2025 update on clinical trials in immune thrombocytopenia
Abstract
Although the development and regulatory approval of the thrombopoietin receptor agonists revolutionized aspects of the immune thrombocytopenia (ITP) treatment landscape over the past two decades, there remain many areas of high unmet need. Therefore, a number of investigational and repurposed agents are currently undergoing clinical development in ITP. In a departure from historical trials, which largely focused on the indefinite treatment of persistent or chronic ITP, ongoing trials run the gamut of disease phases, and include novel agents being evaluated in early phases of the disease to attempt to modify the disease course. Many agents in development target disease pathophysiologic mechanisms not previously targeted by agents in current use, including platelet autoantibody recycling, B-cell maturation and differentiation, long-lived plasma cells, and the complement system, among others. These agents represent promising treatment options for patients with otherwise refractory disease or who are intolerant of currently available therapies. Additionally, with our increasing understanding of the diverse immune mechanisms at play in ITP, the expansion of the therapeutic armamentarium to include agents targeting diverse pathophysiologic mechanisms may allow a more personalized therapeutic selection in the future. This manuscript provides an up-to-date, in-depth overview of recently completed and ongoing clinical trials in ITP.
1 INTRODUCTION
Immune thrombocytopenia (ITP) is an autoimmune platelet disorder resulting in bleeding and constitutional symptoms that afflicts approximately one in 20 000 people worldwide.1 Although initially believed to occur due to the production of glycoprotein-specific platelet autoantibodies leading to destruction of platelets in the reticuloendothelial system, the pathogenesis of ITP is now recognized to be more complex, involving numerous other aspects of the immune system including cytotoxic T cells and complement.2-4 Additionally, the disease is now recognized as one of both increased platelet destruction but also inadequate platelet production, due to the impact of glycoprotein-specific autoantibodies and cytotoxic T cells on bone marrow megakaryocytes. As our understanding of the underlying pathophysiology has grown, so have our therapeutic options to manage ITP. The development, approval, and clinical refinement of the thrombopoietin receptor agonists (TPO-RAs) has revolutionized the management persistent and chronic ITP, offering a highly efficacious, safe, and well-tolerated medical treatment option offering durable disease control to many patients.5, 6 Additionally, robust studies of the off-label anti-CD20 agent rituximab and the development and regulatory approval of the spleen tyrosine kinase (Syk) inhibitor fostamatinib in ITP have further expanded the treatment armamentarium for a disease previously reliant on steroids, splenectomy and salvage therapies.1, 5, 6
However, despite the availability of TPO-RAs, fostamatinib, and rituximab to treat ITP, many unmet needs remain in this disease.7 While the potential for a sustained treatment-free response has been recognized in a minority of chronic ITP patients treated with TPO-RAs for extended periods, no approved medical therapy has demonstrated the ability to modify the disease course—to prevent the progression to chronic disease from early phase ITP, or to durably reduce the severity of the disease such that long-term treatment is not needed. Very few advancements have been made over the past three decades in the treatment of acute ITP exacerbations, which may result in prolonged hospitalizations and bleeding. And patients with ITP refractory and/or intolerant to standard therapies remain a very challenging and often life-defining medical problem with few good solutions.
Thankfully, therapeutic development in ITP has never been more prolific or more promising. Agents targeting entirely different aspects of disease pathophysiology from the presently approved drugs are now in or entering late-stage clinical development, and as a result, multiple new drug approvals are possible over the next few years. These drugs, which include neonatal Fc receptor antagonists, inhibitors of the Bruton's tyrosine kinase (BTK), agents targeting B-cell differentiation and proliferation, complement antagonists, plasma cell-targeted agents, and others hold promise in addressing the various unmet needs in ITP. As of June 2024, the United Stated clinicaltrials.gov study database lists 92 ITP studies that are actively recruiting patients. While it is impossible to describe all of the potential development in ITP, this updates in clinical trials for hematological diseases review will focus on those drugs and studies furthest along in development and holding the greatest promise in addressing the current unmet needs in ITP. Table 1 summarizes the agents discussed in detail in this article.
