Multicentric Castleman disease: A single center experience of treatment with a focus on autologous stem cell transplantation
Funding information: Mayo Clinic
Abstract
Castleman disease (CD) is a rare lymphoproliferative disease characterized by diverse clinical and pathologic features. Due to its rarity, there are limited studies comparing currently available therapies. The role of autologous stem cell transplantation (ASCT) in CD has not yet been established. In this paper, we describe the clinical characteristics, treatment choices, and outcomes in 34 Mayo Clinic patients diagnosed with multicentric CD from July 1, 2003 to April 30, 2018. Eighteen patients (53%) also met the criteria for POEMS, including 14 with the osteosclerotic variant. The first-line treatments included: steroid monotherapy (4), cytotoxic chemotherapy (6), rituximab alone (8) or with chemotherapy (2), anti-IL6 treatment (3), and ASCT (10). The median follow-up was 4.8 (range: 0.1–15.2) years. The 5- and 10-year overall survival rates were 84% and 71%, respectively. Sixteen patients received high-dose chemotherapy followed by ASCT during their disease course. Among those, 14 had multicentric CD associated with POEMS. There were no transplant-related deaths. All patients had at least a partial response to ASCT, most of whom achieved a complete response. The favorable outcomes seen with ASCT in this cohort suggest that transplantation may have a role in multicentric CD, particularly for patients with multicentric CD associated with POEMS.
1 INTRODUCTION
Castleman disease (CD) is a rare lymphoproliferative disease driven by the overproduction of inflammatory cytokines, causing proliferation of benign lymphocytes. Since its initial description in 1954,1 CD has been recognized as a spectrum of diseases with diverse clinical and pathologic features.2-5 The localized unicentric form is usually asymptomatic or associated with mild symptoms.3 However, multicentric Castleman disease (MCD), characterized by involvement of multiple lymph nodes, is a heterogeneous disease; patients present with symptoms of varying severities, cytopenias, autoimmune phenomena, and/or organ dysfunction. MCD is subdivided into two groups based on infection with the human herpes-virus 8 (HHV-8): HHV-8 positive and HHV-8 negative MCD, also known as idiopathic MCD (iMCD).6-8 Clinical and laboratory features of MCD are mediated mainly by the inflammatory cytokine interleukin-6 (IL-6), thus providing a rationale for the use of IL-6 targeting monoclonal antibodies. In HHV-8 positive MCD, viral IL-6 is produced by HHV-8+ infected B cells, which may explain the favorable responses associated with rituximab in this subset of patients.9 In contrast, the pathophysiology of iMCD is not well understood. In the absence of curative treatments, the management of MCD is aimed at symptom control; rituximab-based therapy is the mainstay of treatment for HHV-8 positive MCD,10 while a variety of agents have been utilized in the management of iMCD including immunosuppressive therapies, IL-6 blocking antibodies, and systemic chemotherapy.3, 7, 8, 11 In 2018, the Castleman Disease Collaborative Network (CDCN) developed consensus guidelines for the management of iMCD where anti-IL-6 based treatment (with high-dose steroids in severe cases) was recommended in the first-line setting based on results from a randomized placebo-controlled study demonstrating durable efficacy and safety of siltuximab in patients with iMCD.12, 13 Second-line options include rituximab with steroids +/− immunomodulatory–immunosuppressive agents and various combinations of systemic chemotherapy, depending on disease severity and prior treatment.13, 14 Due to the rarity of this disease, there is a paucity of randomized controlled trials comparing outcomes between the currently available treatment options, and most treatment recommendations are at present derived from systematic literature review, case series and case reports, retrospective studies, and open-label studies. There have been only a few reports on the use autologous hematopoietic stem cell transplantation (ASCT) in MCD. A group of patients with MCD also meet the diagnostic criteria for a coexisting POEMS syndrome,15 where encouraging results have been reported with ASCT.15-17 In this paper, we describe the clinical characteristics and outcomes with treatments, including ASCT in patients seen over a 15-year period in a US center specialized in the management of CD.
