Health-related quality of life in patients with relapsed/refractory multiple myeloma treated with pomalidomide and dexamethasone ± subcutaneous daratumumab: Patient-reported outcomes from the APOLLO trial
Funding information: The APOLLO study was sponsored by the European Myeloma Network (EMN) in collaboration with Janssen Research & Development, LLC. Medical writing and editorial support were provided by Justine Lempart, PhD, and Linda V. Wychowski, PhD, of Eloquent Scientific Solutions and were funded by Janssen Global Services, LLC.
Abstract
In the phase 3 APOLLO trial, daratumumab in combination with pomalidomide and dexamethasone (D-Pd) significantly reduced the rate of disease progression or death by 37% relative to Pd alone in patients with relapsed/refractory multiple myeloma (RRMM) who had received ≥1 prior line of therapy including lenalidomide and a proteasome inhibitor. Here, we present patient-reported outcomes (PROs) from APOLLO. Median treatment duration was 11.5 months with D-Pd and 6.6 months with Pd. PRO compliance rates were high and similar in both groups. No changes from baseline were observed for EORTC QLQ-C30 global health status scores in either group, while physical and emotional functioning, disease symptoms, and adverse effects of treatment remained at baseline levels with D-Pd but worsened with Pd. Reductions (p < 0.05) in pain and fatigue were seen at several time points with D-Pd versus Pd. Overall, these results suggest patients' health-related quality of life remained stable when daratumumab was added to Pd, with several results favoring D-Pd versus Pd. These findings complement the significant clinical improvements observed with D-Pd and support its use in patients with RRMM.
1 INTRODUCTION
Multiple myeloma (MM) is an incurable disease with substantial negative impact on patients' health-related quality of life (HRQoL).1-9 Patients with relapsed/refractory MM (RRMM) often receive multiple regimens, including immunomodulatory drugs and proteasome inhibitors (PIs), which can be associated with adverse events.1, 2, 5 Each line of therapy (LOT) also decreases HRQoL,10 and after multiple lines of treatment, patients often face a poor prognosis and limited treatment options.11, 12 Evaluation of the impact of treatment on patients' HRQoL offers valuable insights to supplement clinical endpoints, can aid in therapeutic decision-making,1, 3, 13, 14 and helps to better understand the effects of MM and its treatment on overall well-being.15
Daratumumab is an anti-CD38 monoclonal antibody with a direct on-tumor16-19 and immunomodulatory20-22 mechanism of action. Daratumumab is approved by the US Food and Drug Administration as monotherapy or in combination with other agents for the treatment of patients with RRMM.23 Based on the results of the EQUULEUS study (NCT01998971), the regimen of intravenous (IV) daratumumab together with pomalidomide and dexamethasone (D-Pd) was approved for the treatment of patients with RRMM who received ≥2 prior LOT and were refractory to their last treatment.24 The phase 3 APOLLO trial (NCT03180736) compared subcutaneous (SC) daratumumab in combination with Pd versus Pd alone in patients with RRMM and ≥1 prior LOT including lenalidomide and a PI.25 At a median follow-up of 16.9 months, D-Pd significantly reduced the rate of progression or death by 37% relative to Pd.25 Here, we present the impact of D-Pd and Pd on HRQoL in APOLLO.
2 METHODS
2.1 Study design and patients
The full methods of APOLLO have been published previously.25 Briefly, APOLLO was a randomized, open-label, active-controlled, multicenter, phase 3 study in patients with RRMM who had previously received ≥1 prior LOT including lenalidomide and a PI. Adult patients were randomized 1:1 to receive D-Pd or Pd and were treated in 28-day cycles until disease progression or unacceptable toxicity. Pomalidomide was given orally as a 4-mg tablet once daily on Days 1–21 of repeated 28-day cycles, and dexamethasone (40 mg) was given orally once daily on Days 1, 8, 15, and 22 of each 28-day treatment cycle. Patients in the D-Pd group received SC daratumumab (1800 mg co-formulated with recombinant human hyaluronidase PH20 [rHuPH20; 2000 U/mL; ENHANZE® drug delivery technology, Halozyme, Inc., San Diego, CA, USA]) or IV daratumumab (16 mg/kg) weekly during Cycles 1 and 2, every 2 weeks for Cycles 3–6, and then every 4 weeks thereafter. All newly enrolled patients in the D-Pd group (n = 142) received only SC daratumumab after Protocol Amendment 1 (approved on 13 October 2017), and those who started on IV daratumumab could switch to SC daratumumab starting on Day 1 of Cycle 3 or later.