| Agent | Structure and mechanism of action | Mode of administration | Stage of development in ITP |
|---|---|---|---|
| Efgartigimod | Modified Fc fragment blocking the neonatal Fc receptor | Intravenous or subcutaneous | Completed phase 3 |
| Rilzabrutinib | Small molecule Bruton's tyrosine kinase inhibitor | Oral | Completed phase 3 |
| Ianalumab | Monoclonal antibody directed against BAFF-R | Intravenous | Ongoing phase 2 and phase 3 |
| Povetacicept | Fc fusion protein that clears BAFF and APRIL | Subcutaneous | Ongoing phase 2 |
| Daratumumab | Anti-CD38 monoclonal antibody | Subcutaneous | Ongoing phase 2 |
| Mezagitamab | Anti-CD38 monoclonal antibody | Subcutaneous | Completed phase 2 |
| CM313 | Anti-CD38 monoclonal antibody | Intravenous | Completed phase 2 |
| Sovlepenib | Small molecule spleen tyrosine kinase inhibitor | Oral | Completed phase 3 |
| Cevidoplenib | Small molecule spleen tyrosine kinase inhibitor | Oral | Completed phase 2 |
| Sutimlimab | Anti-complement C1s monoclonal antibody | Intravenous | Completed phase 1 |
| Iptacopan | Small molecule complement factor B inhibitor | Oral | Ongoing phase 2 |
- Abbreviations: APRIL, a proliferation-inducing ligand; BAFF, B-cell activating factor; BAFF-R, B-cell activating factor receptor.
2 NEONATAL FC RECEPTOR ANTAGONISM
2.1 Background and therapeutic rationale
The neonatal Fc receptor (FcRn) antagonists are a new class of parenteral therapeutic with great promise in the treatment of diverse humoral autoimmune disorders.8 Given the well-documented pathologic role of glycoprotein-specific autoantibodies in ITP,9, 10 their potential benefit has a clear rationale. The FcRn (named as such due to its initial discovery in the gut of neonatal rodents) is an immunoglobulin receptor critical in prolonging the half-life of circulating IgG to its physiologic 21 days due to its role in rescuing IgG from lysosomal degradation, and when inhibited, IgG half-life drops to approximately 7 days (Figure 1).11, 12 It also plays an important role in the passive transfer of IgG antibodies from mother to fetus.12 In generalized myasthenia gravis, ITP, and pemphigus, the FcRn antagonist efgartigimod has demonstrated reliable and durable reductions in serum IgG in the 60%–70% range.13-15 Because pathologic IgG autoantibodies rely on the FcRn for preservation and recycling like all other IgG antibodies, their titers drop to a similar degree. As one author put it, FcRn antagonists have the potential to function like “plasma exchange in a bottle” for certain indications.16 FcRn antagonists do not reduce levels of other immunoglobulin isotypes and the agents presently approved do not impact serum albumin levels, a concern in early development given the FcRn's additional role in recycling albumin at a distinct binding site from IgG.17
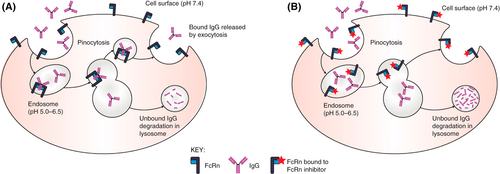
While not eliminating the pathologic autoantibody, a sustained reduction in its serum concentration may be adequate to reduce or eliminate disease manifestations. Of course, because physiologic IgG is also lowered, a fine balance must be achieved to minimize the risk of infections. Two FcRn antagonists, efgartigimod alfa and rozanolixizumab, have achieved regulatory approval for the treatment of generalized myasthenia gravis, and others, including nipocalimab and batoclimab, are in late-stage clinical development for this same indication. The development of the FcRn antagonists in ITP has been less straightforward, however. Data is published for both efgartigimod and rozanolixizumab in ITP, demonstrating both safety and efficacy in humans.14, 18, 19 However, the phase 3 trials of rozanolixizumab in ITP were halted by UCB, their sponsor, due to “a strategic business decision, not a safety decision” (NCT04200456, NCT04224688). While phase 2 and phase 3 randomized clinical trials of intravenous efgartigimod were successful, demonstrating clear safety and efficacy of this agent to treat ITP, the topline results of a phase 3 randomized trial of subcutaneous efgartigimod reported no significant improvement in platelet responses between the efgartigimod group and the placebo group.