2 PATIENTS AND METHODS
This is a retrospective descriptive study, including patients diagnosed histologically with MCD from July 1, 2003 to April 30, 2018. Patients were identified using a prospectively maintained database at Mayo Clinic, and additional clinical and laboratory data were obtained by review of electronic medical records. All patients authorized the use of their medical record data for research purposes. The study was approved by our Institutional Review Board. We collected data from the time of diagnosis on clinical characteristics, histology, common serum and urine tests, serum and urine protein electrophoresis and immunofixation, and serological tests for human immunodeficiency virus (HIV), hepatitis B, and hepatitis C infections. Serum testing for HHV-8 was done using quantitative real-time polymerase chain reaction. To determine HHV-8 status on tissue specimens, immunoperoxidase studies were performed on paraffin sections of lymph node specimens using antibodies directed against HHV-8. We also collected data, when applicable, for concurrent rheumatologic disorders and associated clonal plasma cell disorders including coexistent POEMS syndrome defined based on established criteria.15
For patients with available treatment information, we collected data on first-line and subsequent lines of treatment, including radiation therapy, corticosteroids or other immunosuppressive agents, cytotoxic chemotherapy, rituximab, IL-6 inhibitors (siltuximab, tocilizumab), and/or ASCT. All patients undergoing ASCT were conditioned with high-dose melphalan; one was conditioned with BEAM (carmustine, etoposide, cytarabine, and melphalan) chemotherapy. Response to treatment was reported as documented by the treating physician: stable disease (SD), progressive disease (PD), partial response (PR), or complete response (CR). For patients treated after 2018, treatment response was based on consensus criteria developed by the international working group in 2018.13 Prior to that, response assessments were based on the treating physician's judgment of the composite clinical, biochemical (inflammatory makers, organ function tests) and radiographic responses (lymph node size by positron emission tomography/computed tomography) or response in terms of the predominant disease manifestation. For patients with POEMS and iMCD, POEMS response criteria were utilized if this was the predominant disease manifestation, using laboratory (plasma vascular endothelial growth factor), hematologic (M-spike, bone marrow plasma cells, serum/urine electrophoresis/immunofixation), radiologic, neurologic, and/or organ-specific response criteria, where applicable.14 The time to next treatment (TTNT) was defined as the time from start of the first-line treatment to the time of start of the second-line treatment. Patients who had not started a subsequent line of treatment were censored at their last follow-up. Overall survival (OS) was defined as time from diagnosis to death of any cause; patients who were alive at the last follow-up were censored at their last contact. Survival curves were estimated using the Kaplan–Meier method. All statistical analysis was performed using the JMP Pro Software SAS, NC.
3 RESULTS
3.1 Baseline characteristics
From 2003 to 2018, 34 patients were diagnosed with MCD. Among those, 3 tested positive for HHV-8 on pathological specimens and 19 tested negative; HHV-8 status was unknown for 12 patients, 6 of whom were negative for HIV, and the rest had unknown HIV status. The median age at diagnosis was 56 (range: 24–81) years, and 59% were male. The most common histological type was the plasma cell variant (PCV) (50%). Among all patients, 23 (68%) had peripheral neuropathy, 19 (56%) had skin changes, 26 (76%) had signs of extravascular volume overload, and 24 (71%) patients had endocrine disorders, the most common of which was hypogonadism (50%). HIV testing results were available in 26 patients, including 2 patients who were HIV-positive and had HHV-8 positive MCD; 1 of those patients had Kaposi sarcoma and had been on highly active antiretroviral therapy (HAART) prior to the diagnosis of MCD. The median CD4 lymphocyte count at the time of diagnosis was 788/mm3, and the median viral load was 47 copies/mL. Among 21 patients with available data for hepatitis B and C serologies, one patient had hepatitis C, and none had hepatitis B infection. A monoclonal protein was detected in the serum and/or urine in 22 of 32 evaluable patients (69%) patients; 12 and 8 patients had IgA and IgG isotypes, respectively, 17 had lambda light chain isotype, and 4 had kappa light chain isotype; the light chain type was not available for 1 patient. Three patients had a concurrent plasmacytoma; one patient was diagnosed with angioimmunoblastic T-cell lymphoma 4 years after initial MCD diagnosis.