The study was conducted in accordance with the principles of Declaration of Helsinki, the guidelines for Good Clinical Practice of the International Conference on Harmonization (ICH E6), and all applicable laws/regulations. All patients provided written informed consent.
2.2 Patient-reported outcomes
Patient-reported outcomes (PROs; secondary endpoints in APOLLO) were assessed on Day 1 of each treatment cycle before receiving treatment and were collected using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30-item (EORTC QLQ-C30),26 the EORTC QLQ Multiple Myeloma Module 20-item (MY20),27, 28 and the EQ-5D-5L29 questionnaires. The EORTC QLQ-C30 v3 is a cancer-specific, validated, 30-item instrument comprised of one global health status (GHS) scale, five functional scales (physical, role, emotional, cognitive, social functioning), three symptom scales (fatigue, nausea and vomiting, pain), and six single items (dyspnea, insomnia, appetite loss, constipation, diarrhea, financial difficulties).26 Higher scores represent better GHS and function, but for symptom scales, higher scores represent worse symptoms. The EORTC QLQ-MY20 is an MM-specific PRO measure used in conjunction with the EORTC QLQ-C30 for assessing HRQoL. It contains 20 questions comprising two symptom scales (disease symptoms, side effects of treatment), one function scale (future perspective), and a single item on body image.27, 28 The EQ-5D-5L is a generic measure of health status that includes a visual analog scale (VAS) rating of “health today” and assesses five domains including mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.29 PROs results presented in this manuscript were analyzed for patients on treatment.
2.3 Statistical methods
The PRO analysis set included all patients from the intent-to-treat (ITT) population who were on treatment and had a baseline and ≥1 post-baseline PRO assessment. No imputation of missing data or adjustments for multiplicity were made. Compliance with PRO assessments was summarized for each time point by treatment group and calculated as the number of assessments received divided by the number of assessments expected at each time point. Scale and single-item scores for the EORTC QLQ-C30 and QLQ-MY20 and for the EQ-5D-5L VAS were summarized using descriptive statistics.
Treatment effect for PRO scores was analyzed using a mixed-effects model with repeated measures, with patients as a random effect and baseline value, treatment group, time (weeks), treatment-by-time interaction, and stratification factors (number of prior LOT [1 versus 2–3 versus ≥4] and International Staging System stage [1, 2, 3]) as fixed effects. Results are presented as least-squares (LS) means with 95% confidence intervals (CIs); p-values were based on the difference of the LS mean change from baseline between treatment arms. The percentage of patients with clinically meaningful changes from baseline were defined a priori based on published literature with a threshold of ≥10 points for EORTC QLQ-C30 and QLQ-MY20 scale scores30 and ≥7 points for EQ-5D-5L VAS score.31 The median time to improvement/worsening was calculated using the Kaplan–Meier method; improvement/worsening was defined as an increase/decrease in score ≥50% standard deviation (SD) from baseline values, where SD was calculated from the scores at baseline combining both treatment groups. Hazard ratios (HRs) and 95% CIs were estimated based on the stratified Cox proportional-hazards model and p-values were based on a stratified log-rank test. p-values for PRO endpoints are nominal and were not preplanned to be formally tested.
A post hoc exploratory subgroup analysis was conducted to assess differences in EORTC QLQ-C30 GHS, pain, fatigue, physical and emotional functioning scores by age (<65 and ≥65 years), and number of prior LOT (1, 2–3, and ≥4).
3 RESULTS
3.1 Baseline characteristics and PRO assessment compliance rates
3.1.1 ITT population
Altogether, 304 patients were included in this analysis (D-Pd, 151; Pd, 153) with a median treatment duration of 11.5 months with D-Pd and 6.6 months with Pd. Baseline characteristics were well balanced between treatment groups (Table 1). Median age was 66.0 (range, 35–90) years, 53.0% of patients were male, and 55.3% had an Eastern Cooperative Oncology Group performance status score of 0. Mean baseline scores for the EORTC QLQ-C30 GHS, functional, and symptom scale scores, all EORTC QLQ-MY20 scales, and the EQ-5D-5L VAS were comparable between treatment arms. PRO compliance rates were high and similar in both treatment groups across all time points, with rates >95% at baseline and >93% through Cycle 16 (Table S1). At the time of clinical cutoff, more patients with D-Pd than Pd remained on study treatment (60/151 [40%] versus 33/153 [22%]).