2.2 Efgartigimod alfa
Efgartigimod alfa (Vyvgart, argenx SE, the Netherlands) is a first-in-class FcRn antagonist currently approved to treat generalized myasthenia gravis in adults. After a promising phase 2 study in ITP,19 efgartigimod entered phase 3 with two studies, each evaluating a different mode of administration of the drug. The first study, ADVANCE IV, evaluated intravenous efgartigimod,14 and the second, ADVANCE SC, evaluated subcutaneous efgartigimod in ITP. As a human IgG1 antibody Fc fragment, efgartigimod must be administered parenterally. Via a five amino acid substitution, efgartigimod has been bioengineered to have increased affinity for the FcRn at both neutral and acidic pH,20 facilitating its mechanism of outcompeting IgG for the FcRn and therefore resulting in increased lysosomal destruction of the latter.
In a phase 2 trial of intravenous efgartigimod in 38 adults with ITP (NCT03102593), patients were randomized 1:1:1 to receive four weekly infusions of efgartigimod 5 mg/kg, efgartigimod 10 mg/kg, or placebo.19 This study demonstrated the efficacy of intravenous efgartigimod in significantly improving platelet counts, decreasing bleeding events, and reducing plasma IgG levels in patients with ITP, with a favorable safety profile (headache was the most commonly observed adverse event).
Following the success of this trial, the ADVANCE IV study (NCT04188379), a pivotal phase 3 randomized, double-blind, placebo-controlled trial of intravenous efgartigimod in adults with persistent or chronic primary ITP, was completed.14 This trial was successful, with the main study results initially presented as a plenary abstract presentation at the 2022 American Society of Hematology Annual Meeting and Exposition. ADVANCE IV enrolled and randomized adults with persistent or chronic primary ITP and a platelet count <30 × 109/L at screening 2:1 to weekly intravenous efgartigimod 10 mg/kg or placebo. Concurrent stable doses of certain ITP therapies, such as corticosteroids and TPO-RAs, were allowed, and patients achieving a platelet count of ≥100 × 109/L in three out of the four initial platelet count measurements on drug were allowed to transition to every-other-week study drug infusions. The primary endpoint was a sustained platelet count response, defined as platelet count ≥50 × 109/L for at least four or the six final 2-weekly visits in the main study period without bleeding events. A total of 22% of patients randomized to efgartigimod achieved this primary endpoint, versus 5% of patients randomized to placebo. Notably, as was true in the trials of fostamatinib and in other modern-day chronic ITP trials, patients enrolled in ADVANCE-IV were heavily pretreated in general (with approximately two-thirds of enrolled patients having received three or more prior ITP therapies) and had a long duration of disease (mean time since diagnosis over 10 years). Ultimately, response rates in clinical trials of ITP must be interpreted in the setting of the definition of response (which differs from study to study) and the disease characteristics of the population under study. For example, International Working Group platelet response rates, a secondary trial endpoint, were 51% in the efgartigimod group and 20% in the placebo group. Other secondary endpoints related to platelet response were improved in the efgartigimod group relative to the placebo group, though the rate of bleeding events (rare in both groups) was similar in each group. Responding patients responded within 7 days on average (the first platelet count measurement after initiation). Treatment-emergent adverse events were similar in both groups, and serious treatment-emergent adverse events were reported in double the proportion of patients in the placebo group than the efgartigimod group (16% vs. 8%).
Following ADVANCE-IV, the ADVANCE-SC study (NCT04687072), a pivotal phase 3 randomized, double-blind, placebo-controlled trial of subcutaneous efgartigimod in adults with persistent or chronic primary ITP, was completed. While the main study results have not yet been published (in abstract form or otherwise), the topline results of the trial have been released. The primary endpoint in this study was the same as in ADVANCE-IV. In contrast to ADVANCE-IV, however, the ADVANCE-SC study did not meet the primary endpoint, nor did it meet any of the prespecified secondary endpoints, though it did find a favorable safety and tolerability profile consistent with previous trials. In ADVANCE-SC, 14% of patients randomized to efgartigimod achieved a sustained platelet count response, compared to 16% of patients randomized to placebo. As in ADVANCE-IV, patients in ADVANCE-SC were allowed to continue on concurrent stable doses of certain ITP therapies, including corticosteroids and TPO-RAs. The reasons for the discrepancy between the results of ADVANCE-IV and ADVANCE-SC are presently unknown and are currently a topic of great interest among clinical investigators in ITP. Intravenous efgartigimod has achieved regulatory approval in Japan for adult ITP, where it is available for routine use. Its mechanism of action is novel relative to currently approved therapies and it has been clearly demonstrated to have efficacy in heavily pretreated patients refractory to TPO-RAs and rituximab, solidifying its potential as a promising treatment for patients with difficult-to-treat ITP.