Among all 34 patients with MCD, 18 (53%) also met the criteria for POEMS. Radiological evidence for sclerotic and/or mixed lytic and sclerotic lesions was found in 14 patients. Three patients had autoimmune hemolytic anemia, and none of the patients had evidence of bronchiolitis obliterans organizing pneumonia or TAFRO syndrome (thrombocytopenia, anasarca, fever, reticulin myelofibrosis, renal dysfunction, and organomegaly). The clinical and laboratory characteristics of MCD patients with or without POEMS are shown in Tables 1 and 2, respectively. All patients with MCD and POEMS had peripheral neuropathy, endocrinopathies, and extravascular volume overload; these patients were also more likely to have skin changes, bone lesions, and the presence of a monoclonal protein compared to patients with MCD without POEMS. When a monoclonal protein was present, IgG was the predominant heavy chain isotype in MCD without POEMS, while IgA was the most common isotype among patients with MCD with POEMS. In the latter group, all monoclonal proteins were of lambda light chain isotype. Patients with MCD without POEMS had lower hemoglobin concentration, platelet count and albumin level, and higher LDH. The levels of inflammatory markers (erythrocyte sedimentation rate) and C-reactive protein) were higher in this group compared to patients with MCD and POEMS, but the difference was not statistically significant. PCV histology was the predominant histology in both groups.
| MCD without POEMS (N = 16) | MCD and POEMS (N = 18) | p value | |
|---|---|---|---|
| Variable | N (%) | N (%) | |
| Median age at diagnosis, years (range) | 57 (29–81) | 54 (24–81) | NS |
| Male sex | 8 (50) | 12 (67) | NS |
| Constitutional symptoms | 12 (75) | 12 (75) | NS |
| Fever | 4 (25) | 1 (6) | |
| Night sweats | 7 (44) | 5 (28) | |
| Anorexia | 4 (25) | 4 (22) | |
| Fatigue | 12 (75) | 8 (44) | |
| Dyspnea | 5 (31) | 5 (28) | |
| Arthralgia | 4 (25) | 3 (17) | NS |
| GI symptoms (vomiting/diarrhea) | 1 (6) | 1 (6) | NS |
| ECOG performance status | |||
| 0–1/2/≥3 | 7 (44)/2 (13)/2 (13) | 8 (44)/3 (17)/1 (6) | |
| Unknown | 5 (31) | 0 (0) | |
| Peripheral neuropathy | 5 (31) | 18 (100) | <.001 |
| Sensory/sensorimotor | 5 (31)/1 (6) | 18 (100)/14 (78) | |
| Skin changes | 5 (31) | 14 (78) | .014 |
| Rash | 3 (19) | 1 (6) | |
| Rubor | 1 (6) | 2 (11) | |
| Hyperpigmentation | 0 (0) | 5 (28) | |
| Cherry angioma | 3 (19) | 6 (33) | |
| Cyanosis/acrocyanosis | 1 (6) | 8 (44) | |
| Skin thickening | 0 (0) | 2 (11) | |
| Raynaud's | 0 (0) | 4 (22) | |
| Hypertrichosis | 0 (0) | 3 (17) | |
| Nail changes (white nail/clubbing) | 0 (0) | 4 (22) | NS |
| Bleeding/Bruising | 1 (6) | 0 (0) | NS |
| Endocrinopathy | 6 (38) | 18 (100) | <.001 |
| Adrenal insufficiency | 0 (0) | 4 (22) | |