| Characteristic | D-Pd (n = 151) | Pd (n = 153) |
|---|---|---|
| Age, years, n (%) | ||
| <65 | 63 (41.7) | 60 (39.2) |
| 65–74 | 63 (41.7) | 62 (40.5) |
| ≥75 | 25 (16.6) | 31 (20.3) |
| Sex, n (%) | ||
| Female | 72 (47.7) | 71 (46.4) |
| Male | 79 (52.3) | 82 (53.6) |
| Baseline ECOG PS, n (%) | ||
| 0 | 91 (60.3) | 77 (50.3) |
| 1 | 54 (35.8) | 57 (37.3) |
| 2 | 6 (4.0) | 19 (12.4) |
| Number of prior LOT, n (%) | ||
| 1 | 16 (10.6) | 18 (11.8) |
| 2–3 | 114 (75.5) | 113 (73.9) |
| ≥4 | 21 (13.9) | 22 (14.4) |
| EORTC QLQ-C30 scores,a mean (SD) | ||
| GHS | 60.9 (22.9) | 60.5 (20.3) |
| Functional scales | ||
| Physical functioning | 71.2 (25.1) | 69.3 (24.7) |
| Role functioning | 68.0 (31.8) | 66.9 (30.5) |
| Emotional functioning | 80.7 (17.5) | 78.1 (19.8) |
| Cognitive functioning | 85.8 (19.5) | 84.9 (19.5) |
| Social functioning | 73.1 (28.3) | 76.6 (26.8) |
| Symptom scales | ||
| Pain | 30.8 (30.9) | 34.9 (27.6) |
| Fatigue | 35.5 (27.1) | 38.1 (25.5) |
| Nausea/vomiting | 4.7 (13.7) | 3.9 (10.7) |
| EORTC QLQ-MY20 scores,a mean (SD) | ||
| Future perspective | 62.3 (26.2) | 61.8 (26.8) |
| Body image | 83.2 (27.7) | 80.0 (27.4) |
| Disease symptom | 23.6 (23.0) | 25.8 (22.4) |
| Adverse effects of treatment | 15.6 (14.9) | 17.7 (15.4) |
| EQ-5D-5L scores, mean (SD) | ||
| VASb | 64.7 (20.1) | 65.7 (19.3) |
- Abbreviations: D-Pd, daratumumab, pomalidomide, and dexamethasone; ECOG PS, Eastern Cooperative Oncology Group performance status; EORTC, European Organization for Research and Treatment of Cancer; GHS, global health status; ITT, intent-to-treat; LOT, line of therapy; Pd, pomalidomide and dexamethasone; QLQ-C30, Quality of Life Questionnaire Core 30-item; QLQ-MY20, Quality of Life Questionnaire Multiple Myeloma Module; SD, standard deviation; VAS, visual analog scale.
- a Scores range from 0 to 100; higher scores represent better health status, better physical functioning, and more (worse) symptoms.
- b Scores range from 0 to 100; higher scores represent better self-evaluated health status.
3.2 Treatment effect on PRO measures
3.2.1 ITT population
No change from baseline in LS mean GHS scores was observed in either treatment group, although point estimates generally favored D-Pd. The greatest between-group difference in LS mean change from baseline [95% CI] was observed at Cycle 10 (3.1 [−1.0, 7.2] for D-Pd versus −1.9 [−6.4, 2.6] for Pd; p = 0.0836) (Figure 1A). Physical and emotional functioning were also unchanged from baseline with D-Pd but worsened with Pd (maximum worsening at Cycle 15 [7.2 points] for physical functioning and at Cycle 14 [9.1 points] for emotional functioning). Between-group differences favored D-Pd at all time points, with nominal p-values <0.05 at Cycles 10, 11, and 15 for physical functioning and at Cycles 5, 6, 8–11, 13, and 14 for emotional functioning (Figure 1B,C).
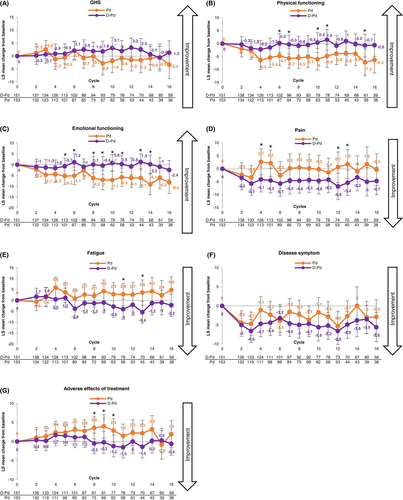
There was a trend toward reduction in pain for the D-Pd group, with point estimates favoring D-Pd compared with Pd at all time points. Maximum LS mean improvement from baseline was 6.8 points with D-Pd at Cycle 12 (p = 0.0443); maximum LS mean worsening from baseline was 2.7 points with Pd at Cycle 4 (p = 0.0286) (Figure 1D). The LS mean (95% CI) change in fatigue scores was −4.0 (−8.7, −0.6) for D-Pd versus 2.8 (−2.4, 7.9) for Pd (p = 0.0372) at Cycle 11 and − 5.4 (−10.4, −0.4) versus 3.6 (−2.2, 9.4) (p = 0.0152) at Cycle 13. At other assessment points, point estimates favored D-Pd (Figure 1E).