3 BTK INHIBITION
3.1 Background and therapeutic rationale
Inhibitors of the BTK have revolutionized the management of B-cell malignancies over the past decade and are now being investigated in the treatment of autoimmune diseases, including ITP, rheumatoid arthritis, Sjogren syndrome, and pemphigus.21-23 The first BTK inhibitor approved to treat B-cell malignancies, ibrutinib, is also the first BTK inhibitor approved to treat a nonmalignant immune-mediated disorder, graft-versus-host disease.24 The BTK plays crucial roles in B-cell maturation, antibody production, and macrophage Fcγ receptor-mediated signaling pathways.25 In contrast to the FcRn antagonists, BTK inhibitors are administered orally, and their multifaceted impacts on various aspects of the autoimmune response particularly important in ITP, including B-cell development, autoantibody production, and phagocyte function make them quite promising in the management of ITP.
A major barrier to the use of BTK inhibition in ITP is the nonselective nature of currently approved BTK inhibitors. As tyrosine kinase inhibitors, BTK inhibitors used to treat malignancies, such as ibrutinib and acalabrutinib, inhibit a number of other related tyrosine kinases including several important in normal platelet function, thereby resulting in a side-effect of inhibition of platelet aggregation and adhesion mechanisms downstream of the collagen receptor GPVI, GPIb and integrin αIIbβ3.26 Ibrutinib, which inhibits 21 kinases by ≥90%, has in particular been demonstrated to increase the risk of bleeding in patients receiving it, including those with normal platelet counts. Therefore, for BTK inhibition to be viable to treat patients with existing profound thrombocytopenia, inhibitors with greater specificity for the BTK such that platelet function is not significantly adversely affected are necessary. This is supported by the observation that patients with Bruton's agammaglobulinemia, an isolated hereditary BTK defect, do not have a bleeding propensity.27
3.2 Rilzabrutinib
Rilzabrutinib (PRN1008, Sanofi, France) is an oral inhibitor of the BTK being developed to treat autoimmune conditions instead of hematologic malignancies.28 Rilzabrutinib differs from typical BTK inhibitors currently used to treat lymphoid malignancies in multiple key ways. First, it is highly selective: in an evaluation of rilzabrutinib against a panel of 251 kinases, only six (including BTK) were >90% inhibited by the drug (in contrast to 21 that were >90% inhibited by ibrutinib).28 This greater selectivity allows for potent BTK inhibition while preserving platelet function, unlike ibrutinib and other BTK inhibitors which result in a platelet function defect (Figure 2). Furthermore, the fact that this selectivity allows rilzabrutinib to avoid significant inhibition of the PI3K-AKT pathway may decrease the risk of atrial arrhythmias with its use, a well-known and significant toxicity of ibrutinib.28 Secondly, rilzabrutinib binds the BTK covalently, inducing a potent inhibition rapidly after drug exposure, but is then rapidly cleared from the circulation. This rapid clearance may mean that potential off-target impacts of the drug are lower compared with other BTK inhibitors.23
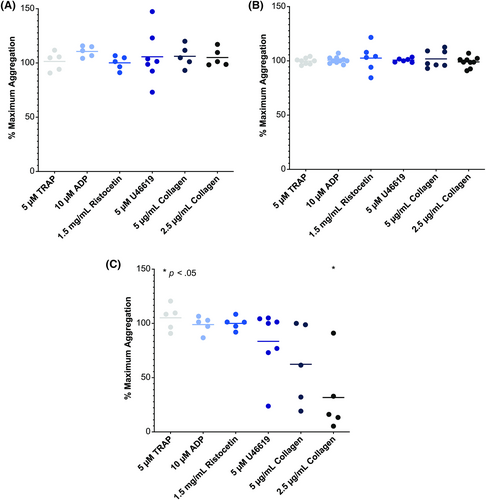
Two major clinical trials of rilzabrutinib in ITP have been completed and both reported positive results. The first (NCT03395210) was an adaptive, open-label, dose-finding phase 1/2 clinical trial of rilzabrutinib in 60 adults with chronic ITP.29 This clinical trial enrolled a refractory chronic ITP population, with one of the key eligibility criteria stating that patients must be “relapsed or refractory patients with no available and approved therapeutic options.” The characteristics of the enrolled patients certainly reflected this criterion, with a median of 4 prior ITP-directed therapies prior to enrollment, a median disease duration of over 6 years, and a median baseline platelet count