| Hypogonadism | 5 (31) | 12 (67) | |
| Hypothyroidism | 3 (19) | 11 (61) | |
| Hyperprolactinemia | 0 (0) | 3 (19) | |
| Hyperparathyroidism | 0 (0) | 2 (11) | |
| Extravascular volume overload | 8 (50) | 18 (100) | <.001 |
| Edema | 4 (25) | 16 (89) | |
| Ascites | 3 (19) | 6 (33) | |
| Pleural effusion | 4 (25) | 5 (28) | |
| Pericardial effusion | 4 (25) | 4 (22) | |
| Papilledema | 0 (0) | 2 (11) | NS |
| Bone lesions | 2 (20) | 12 (67) | .046 |
| Sclerotic lesions | 2 (20) | 11 (61) | .054 |
| Lytic lesions | 1 (10) | 4 (22) | NS |
| Mixed lytic/sclerotic | 1 (10) | 5 (28) | NS |
| Pulmonary hypertension | 0 (0) | 3 (19) | NS |
| Cardiomegaly/cardiomyopathy | 1 (6) | 1 (6) | NS |
| Hepatomegaly | 2 (13) | 7 (39) | NS |
| Splenomegaly | 7 (44) | 13 (72) | NS |
| Paraneoplastic pemphigus | 0 (0) | 0 (0) | — |
| Autoimmune hemolytic anemia | 3 (19) | 0 (0) | NS |
| Rheumatologic disease | 0 (0) | 1 (6) | NS |
- Abbreviations: ECOG, Eastern Cooperative Oncology Group; GI, gastrointestinal; MCD, multicentric Castleman disease; NS, not significant.
| MCD without POEMS (N = 16) | MCD and POEMS (N = 18) | p value | |
|---|---|---|---|
| Variable | Median (range) | Median (range) | |
| Histology, n (%) | NS | ||
| Hyaline vascular | 3 (19) | 5 (28) | NS |
| Plasma cell variant | 10 (63) | 7 (39) | NS |
| Mixed variant | 3 (19) | 3 (17) | |
| Unknown | 0 (0) | 3 (17) | |
| HIV status, n (%) | NS | ||
| Positive | 2 (13) | 0 (0) | |
| Negative | 11 (69) | 13 (72) | |
| Unknown | 3 (19) | 5 (28) | |
| Kaposi Sarcoma | 1 (6) | 0 (0) | NS |
| Hemoglobin (g/dL) | 11.2 (5–17.1) | 13.2 (8.6–17.2) | .016 |
| Platelets ×109/L | 211 (38–474) | 378 (166–722) | .007 |
| ESR mm/hr | 61.5 (30–138) | 28 (4–67) | NS |
| CRP mg/L | 21.95 (0.3–282.7) | 5.85 (0.3–234) | NS |
| Creatinine mg/dL | 1.1 (0.7–2.0) | 0.9 (0.6–1.6) | NS |
| Albumin g/dL | 2.7 (1.9–3.5) | 3.5 (2.7–4.3) | <.001 |
| Urine protein (g/24 h) | 0.2375 (0.004–0.496) | 0.226 (0.054–3.1) | NS |
| Uric acid mg/dL | 5.9 (2.3–10.6) | 6.9 (3.6–9.8) | NS |
| B2M mg/L | 3.23 (0.8–9.11) | 4.2 (1.9–8.8) | NS |
| LDH IU/L | 229 (114–370) | 125 (69–238) | .004 |
| BMPCs (%) | 4.5 (2.0–90) | 3.5 (1–10) | NS |
| VEGF pg/mL | 104.5 (38–576) | 389 (60–1847) | .068 |
| IL-6 pg/mL | 9.2 (6.4–21.9) | 6.5 (2.7–627.7) | NS |
| Serum/urine monoclonal protein, N (%) | |||
| Yes | 6 (38) | 16 (89) | .008 |
| No | 8 (50) | 2 (11) | |
| Unknown | 2 (13) | 0 (0) | |
| Monoclonal protein isotype | |||
| Heavy chain: IgA/IgG | 0/5 | 12/3 | .004 |
| Light chain: Lambda/Kappa | 2/4 | 15/0 | .003 |
- Abbreviations: B2M, Beta 2 Microglobulin, BMPCs, bone marrow plasma cells, IL-6, interleukin-6; MCD, multicentric Castleman disease; NS, not significant; VEGF, vascular endothelial growth factor.