Differences between groups favoring D-Pd (nominal p-values <0.05) were also observed in role functioning, cognitive and social function scores, symptom scores of nausea/vomiting, and single-item scores (dyspnea, insomnia, appetite loss, constipation, diarrhea, financial difficulties) at various time points (Figure S1).
No changes were observed in disease symptoms or treatment-related adverse effects in patients treated with D-Pd; however, patients treated with Pd had worsening of adverse effects of treatment (maximum LS mean worsening: 4.0 points at Cycle 9; Figure 1F,G). Results for EQ-5D-5L VAS were similar with those observed for EORTC QLQ-C30 GHS scores, with point estimates generally favoring D-Pd (data not shown).
3.2.2 Subgroup analyses
LS mean changes from baseline in EORTC QLQ-C30 GHS, pain, fatigue, physical, and emotional functioning scores in subgroups based on age (<65 versus ≥65 years) and number of prior LOT (1, 2–3, ≥4) were similar with the overall population (Figure S2). Patients in both age groups showed a trend toward improved PROs with D-Pd compared with Pd. Patients aged ≥65 years were most likely to demonstrate significant differences in LS mean change from baseline in favor of D-Pd for GHS (maximum difference: −9.7 points at Cycle 16), pain (11.8 points at Cycle 15), physical functioning (−9.1 points at Cycle 4), and emotional functioning (−12.1 points at Cycle 16) scores. Except for GHS scores in patients with one prior LOT, patients in the D-Pd group experienced improvements regardless of number of prior LOT compared with Pd alone. Differences favoring D-Pd over Pd were more pronounced with increasing numbers of prior LOT, with the most pronounced differences with ≥4 prior LOT.
3.3 Proportion of patients with meaningful change from baseline
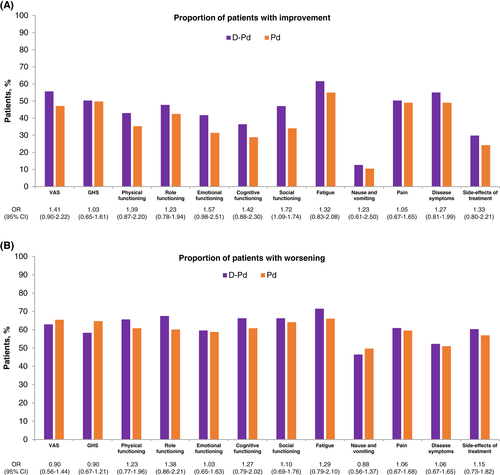
Meaningful improvement with D-Pd versus Pd from baseline was seen in 55.0% of patients versus 49.0% for disease symptoms, 43.0% versus 35.3% for physical functioning, and 41.7% versus 31.4% for emotional functioning, respectively (Figure 2).
3.4 PROs and clinical outcomes
Improvement in the EORTC QLQ-C30 GHS, pain, and fatigue subscales showed some correlation with clinical outcomes (Figure S3). Patients with a very good partial response or better reported an overall improvement in pain symptoms. Similarly, GHS and fatigue improved with depth of clinical response (except in patients with a stringent complete response [CR]). Patients with stable disease or progressive disease reported no change in their health state, as assessed by their GHS, fatigue, or pain subscale scores.
3.5 Median time to improvement and worsening
3.5.1 ITT population
Across all measurement scales, the median time to improvement ranged between ~4 and 21 months across both treatment arms. Median time to worsening was similar between treatments for all subscales and ranged between ~3 and 6 months except for nausea/vomiting (range, ~7–13 months), the disease symptoms subscale (~8–10 months), and future perspective score (~14–21 months) (Table 2). Median time to improvement in GHS score was 5.62 versus 3.81 months with D-Pd versus Pd (HR 0.88; 95% CI 0.64–1.21; p = 0.4346) and the median time to meaningful worsening in GHS score was 4.01 versus 3.84 months, respectively (HR 0.90; 95% CI 0.67–1.21; p = 0.4810). Median time to improvement for other EORTC QLQ-C30 subscales was comparable between the treatment groups for pain, showed slightly shorter times for D-Pd versus Pd for physical, role, and emotional functioning, and was not yet reached for cognitive and social functioning, and nausea/vomiting. The median time to worsening showed no differences between D-Pd and Pd treatment across all EORTC QLQ-C30 scales and ranged between ~3 and 6 months except for time to worsening for nausea/vomiting (D-Pd versus Pd, 12.91 versus 7.43 months; HR 0.86; 95% CI 0.62–1.19; p = 0.3579).