of 15 × 109/L. Like other trials of heavily pretreated patients, patients could enroll with a stable low dose of chronic corticosteroid or stable dose of a TPO-RA, which was to be kept unchanged over the study period. The primary endpoint of a platelet response (defined here as a platelet count of ≥50 × 109/L with an increase of at least 20 × 109/L from the pretreatment baseline) was observed in 40% (24 of 60) patients enrolled. Higher doses of rilzabrutinib were more efficacious, with the highest evaluated dose of 400 mg twice daily having the best efficacy. Response was relatively rapid for a noncorticosteroid immunomodulating drug, with a median time to response of 11.5 days, and responses were quite durable and stable in those patients who responded; the mean percentage of weeks with a platelet count ≥50 × 109/L was 65%. Irrespective of the prespecified subgroup analysis (e.g., splenectomy vs. no splenectomy, receipt of concurrent ITP therapy vs. not, etc.), response rates hovered around 40%. Rilzabrutinib was also well tolerated, with no treatment-related bleeding events or thromboembolic events of grade 2 or higher, and no evidence of increased infection risk, cardiac arrhythmias, or other major organ toxicity (unlike BTK inhibitors currently used to treat lymphoid malignancies). All treatment-related adverse events were mild to moderate (grade 1 or 2) and relatively transient, with gastrointestinal events (diarrhea and nausea) occurring in approximately one-third of patients.
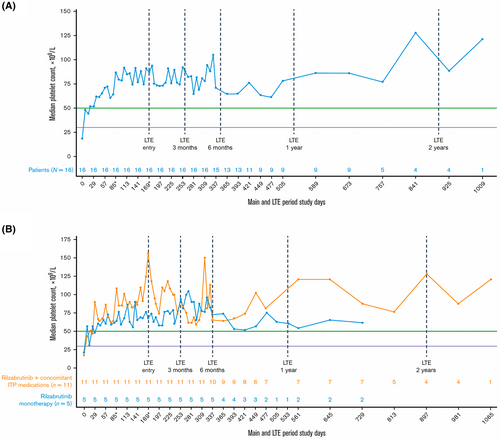
Results from the long-term extension of this phase 1/2 clinical trial have additionally been published.30 Sixteen patients who were responders in the double-blind portion of the study continued to the open-label extension; at a median treatment duration of 1.3 years, 11 of 16 patients continued to receive rilzabrutinib, and platelet count of ≥50 × 109/L was observed in 93% of these patients for over half of their monthly visits. Notably, 100% of platelet counts measured in the long-term extension were ≥30 × 109/L (Figure 3), and adverse events related to treatment continued to be grade 1 or 2 and transient, with no bleeding, thrombotic, or serious adverse events.
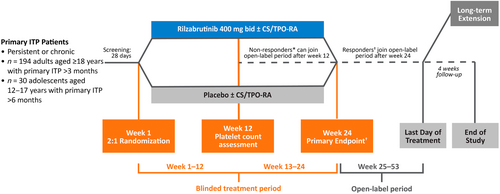
Following on the success of this initial trial, a phase 3, double-blind, randomized, global clinical trial of rilzabrutinib in ITP began (LUNA 3, NCT04562766).31 The design of the LUNA 3 study is illustrated in Figure 4. This study set out to enroll 194 adults with persistent or chronic ITP plus an additional pediatric arm of 30 children aged 12–17 years with primary ITP of a duration of greater than 6 months. The primary endpoint of LUNA 3 is a durable platelet response, defined as a platelet count ≥50 × 109/L for eight or more of the final 12 weeks of the 23-week double-blind treatment period without requiring rescue medication. As of the time of writing, topline results from the adult part of this trial have been announced in press release form. While the announcement did not contain quantitative results, it did state that the LUNA 3 study did achieve its primary endpoint of a durable platelet response in adults with persistent or chronic ITP, with a safety profile consistent with prior studies. Patients in this study had very similar baseline characteristics to the Phase I/II study, with a median of four prior ITP-directed therapies prior to enrollment and a median baseline platelet count of 15 × 109/L. The press release also stated that the study achieved positive results on key secondary endpoints. The complete results from LUNA 3 are eagerly awaited (including the pediatric portion, which is still ongoing) but given the impressive phase 1/2 study results and the known success of the phase 3 study (which incorporated a more rigorous primary endpoint), rilzabrutinib is likely to emerge as an efficacious and attractive oral treatment option for patients with persistent and chronic ITP.