3.2 Treatment
Treatment data were available for 33 patients; first-line treatments and treatment responses for MCD patients with and without POEMS are shown in Table 3. Treatment was initiated after a median of 1.6 (range: 0–102.8) months from diagnosis. Among patients with MCD without POEMS (n = 15), the most common treatment was rituximab +/− steroids (n = 7), with most patients receiving this treatment achieving a CR (n = 4). The most common first-line treatment strategy among patients with MCD and POEMS (n = 18) was ASCT (N = 9); all of these patients achieved at least a PR, including a CR in seven patients.
| MCD without POEMS (N = 15) | MCD with POEMS (N = 18) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | CR | PR | SD/PD | NK | N | CR | PR | SD/PD | NK | |
| Rituximab ± CS | 7 | 4 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
| CS | 2 | 0 | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 1 |
| Anti-IL-6 ± CS | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 |
| CT ± CS | 1 | 0 | 0 | 0 | 1 | 5 | 2 | 0 | 1 | 2 |
| CT + Rituximab ± CS | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| ASCT | 1 | 1 | 0 | 0 | 0 | 9 | 7 | 2 | 0 | 0 |
| Total | 15 | 7 | 1 | 3 | 4 | 18 | 9 | 3 | 3 | 3 |
- Note: First-line treatments and responses in patients with MCD with (N = 18) and without POEMS (N = 15).
- Abbreviations: ASCT, autologous stem cell transplant; CR, complete response; CS, corticosteroids; CT, chemotherapy (agents used were etoposide, cyclophosphamide, bortezomib, lenalidomide, and thalidomide); NK, not known; PR, partial response; SD/PD, stable disease/progressive disease.
The 10 patients who underwent ASCT as the first-line treatment did so after a median of 7.0 (range: 2.2–102.8) months from diagnosis. Four received upfront ASCT without prior systemic treatment, while the others received prior induction with dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide (D-PACE) (one patient), cyclophosphamide (two patients), lenalidomide, and dexamethasone (one patient), few doses of bortezomib stopped due to side effects (one patient) and bevacizumab (one patient). One-hundred-day ASCT treatment-related mortality was zero. All patients had at least a PR to ASCT, including CR in eight patients.
Among those 10 patients, 1 patient had MCD without POEMS; his lymph node pathology at the time of diagnosis showed PCV MCD with a clonal lambda light chain restricted plasma cell infiltrate (5%) and an additional CD5 positive marginal zone B-cell proliferation, and his main disease symptoms were related to spinal compression from a T10 spinal lesion. He underwent induction with D-PACE, followed by ASCT after 7.1 months from diagnosis. Posttransplant, he received maintenance with Velcade, dexamethasone, and thalidomide, achieving a CR with a decrease in the size of his lymph nodes after 3 months and complete regression in 6 months. He was still alive and in CR at the last follow-up 9.4 months from his transplant.
TTNT was 11.5 (95% CI: 4.5-NR) years in patients who underwent transplant as part of first-line treatment and 2.0 (95% CI: 0.6-NR) years in patients who did not (p = .009) (Figure 1A), but OS did not differ between the two groups (Figure 1B). The 5- and 10-year OS rates in the entire cohort were 84% and 71%, respectively. Eastern Cooperative Oncology Group (ECOG) performance status at diagnosis was available for 17 (of 23) patients who did not undergo transplant as part of first-line treatment, and 6 (of 10) patients who did. Among the two groups, 65% (11 of 17) and 67% (4 of 6) patients had ECOG performance status <2, respectively. The difference was not statistically significant (p = .93).