| Scale | D-Pd, months, median (95% CI) (n = 151) | Pd, months, median (95% CI) (n = 153) | HR (95% CI) | p value |
|---|---|---|---|---|
| Median time to improvement | ||||
| EORTC QLQ-C30 | ||||
| GHS | 5.62 (3.22–17.48) | 3.81 (2.79–5.82) | 0.88 (0.64–1.21) | 0.435 |
| Physical functioning | 12.22 (6.47–NE) | 20.73 (6.47–NE) | 1.10 (0.76–1.59) | 0.602 |
| Role functioning | 6.28 (3.35–NE) | 9.30 (3.75–NE) | 1.01 (0.72–1.41) | 0.972 |
| Emotional functioning | 13.44 (7.00–NE) | 20.11 (16.56–NE) | 1.12 (0.76–1.63) | 0.573 |
| Cognitive functioning | NE (11.40–NE) | NE (NE–NE) | 1.16 (0.77–1.73) | 0.476 |
| Social functioning | 5.59 (3.98–NE) | NE (8.35–NE) | 1.35 (0.94–1.94) | 0.096 |
| Pain | 4.63 (2.83–15.74) | 4.14 (2.23–7.49) | 0.92 (0.66–1.27) | 0.595 |
| Fatigue | 14.06 (6.80–27.70) | NE (11.43–NE) | 1.17 (0.80–1.70) | 0.417 |
| Nausea/vomiting | NE (NE–N E) | NE (NE–NE) | 1.09 (0.56–2.13) | 0.791 |
| EORTC QLQ-MY20 | ||||
| Future perspective | 9.30 (4.63–NE) | 6.51 (3.22–11.14) | 0.83 (0.59–1.16) | 0.274 |
| Body image | NE (NE–NE) | NE (NE–NE) | 0.78 (0.50–1.21) | 0.254 |
| Disease symptoms | NE (3.55–NE) | 8.87 (3.02–NE) | 0.87 (0.62–1.24) | 0.441 |
| Adverse effects of treatment | NE (NE–NE) | NE (NE–NE) | 1.06 (0.69–1.65) | 0.782 |
| EQ-5D-5L | ||||
| VAS | 3.81 (2.10–10.25) | 5.55 (3.75–NE) | 1.15 (0.83–1.59) | 0.391 |
| Median time to worsening | ||||
| EORTC QLQ-C30 | ||||
| GHS | 4.01 (2.20–7.59) | 3.84 (3.02–5.91) | 0.90 (0.67–1.21) | 0.481 |
| Physical functioning | 3.78 (2.86–5.62) | 3.75 (2.86–6.01) | 0.99 (0.74–1.32) | 0.952 |
| Role functioning | 2.83 (2.00–4.76) | 3.22 (2.79–5.55) | 1.05 (0.79–1.40) | 0.740 |
| Emotional functioning | 5.82 (4.40–9.26) | 3.88 (2.86–6.14) | 0.84 (0.62–1.13) | 0.252 |
| Cognitive functioning | 4.17 (2.86–6.05) | 4.70 (2.76–5.62) | 0.95 (0.72–1.27) | 0.751 |
| Social functioning | 3.81 (2.23–5.59) | 2.76 (2.00–3.94) | 0.88 (0.66–1.18) | 0.398 |
| Pain | 4.01 (2.89–7.43) | 3.71 (2.83–5.13) | 0.89 (0.66–1.20) | 0.435 |
| Fatigue | 5.59 (3.78–8.84) | 4.63 (2.96–7.66) | 0.99 (0.73–1.34) | 0.951 |
| Nausea/vomiting | 12.91 (6.05–16.79) | 7.43 (4.83–10.18) | 0.86 (0.62–1.19) | 0.358 |
| EORTC QLQ-MY20 | ||||
| Future perspective | 21.49 (10.81–NE) | 14.09 (6.87–NE) | 0.94 (0.64–1.37) | 0.732 |
| Body image | 4.73 (4.01–6.77) | 7.39 (4.86–20.96) | 1.27 (0.92–1.76) | 0.142 |
| Disease symptoms | 10.41 (5.78–NE) | 8.67 (4.90–21.45) | 0.90 (0.64–1.27) | 0.559 |
| Adverse effects of treatment | 4.01 (2.86–5.78) | 3.94 (2.83–5.55) | 0.99 (0.73–1.34) | 0.965 |
| EQ-5D-5L | ||||
| VAS | 3.78 (2.83–6.54) | 2.86 (1.94–3.71) | 0.82 (0.62–1.10) | 0.178 |
- Abbreviations: CI, confidence interval; D-Pd, daratumumab, pomalidomide, and dexamethasone; EORTC, European Organization for Research and Treatment of Cancer; GHS, global health score; HR, hazard ratio; NE, not evaluable; QLQ-C30, Quality of Life Questionnaire Core 30-item; QLQ-MY20, Quality of Life Questionnaire Multiple Myeloma Module; Pd, pomalidomide and dexamethasone; VAS, visual analog scale.