4 B-CELL ACTIVATING FACTOR PATHWAY INHIBITION
4.1 Background and therapeutic rationale
Blockade of the signaling pathways critical to the activation, differentiation, and survival of B-cells remains a promising means to treat autoimmune diseases. Multiple cytokines and receptors are involved in this complex physiologic process, including the cytokines B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL), and the BAFF receptor and transmembrane activator and CAML interactor (TACI).32 Agents targeting these pathways have been approved for systemic lupus erythematosus (e.g., belimumab, a monoclonal antibody binding to BAFF) and are being actively investigated in other B-cell-mediated autoimmune diseases such as Sjogren syndrome. Patients with ITP have elevated levels of both BAFF and APRIL,33, 34 and a prior study evaluating the combination of rituximab and belimumab in patients with ITP has been completed, with promising results.35 Moreover, two novel agents targeting these pathways, ianalumab and povetacicept, are currently under investigation for the management of autoimmune cytopenias, including ITP, warm autoimmune hemolytic anemia, and cold agglutinin disease.
4.2 Ianalumab
Ianalumab (VAY736, Novartis) is a fully human IgG1 monoclonal antibody targeting BAFF receptor, thereby preventing activation and differentiation of B-cells and therefore the induction of long-lived plasma cells. Due to its specialized glycoengineering (afucosylation), it also functions as a potent mediator of antibody-dependent cell-mediated cytotoxicity against B-cells, allowing for a potent and sustained B-cell depletion in blood and tissues.36 Ianalumab is currently under investigation in a number of systemic autoimmune disorders, including systemic lupus erythematosus, pemphigus vulgaris, rheumatoid arthritis, Sjogren syndrome, ITP, and warm autoimmune hemolytic anemia.
Although no data in patients with ITP has been published, positive results in Sjogren syndrome have been published,37 and three clinical trials of ianalumab in ITP are presently ongoing (Figure 5). While the phase II VAYHIT3 study (NCT05885555) is an open-label, single-arm study evaluating ianalumab in 40 patients with ITP previously treated with at least a corticosteroid and a TPO-RA,38 capturing a population of subjects with more chronic, relapsed primary ITP (Figure 5C), the other two clinical trials take an entirely different approach. These large, pivotal phase III studies are evaluating the potential of ianalumab to modify the natural history of ITP in adults, seeking to provide for sustained responses off treatment via intervening to prevent development of long-lived autoantibody-producing plasma cells early in the disease course. The VAYHIT1 trial is a multicenter, double-blind, placebo-controlled phase III clinical trial randomizing 225 adults with newly diagnosed primary ITP 1:1:1 to lower-dose ianalumab plus corticosteroids, higher-dose ianalumab plus corticosteroids, or placebo plus corticosteroids (Figure 5A).39 Similarly, the VAYHIT2 trial is a multicenter, double-blind, placebo-controlled phase III clinical trial randomizing 150 adults with primary ITP with an insufficient response to or relapse after first-line corticosteroid or IVIG therapy 1:1:1 to lower-dose ianalumab plus eltrombopag, higher-dose ianalumab plus eltrombopag, or placebo plus eltrombopag (Figure 5B).40 Both VAYHIT1 and VAYHIT2 will use 4 monthly doses of ianalumab or placebo in each treatment arm, with tapering of corticosteroids (VAYHIT1) or eltrombopag (VAYHIT2). The primary endpoint for both VAYHIT1 and VAYHIT2 is the time from randomization to treatment failure, defined as a platelet count measured <30 × 109/L or need for rescue treatment later than 8 weeks from randomization (with additional criteria depending on the trial).

4.3 Povetacicept
Povetacicept (ALPN-303, Alpine Immune Sciences) is a dual inhibitor of the cytokines BAFF and APRIL. Structurally, it is an Fc fusion protein of a modified TACI domain engineered for increased affinity to both BAFF and APRIL, exceeding the affinity of wild-type TACI-Fc or of other BAFF or APRIL-specific monoclonal antibodies.41 Because both BAFF and APRIL are involved in B-cell activation, maturation, and differentiation but are nonredundant in their biological functions, simultaneous inhibition of both BAFF and APRIL may allow for an enhanced treatment effect over inhibition of one of these two critical cytokines alone (Figure 6).