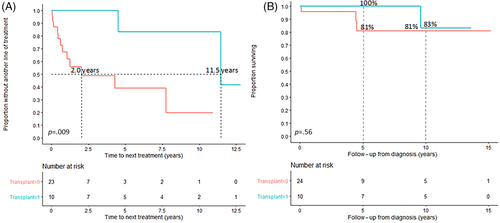
With a median follow-up in the entire cohort of 6.0 (95% CI: 3.0–10.2) years, 14 patients had initiated a second treatment after a median of 1.2 (95% CI: 0.6–4.8) years from the time of initiation of the first treatment. Among those, eight had MCD with POEMS. Within this group, the reasons for initiating a second-line of treatment, available for seven patients, were predominantly inadequate clinical and/or radiologic control of POEMS-related disease features.
Second-line treatments included anti-IL 6 (siltuximab) +/− steroids in three patients, rituximab in one patient, chemotherapy (with or without steroids) in five patients, chemotherapy with rituximab and steroids in one patient, and ASCT in three patients; one patient received additional radiation therapy after 4.5 years from initiation of first treatment (ASCT), and subsequently received rituximab 5.4 years later. Beyond second-line treatment, nine patients had received one or more subsequent lines of treatment by the last follow-up, including ASCT in three patients, for a total of six patients receiving ASCT as a subsequent therapy.
The six patients who underwent ASCT as a subsequent line of treatment did so after a median of 1.3 (range: 0.4–5.3) years from diagnosis. All five patients with known response to ASCT achieved at least a PR, including CR in two patients. One-hundred-day ASCT treatment-related mortality was zero. Five of the six patients had MCD with POEMS; among those, one initiated a subsequent line of treatment with chemotherapy (daratumumab, bortezomib, and dexamethasone) after 14.6 years from ASCT, one patient had a second ASCT after 7.3 years and was still alive at the last follow-up 3.3 years from his second transplant, and three patients had not initiated another line of treatment and were still alive at their last follow-up 5.2, 5.3, and 6.6 years from ASCT.
The one patient who did not have coexistent POEMS and received ASCT beyond first-line therapy was initially treated with partial resection of a mediastinal mass followed by prednisone and tocilizumab, and then received chemotherapy (lenalidomide and dexamethasone) 7 months later with no response. ASCT with BEAM conditioning was performed 1.8 years from diagnosis, and he achieved a PR. A combination of pomalidomide and dexamethasone was given for 9 months posttransplant as consolidation. He was still alive without progression at the last follow-up 1.2 years from ASCT.
4 DISCUSSION
MCD is a rare disease characterized by marked heterogeneity in clinical characteristics and outcomes. The cohort described in this paper, including 34 Mayo Clinic patients diagnosed with MCD over a period of 15 years, portrays the heterogeneity of this disease and of therapy. Nearly half of patients received ASCT as part of their therapy during the course of their disease. There was no transplant-related mortality, and both TTNT and OS were excellent. Most of the patients undergoing ASCT had coexistent POEMS syndrome; two patients did not.
The clinical phenotype of MCD patients with and without POEMS syndrome features were as expected: by definition, patients with MCD and POEMS were more likely to present with peripheral neuropathy, endocrinopathy, skin changes, bone lesions, and extracellular volume overload compared to patients with MCD without POEMS and were more likely to have a monoclonal protein in the serum and/or urine. Although patients with MCD and POEMS had a higher rate of pulmonary hypertension, hepatomegaly, and splenomegaly, the differences were not statistically significant, which may be attributed to small sample size. We did not observe differences in histology between the two groups. Laboratory differences were also observed and were consistent with prior studies,18 where the MCD without POEMS group had lower hemoglobin concentration and platelet counts, but higher levels of inflammatory markers (although not statistically significant) compared to patients with MCD and POEMS. In addition, lower albumin and higher lactate dehydrogenase (LDH) levels were observed in this group.