Time to improvement for EORTC QLQ-MY20 scales was not yet reached for most subscales while time to worsening ranged from 3.9 to 7.4 months, with the exception of time to worsening of the future perspective scale (D-Pd versus Pd, 21.49 versus 14.09 months; HR 0.94; 95% CI 0.64–1.37; p = 0.7316) and the disease symptoms scale (D-Pd versus Pd, 10.41 versus 8.67 months; HR 0.90; 95% CI 0.64–1.27; p = 0.5708).
Median time to meaningful improvement for EQ-5D-5L VAS score was 3.81 versus 5.55 months with D-Pd versus Pd (HR 1.15; 95% CI 0.83–1.59; p = 0.3913), and the median time to meaningful decrease in VAS score was 3.78 versus 2.86 months, respectively (HR 0.82; 95% CI 0.62–1.10; p = 0.1780).
3.5.2 Subgroups
Median time to worsening and improvement for EORTC QLQ-C30 GHS, fatigue, pain, and physical and emotional functioning scores were mostly similar regardless of age and number of prior LOT and were comparable to the overall population (Table S2).
4 DISCUSSION
The results showed no decrement in HRQoL when daratumumab was added to Pd in patients with RRMM who had received ≥1 prior LOT. Although the between-group differences for D-Pd and Pd were largely nonsignificant, overall point estimates generally favored D-Pd, and patients in the D-Pd group reported reduction in pain, whereas patients in the Pd group experienced worsening of physical functioning. The improvements in pain are noteworthy, as pain has been shown to have a particularly detrimental effect on HRQoL in patients with MM.13 Compared with the significantly better clinical outcomes (progression-free survival, and rates of ≥CR) observed with D-Pd versus Pd in the primary analysis of APOLLO,25 the numerically small between-group differences in PROs in this study might be explained by the use of an on-treatment analysis approach. More patients discontinued treatment in the Pd group (78%) compared with the D-Pd group (60%). Patients who remained on study treatment were those who had achieved a clinical response, whereas those who relapsed and transitioned to a subsequent LOT were not included in these a priori analyses. Another possible explanation is most baseline EORTC QLQ-C30 scores in patients from APOLLO were better than a typical population of patients with RRMM,32 thus leaving less room for improvement following treatment.
Although baseline EORTC QLQ-C30 scores in APOLLO were better than typically seen in patients with RRMM,25 they were still worse than those of patients without cancer,33 so improving or maintaining HRQoL is an important treatment goal in this patient population. As most patients in APOLLO received ≥2 prior LOT25 and these patients experienced clinical benefit from D-Pd, this could be particularly relevant as it further underscores the addition of daratumumab to a later-line Pd regimen as a useful option for salvage therapy. Subgroup analyses according to the number of prior LOT, including patients treated with ≥4 prior LOT, generally confirmed the results of the primary analysis, demonstrating numerically greater improvement in HRQoL with D-Pd versus Pd. Improvements were also observed regardless of age, indicating that the addition of daratumumab did not negatively affect HRQoL, even in potentially frail and elderly or heavily pretreated patients. Improvement in HRQoL is particularly important in older patients with greater health impairment, including comorbidities and a lower overall likelihood of achieving treatment benefits.5
There are few published studies reporting the impact of treatment on HRQoL in patients with RRMM treated with Pd-based regimens. In general, these studies have found baseline levels of HRQoL were maintained rather than improved. OPTIMISMM compared pomalidomide, bortezomib, and dexamethasone (P-Vd) versus Vd alone until disease progression or treatment discontinuation in patients who received an average of two prior LOT.8 Mean changes from baseline in GHS, physical functioning, and fatigue scores were similar between treatments. The proportion of patients who experienced clinically meaningful worsening of GHS scores was generally similar in both groups; although worsening was significantly greater with P-Vd versus Vd at Cycles 5 and 9, those differences were not clinically meaningful. The proportion of patients with worsening physical functioning and fatigue scores decreased over time in the P-Vd group and increased in the Vd group. NIMBUS compared pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone in patients who were refractory to their last treatment and had failed lenalidomide and bortezomib after ≥2 consecutive cycles of each (alone or in combination).34 Patients who received pomalidomide plus low-dose dexamethasone were more likely to experience HRQoL improvement than patients treated with high-dose dexamethasone. Median time to first worsening was longer with pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone in several scales. The ICARIA study compared isatuximab plus Pd with Pd alone in patients with RRMM who failed ≥2 prior therapies including lenalidomide and a PI.35 Baseline HRQoL was maintained with isatuximab plus Pd, and less decrement was observed in GHS, fatigue, and physical functioning scores compared with Pd alone.