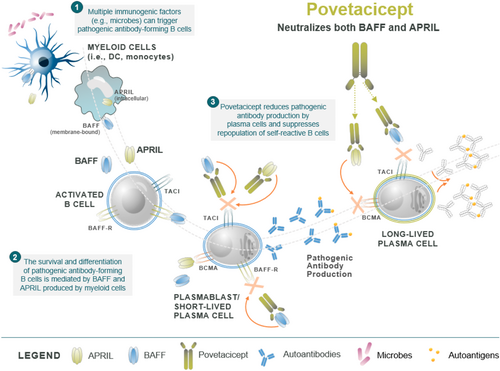
Povetacicept is currently under evaluation in a multicenter, group-sequential, parallel cohort phase 1b basket study of adults with ITP, warm autoimmune hemolytic anemia, or cold agglutinin disease (RUBY-4).42 In the ITP cohort, patients are required to have sustained persistent or chronic ITP and have a history of failure or relapse on two or more prior treatments including a TPO-RA. Participants are receiving povetacicept 240 mg subcutaneously once every 4 weeks for 24 weeks, with an optional extension period of 24 additional weeks. Each cohort aims to enroll seven to 14 patients. The primary objective of this study is safety, with efficacy, pharmacodynamics, and pharmacokinetics being evaluated as secondary objectives.
5 OTHER MECHANISMS: PLASMA CELL DEPLETION, NOVEL SYK INHIBITORS, AND COMPLEMENT INHIBITION
5.1 Plasma cell depletion (anti-CD38 agents)
Given the critical role of platelet autoantibodies in the pathophysiology of ITP and the fact that agents targeting B-cells do not deplete or inhibit long-lived plasma cells, the rationale for plasma cell-targeted therapeutics for the treatment of ITP is clear. CD38 (also known as cyclic ADP ribose hydrolase) is an enzymatic glycoprotein highly expressed on the surface of plasma cells.43 Agents targeting CD38, such as daratumumab, have been in use to treat plasma cell neoplasms for many years and are now under investigation for the treatment of ITP given their ability to deplete plasma cells via antibody-dependent cellular toxicity, antibody-dependent cellular phagocytosis, complement-dependent cytotoxicity, and direct apoptosis (Figure 7).43, 44
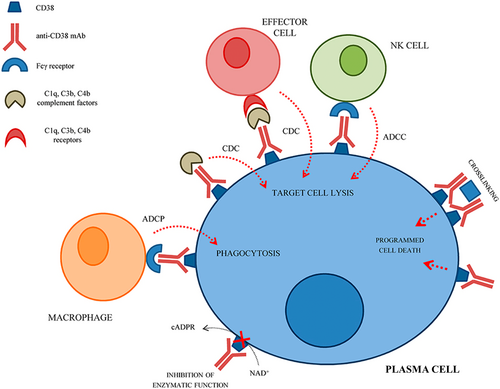
Three anti-CD38 antibodies are currently under investigation in the treatment of ITP. Following the publication of a number of case studies suggesting potential effectiveness of daratumumab in the management of autoimmune cytopenias,45-48 a phase II clinical trial of daratumumab in ITP, the DART study (NCT04703621), began and is presently ongoing.49 This study is evaluating the safety and efficacy of weekly subcutaneous daratumumab in adults with primary ITP who have progressed beyond second-line therapy (either a TPO-RA or rituximab). A second and anti-CD38 monoclonal antibody, mezagitamab (TAK-079, Takeda)50 is also under evaluation for the treatment of ITP. A phase 2, randomized, double-blind, placebo-controlled study of mezagitamab in adults with persistent or chronic primary ITP (TAK-079-1004, NCT04278924) is ongoing, with positive topline results from a prespecified interim analysis reported in press release form. This study is evaluating three different doses of subcutaneous mezagitamab versus placebo given once weekly for 8 weeks. All three doses tested demonstrated a higher platelet response with placebo, and dose-dependent responses with the greatest response in the highest dose group. The platelet response occurred “rapidly” and was maintained post-therapy in patients treated with mezagitamab. The drug was reported to be safe and well-tolerated across all three dose cohorts. Given these positive results, a global Phase 3 trial of mezagitamab in ITP is now planned. Lastly, CM313, a novel anti-CD38 monoclonal antibody administered intravenously, is currently in development in China. A recent 22-patient phase 1/2 study of CM313 (NCT05694767) in adults with persistent or chronic ITP who had previously failed glucocorticoids and at least 1 second-line ITP treatment found high response rates in this population and was well-tolerated.51 A larger phase 2 randomized, placebo-controlled study of CM313 in ITP is currently ongoing in China (NCT06199089).