The heterogeneity in clinical characteristics was paralleled by an assortment of treatments, reflecting the paucity of comparative studies to guide management of this rare disease. Variability in the timing of treatment initiation and TTNT was also seen, with some patients not requiring treatment for several years from the first-line treatment. The treatment armamentarium has expanded in the last decade; in the Mayo Clinic/University of Nebraska study spanning the period before 2002, patients primarily received steroids and alkylator-based treatments; only one subject received rituximab in second-line setting. The changes in treatment over time are reflected in a recent study evaluating treatments and treatment responses in patients with POEMS and Castleman's from 2000 to 2018 in Japan; among 14 evaluable patients, 10 received thalidomide-based treatment in the first-line setting, and 6 underwent ASCT after proteasome inhibitor- or immunomodulatory-based induction, with responses achieved in the vast majority.19 The treatment used in the Mayo cohort included cytotoxic chemotherapy, proteasome inhibitors, immunomodulatory agents, rituximab (alone or in combination with chemotherapy), and IL-6 targeting treatments. Furthermore, ASCT was utilized in approximately 50% of patients with treatment data, mainly those with MCD and POEMS. All patients who underwent transplant achieved at least a PR to treatment and there were no deaths within 100 days of transplantation, and patients who underwent transplant in the first-line setting had a 5-year TTNT of 83%. We observed improved TTNT in patients who underwent ASCT in the frontline setting, but without significant improvement in OS, which may be due to the small sample size and/or inadequate follow-up. Although, we did not observe significant differences in performance status at diagnosis between patients who underwent ASCT and those who did not (p = .93), the risk of selection bias, where more fit patients undergo transplant, cannot be excluded.
ASCT is considered one of the treatment modalities in management of POEMS syndrome, with high rates of clinical, hematologic, and VEGF responses, significant improvement in neuropathy, and long progression-free survival reported in retrospective studies.15-17, 19-21 There are no randomized controlled studies comparing upfront treatment with ASCT versus other regimens, but a retrospective study by Zhao et al. comparing upfront ASCT, melphalan and dexamethasone combination, and lenalidomide and dexamethasone combination among 347 patients with POEMS treated over a 17-year period showed that ASCT was associated with higher response and 5-year progression-free survival rates especially among medium-high risk patients.21 Although results from this and other retrospective studies may be confounded by selection bias with more fit patients and those with lower risk undergoing ASCT, favorable long-term outcomes reported in these studies suggest that ASCT should be considered in the upfront setting in eligible patients with disseminated bone marrow involvement albeit with close monitoring for periengraftment syndrome.14, 22 The role of ASCT in MCD without POEMS has not yet been established, with only a few case reports and mostly after failure of other regimens. These are summarized in Table 4 and include six cases of ASCT and two cases of allogeneic stem cell transplant. Responses with ASCT appeared to be durable, ranging from 15+ to 50+ months. In the present study, we highlighted the clinical course of the two patients with MCD without POEMS who underwent transplant; one patient underwent ASCT as first-line treatment followed by maintenance chemotherapy and was still alive and in CR at the last follow-up 9.4 months from his transplant. The second patient who underwent transplant as salvage therapy achieved a PR was still alive by last follow-up 1.2 years from transplant.