The results of the present study are also consistent with other studies that have examined the impact of daratumumab-based regimens on HRQoL in patients with RRMM. In the phase 3 CASTOR study, patients (median of 2 prior LOT; range, 1–10) treated with daratumumab, bortezomib, and dexamethasone maintained baseline levels of HRQoL throughout eight treatment cycles; thereafter, patients receiving long-term daratumumab treatment reported improvements in some PROs, including GHS, pain, and VAS scores.36, 37 In the phase 3 POLLUX study, patients (median of 1 prior LOT; range, 1–11) with RRMM treated with daratumumab, lenalidomide, and dexamethasone (D-Rd) experienced significant between-group differences from baseline in EORTC QLQ-C30 GHS, functional, and symptom scores, and EQ-5D-5L VAS scores at several time points over the course of treatment compared with patients treated with Rd alone.38, 39 Notably, the lack of negative impact of long-term therapy illustrates that the clinical benefits observed with D-Rd were not at the expense of patients' HRQoL.
The study findings are strengthened by the high compliance rate. Limitations of this study include that this was an on-treatment analysis with higher rates of discontinuation in the Pd group. Furthermore, patients in APOLLO were aware of their treatment assignment, which may have influenced their responses to HRQoL assessments, although there are limited data in the literature suggesting widespread open-label bias regarding PRO measures in the oncology setting.40 The EORTC QLQ-MY20 contains 10 items that make up the adverse events of treatment subscale. These 10 patient-reported items are not intended to be reconciled or replace the clinician-reported adverse events that are part of the clinical trial safety reporting. Patient-reported results presented here should not be compared with the safety/adverse event results presented in the primary APOLLO manuscript.25
5 CONCLUSION
Overall PRO results suggested no statistically significant changes from baseline in patients' HRQoL when daratumumab was added to Pd, although between-group differences favored D-Pd versus Pd for most data points, with a notable reduction in pain in favor of D-Pd, and reduction in functional status and increase in symptoms in patients treated with Pd, thereby favoring D-Pd. These results complement the significant clinical improvements observed with D-Pd in patients with RRMM, such as prolonged progression-free survival, and further support its use in this population.
ACKNOWLEDGMENTS
The authors thank the patients who participated in this study and their families, the staff members at the study sites, the data and safety monitoring committee, and the staff members involved in data collection and analysis.
