Myelodysplastic/myeloproliferative neoplasm with neutrophilia (atypical chronic myeloid leukemia—aCML)
Giovanni Martino and Martina Quintini contributed equally to this study.
Graphical Abstract
A 75-year-old male patient presented with persistent neutrophilia, anemia, and mild splenomegaly (155 × 75 mm at US examination). CBC resulted in WBC 15.07 × 109/L (N 76% - 11,45 × 109/L, L 14%, M 7%, E 1,5%, B 1,5%), Hb 9.7 g/dL, MCV 94 fL, and PLT 203 × 109/L. Molecular analysis for JAK2, MPL, CALR, CSF3R gene mutations, and BCR/ABL rearrangement on peripheral blood tested negative. Bone marrow (BM) examination with biopsy and aspirate was then performed.
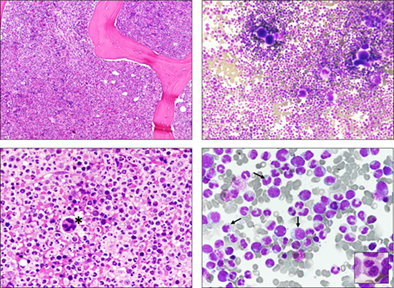
BM was markedly hypercellular (Figure 1: top left panel, H&E, 100×; top right panel, MGG, 100×) due to a strong increase in granulocyte lineage precursors, showing diffuse signs of dysgranulopoiesis and dismegakaryopoiesis (Figure 1: bottom left panel, H&E, ×400 magnification. *: dysplastic megakaryocyte; bottom right panel, MGG, ×400 magnification: note the striking reduction in cytoplasmic granules in myelocytes and promyelocytes indicated by black arrows. Inset in bottom right panel: neutrophil with pseudo-Pelger-Huët anomaly - 600×). Blast count was <10%. Granulocytic lineage accounted for 72% of the total cells. Dysplastic features were also observed in erythroid precursors and megakaryocytes. Reticulin fibrosis: MF-1. Karyotype resulted in trisomy 13. I-FISH (probes for PDGFRA/4q12, PDGFRB/5q33, FGFR1/8p11, ETV6/12p13, BCR-ABL1, C-KIT/4q12, TET2/4q24, FLT3/13q12, and JAK2/9p24) showed FLT3/13q12 trisomy in 11% of the nuclei. Next-Generation Sequencing examination pointed out the following pathogenic mutations: SETBP1 c.2608G > A p.(Gly870Ser) COSV56311834 VAF 41%; ASXL1 c.1450G > T p.(Glu484*) VAF 46.6%; TET2 c.5707C > T p.(Glu1903*) VAF 45.9%; SRSF2 c.284C > T p.(Pro95Leu) COSV57969830 VAF 45.6%; and PTPN11 c.1510A > G p.(Met504Val) COSV61012722 VAF 27.7%. A diagnosis of myelodysplastic/myeloproliferative neoplasm with neutrophilia—atypical chronic myeloid leukemia (aCML)—was rendered. The patient is currently in decent clinical condition and is receiving outpatient follow-up. No therapy was started.
Myelodysplastic/myeloproliferative neoplasm with neutrophilia, formerly known as atypical chronic myeloid leukemia BCR::ABL1 negative (aCML), is a rare myelodysplastic/myeloproliferative neoplasm arising from clonal hematopoiesis with poor prognosis, occurring mainly in elderly individuals. It has been renamed in the recent 5th edition of WHO classification of hematolymphoid tumors to avoid potential confusion with “classical” CML and the other myeloproliferative neoplasms.1 However, the term “atypical chronic myeloid leukemia” has been maintained in the 2022 International Consensus Classification (ICC) of Myeloid Neoplasms and Acute Leukemia, dropping off the notation BCR::ABL1 negative.2 The diagnosis is largely based on the exclusion of other causes of clonal (e.g., typical CML and MPN) and reactive neutrophilia. Finding of SETBP1 and/or ETNK1 gene mutations are considered desirable diagnostic criteria according to the 5th edition of WHO classification. Indeed, SETBP1 and ASXL1 gene mutations with CSF3R “wild type” status support aCML diagnosis according to the ICC. Identifying potentially treatable patients (i.e., with allo-HCT) with a prompt diagnosis is necessary due to the frequent progression to AML, reported in about 40% of cases.
AUTHOR CONTRIBUTIONS
Giovanni Martino and Martina Quintini both collected the data, wrote, and reviewed the manuscript. Maria Paola Martelli, Stefano Ascani, Stefano Lazzi, and Cristina Mecucci participated in writing and reviewing the manuscript.
FUNDING INFORMATION
No funding received for this manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
No data are available for this manuscript. Medical reports for this case are available upon reasonable request.