Merck, Cullinan Oncology, Astex, and NuProbe. NP sits on the Board of Directors of Dan's House of Hope, has received consulting or advisory role fees from AbbVie, Aplastic Anemia & MDS International Foundation, Aptitude Health, Astellas Pharma US, Blueprint Medicines, Bristol-Myers Squibb Pharmaceuticals, CancerNet, CareDx, Celgene, Cimeio Therapeutics AG, ClearView Healthcare Partners, CTI BioPharma, Curio Science, Dava Oncology, EUSA Pharma, Harborside Press, Imedex, Immunogen, Intellisphere, Karyopharm, Magdalen Medical Publishing, Medscape, Menarini Group, Morphosys, Neopharm, Novartis Pharmaceuticals, OncLive, Pacylex, Patient Power, PeerView Institute for Medical Education, Pharma Essentia, Physician Education Resource (PER), and research grant support from the United States Department of Defense (DOD) and National Institute of Health/National Cancer Institute (NIH/NCI). NGD has received grants or contracts from Hanmi, Trovagene, Fate Therapeutics, Novimmune, and GlycoMimetics; consulting fees from Arog, Novartis, Jazz, Celgene, Syndax, Shattuck Labs, and Agios; and grants or contracts and consulting fees from Daiichi-Sankyo, Bristol-Meyers Squibb, Pfizer, Gilead Sciences, Inc., Servier, Genentech, Astellas, AbbVie, ImmunoGen, Amgen, and Trillium. LM is on the Advisory Board for MorhoSys. HMK has received honoraria, consulting, or advisory role fees from AbbVie, Amgen, Amphista, Ascentage, Astellas, Biologix, Curis, Ipsen Biopharmaceuticals, KAHR Medical, Labcorp, Novartis, Pfizer, Shenzhen Target Rx, Stemline, Takeda, and research grants from AbbVie, Amgen, Ascentage, BMS, Daiichi-Sankyo, Immunogen, Jazz, and Novartis. WYJ, GB, and RG declare no relevant conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are not publicly available due patient confidentiality, but are available from the corresponding author on reasonable request.