CONFLICT OF INTEREST
Evangelos Terpos served in a consulting or advisory role for Amgen, Celgene, Genesis Pharmaceuticals, Janssen-Cilag, Sanofi, and Takeda, received honoraria from Amgen, Bristol-Myers Squibb, Celgene, Genesis Pharmaceuticals, Janssen-Cilag, Novartis, Sanofi, and Takeda, received travel funding from Amgen, Genesis Pharmaceuticals, Janssen-Cilag, and Takeda, and received research funding from Amgen, Genesis Pharmaceuticals, Janssen-Cilag, Sanofi, and Takeda. Meletios A. Dimopoulos served in a consulting or advisory role for Amgen, BeiGene, Bristol-Myers Squibb, Janssen-Cilag, and Takeda, and received honoraria from Amgen, BeiGene, Bristol-Myers Squibb, Janssen-Cilag, and Takeda. Mario Boccadoro served in a consulting or advisory role for GlaxoSmithKline and Janssen, received honoraria from Amgen, AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Novartis, and Sanofi, and received research funding from Amgen, Bristol-Myers Squibb, Celgene, Janssen, Mundipharma, Novartis, and Sanofi. Sosana Delimpasi served in a consulting or advisory role for Amgen, Janssen, and Takeda, and served in speakers bureaus for Amgen, Janssen, and Takeda. Meral Beksac served in a consulting or advisory role for Amgen, Celgene, Janssen-Cilag, Oncopeptides, Sanofi Pasteur, and Takeda, and served in speakers bureaus for Amgen, Celgene, Janssen-Cilag, and Sanofi Pasteur. Eirini Katodritou served in a consulting or advisory role for Amgen and Janssen-Cilag, received travel funding from Genesis Pharma and Takeda, received honoraria from Amgen, Genesis Pharma, Janssen-Cilag, and Takeda, and received research funding from Amgen, Genesis Pharma, Janssen-Cilag, and Takeda. Philippe Moreau served in a consulting or advisory role for Amgen, Celgene, GlaxoSmithKline, Janssen, and Takeda, and received honoraria from Amgen, Celgene, GlaxoSmithKline, Janssen-Cilag, Novartis, and Takeda. Alessandra Pompa has nothing to disclose. Argiris Symeonidis served in a consulting or advisory role for AbbVie, Bristol-Myers Squibb, Celgene, Gilead Sciences, Janssen-Cilag, Novartis, Pfizer, Sanofi-Aventis, Roche, and Takeda, served in speakers bureaus for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Novartis, Pfizer, Roche, Sanofi-Aventis, and Takeda, received honoraria from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Novartis, Pfizer, and Takeda, and received research funding from AbbVie, Amgen, Astellas Pharma, Bayer, Celgene/Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, MSD Oncology, Novartis, Pfizer, Roche, Sanofi-Aventis, Takeda, and WinMedica. Jelena Bila served in a consulting or advisory role for Amgen, Janssen, and Takeda, received honoraria from Amgen, Janssen, and Takeda, and received research funding from Janssen Pharmaceutical Companies of Johnson & Johnson. Albert Oriol served in a consulting or advisory role for Amgen, Celgene, GlaxoSmithKline, Karyopharm, Oncopeptides and Sanofi, and served in speakers bureaus for Amgen and Celgene. Maria-Victoria Mateos served in a consulting and advisory role for Amgen, AbbVie, Celgene, GlaxoSmithKline, Janssen-Cilag, Pfizer, Regeneron, Roche/Genentech, and Takeda, and received honoraria from Amgen, AbbVie, Adaptive Biotechnologies, Celgene, GlaxoSmithKline, Janssen-Cilag, Roche, and Takeda. Hermann Einsele served in a consulting and advisory role for Amgen, Bristol-Myers Squibb, Celgene, Janssen, Novartis, and Takeda, received travel, accommodations, and expenses from Amgen, Bristol-Myers Squibb, Celgene, Janssen, and Takeda, received honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, Novartis, and Takeda, and received research funding from Amgen, Bristol-Myers Squibb, Celgene, and Janssen. Ioannis Orfanidis is an employee of Health Data Specialists, and equity holder in a private company. Katharine S. Gries, Kevin Liu, Jianming He, Tobias Kampfenkel, and Robin Carson are employees of Janssen. John Fastenau and Himal Amin are employees of Janssen and equity holders in a publicly traded company. Yanping Qiu was an employee of Janssen at the time this research was conducted and equity holder in a publicly traded company. Pieter Sonneveld served in a consulting or advisory role for Amgen, CARsgen Therapeutics, Celgene, Janssen, and Karyopharm Therapeutics, and received research funding from Amgen, Janssen, and Skyline Diagnostics.
AUTHOR CONTRIBUTIONS
Evangelos Terpos, Meletios A. Dimopoulos, Mario Boccadoro, Sosana Delimpasi, Meral Beksac, Eirini Katodritou, Philippe Moreau, Alessandra Pompa, Argiris Symeonidis, Jelena Bila, Albert Oriol, Maria-Victoria Mateos, Hermann Einsele, Ioannis Orfanidis, and Pieter Sonneveld contributed to the conception and design, collection and assembly of data, and data analysis and interpretation; Katharine S. Gries, John Fastenau, Kevin Liu, Jianming He, Tobias Kampfenkel, Yanping Qiu, Himal Amin, and Robin Carson contributed to the conception and design and data analysis and interpretation; all authors drafted and reviewed the manuscript, approved the final version for submission, and vouch for data accuracy and completeness.
PATIENT CONSENT
All patients provided written informed consent.
ETHICS STATEMENT
Each study site's local independent ethics committee or institutional review board approved the study protocol. The study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines.
Open Research
DATA AVAILABILITY STATEMENT
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinicaltrials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.