5.2 Novel Syk inhibitors
Syk inhibition is a therapeutic mechanism already known to be efficacious in ITP, as the Syk inhibitor fostamatinib is U.S. FDA- and EMA-approved for the treatment of patients with chronic ITP who have had an insufficient response to a previous treatment.52, 53 Since the 2018 approval of fostamatinib (based on the FIT-1 and FIT-2 trials, which enrolled a population with heavily pretreated and chronic disease), real-world data has been published demonstrating much higher response rates to fostamatinib than was observed in the FIT-1 and FIT-2 trials.54 Syk signaling is important in a number of immune processes, most notably phagocytosis by splenic macrophages but also cytokine production and B-cell maturation. Two novel Syk inhibitors are currently in the development for the treatment of ITP.
Sovleplenib (HMPL-523, Hutchmed) is a novel, investigational Syk inhibitor that has been in development for ITP in China for a number of years with a positive phase 1b/2 trial (NCT03951623) published and positive topline results from a phase 3 trial (NCT05029635) recently reported. Outside of China, a phase 1b trial of sovleplenib in adults with ITP is currently ongoing in the U.S., Europe, and Australia (NCT06291415). The phase 1b/2 trial randomized adults with primary ITP of at least 6 months duration that did not respond or relapsed after prior first-line treatment or splenectomy 3:1 to sovleplenib or placebo.55 A response was defined as an improvement in platelet count to 30 × 109/L and at least double the baseline at two consecutive visits during the first 8 weeks of the trial without rescue therapy. Eighteen of 34 patients randomized to sovleplenib achieved a response (53%), versus one of 11 patients randomized to placebo (9%). The most common adverse events (in contrast to trials of fostamatinib in ITP) were increased lactate dehydrogenase, hematuria, and urinary tract infection, which occurred in seven of the 34 patients in the sovleplenib groups versus one of the 11 patients in the placebo group. The completed phase 3 trial of sovleplenib in China randomized 188 patients to sovleplenib or placebo, and positive topline results (primary endpoint met and certain secondary endpoints met) were announced in a press release. Full results from this trial are awaited.
Cevidoplenib (SKI-O-703, Genosco) is a novel, investigational Syk inhibitor in development for ITP for which an international randomized phase 2 trial (NCT04056195) has been completed, with positive topline results announced in press release form. Full results from this trial are awaited.
5.3 Complement inhibition
Platelet autoantibodies are capable of fixing complement and resulting in complement deposition on the platelet surface, thereby resulting in platelet opsonization and destruction as well as direct platelet destruction via action of the membrane attack complex.56 Therefore, complement inhibition may be an untapped therapeutic modality in ITP. Sutimlimab (BIVV009, Enjaymo, Sanofi) a humanized anti-C1s monoclonal antibody already approved for cold agglutinin disease, was evaluated in a phase 1 trial (NCT03275454) in patients with ITP with promising results.57 Notably, clinically relevant platelet count improvements were observed within hours of intravenous sutimlimab infusion in some patients, which is promising for the potential use of sutimlimab as a rescue therapy (although this is not what the study evaluated). Unfortunately, no further studies of sutimlimab in ITP are presently ongoing. Notably, there was a phase 2a study (NCT04669600) of a similar compound (BIVV020) that was conducted in ITP and in contrast to the phase 1 study of sutimlimab, this study was largely negative, with only one patient out of 12 achieving a durable platelet response (results not formally published but available online on clinicaltrials.gov). Lastly, iptacopan (LNP023, Fabhalta, Novartis), an oral complement factor B inhibitor already approved for paroxysmal nocturnal hemoglobinuria,58 completed a phase 2 basket study in patients with ITP and CAD (NCT05086744). Formal results of this trial are awaited.
AUTHOR CONTRIBUTIONS
H.A. was responsible for all aspects of this manuscript from conception to completion.
ACKNOWLEDGMENTS
H.A. is funded by the National Heart, Lung, and Blood Institute (1K23HL159313).
CONFLICT OF INTEREST STATEMENT
Dr. Al-Samkari reports research funding to his institution (Agios, Sobi, Novartis, Vaderis, Amgen) and consultancy (Agios, Sobi, Alnylam, Novartis, Alpine, argenx, Amgen, Pharmacosmos).
PATIENT CONSENT STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