| Study | MCD type | Age (y)/Sex | HHV-8/HIV status | Prior treatments and responses | Dx to ASCT (m) | ASCT-related toxicity | FU from ASCT, m | Disease status at last follow-up |
|---|---|---|---|---|---|---|---|---|
| Repetto 198623 | HV | 42/M | NA/ NA | 1. VAP ➔ resolution of Sx and LN regression × 6 m 2. CHOP alternating with etoposide, vindesine, doxorubicin and prednisolone × 8 courses ➔ LN regression. Response lasted 1 m 3. ASCTa ➔ CR 16 weeks after treatment |
NA | NA | 15 | CR |
| Advani 199924 | Mixed | 38/M | NA/ Neg | 1. Prednisone 80 mg/day ➔ significant response lasting 5 months 2. CVP × 6 cycles ➔ CR lasting 5 m ➔ follicular mixed lymphoma 3. ASCTb ➔ CR |
18 | NA | 48 | CR |
| Ogita 200725 | PCV, with AA amyloidosis | 39/F | NA/Neg | 1. Double-filtration plasmapheresis twice monthly and oral daily cyclophosphamide and prednisolone ➔ response lasting 6 m 2. ASCTc ➔ CR |
NA | intestinal TMA, Transient dialysis | 42 | CR |
| Tal 201126 | PCV | 52/M | Neg/Neg | 1. R-CVP 6 cycles➔ CR 2. Rituximab and vinblastine × 2 months ➔ ASCTd consolidation |
32 | Neutropenic fever day 6 | 50 | CR |
| Jerkeman 201527 | PCV | 55/F | Neg/Neg | 1. R-CHOP × 3 cycles followed by weekly Ritux × 4 ➔ NR 2. Steroids 3. Tocilizumab at 2-wk intervals × 17 doses ➔ CR ➔ ASCTa consolidation |
~15 | None | 30 | CR |
| Jerkeman 201527 | PCV | 63/M | Neg/Neg | 1. Rituximab × 4 doses ➔ NR 2. R-CHOP × 6 courses ➔ NR 3. Tocilizumab × 12 doses ➔ consolidation ASCTa |
~ 50 | None | 18 | CR |
| Angenendt 201528 | HV | 25/M | Neg/Neg | 1. Rituximab weekly × 4 cycles ➔ CR lasting 18 months 2. Rituximab × 4 cycles ➔ CR × 21 months 3. R-CHOP × 6 cycles ➔ CR × 1 month 4. R-Dexa-BEAM × 2 cycles (intent for ASCT consolidation) ➔ PD 5. Rituximab and tocilizumab ➔ Sx resolved but no radiologic response, then PD after 5 cycles 6. AlloSCTe from a matched-unrelated donor |
~ 72 | GI GvHD | ~ 6 | CR |
| Wang 202029 | Mixed | 17/F | Pos/Neg | 1. CHOEP × 3 cycles ➔ NR 2. Hyper-CVAD-B ➔ NR 3. COAP ➔ NR 4. VAD × 2 ➔ CR lasting 6 m 5. VAD ➔ NR 6. Bortezomib and dexamethasone × 2 cycles ➔ PR 7. Allo-HSCTf from HLA-matched unrelated donor |
12 | Premature menopause | 84 | CR |
| Current report—Patient 1 | PCV | 66/M | Neg/Neg | 1. D-PACE, followed by ASCT and maintenance with Velcade, dexamethasone and thalidomide ➔ CR | 7.1 | None | 9.4 | CR |
| Current report—Patient 2 | Mixed | 42/M | Neg/NA | 1. Partial resection of a mediastinal mass, prednisone and tocilizumab 2. Lenalidomide and dexamethasone ➔ NR 3. ASCTg and Pomalidomide and dexamethasone consolidation × 9 ➔ PR |
21.4 | None | 15.1 | PR |
- Abbreviations: ASCT, transplant; BEAM, carmustine, etoposide, cytarabine and melphalan; CHOEP, cyclophosphamide, vincristine, methylprednisolone, and epirubicin; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone; COAP, cyclophosphamide, vincristine, methylprednisolone, and cytarabine; CR, complete remission; CVP, cytoxan, vincristine, prednisone; HV, hyaline vascular; Hyper-CVAD-B, high-dose methotrexate and cytarabine; LN, lymph node; MCV, multicentric Castleman's disease; NA, not available; NHL, non-Hodgkin's lymphoma; PCV, plasma cell variant, Sx, symptoms; TMA, thrombotic microangiopathy; VAD, vincristine, doxorubicin, and dexamethasone; VAP, Prednisolone 70 mg/day × 5 weeks, then vincristine 2 mg/week × 3 weeks, then doxorubicin 30 mg/week × 3 weeks.
- a Conditioning with Melphalan 200 mg/m2.
- b Conditioning with total body radiation 1200 cGy, etoposide 60 mg/kg and cyclophosphamide 100 mg/kg.
- c Conditioning with Melphalan 140 mg/m2.
- d Conditioning with etoposide, thiotepa, cytosine arabinoside, cyclophosphamide, and melphalan.
- e Reduced intensity conditioning with fludarabine, cyclophosphamide, total body irradiation, and antithymocyte globulin.
- f Conditioning with Busulfan, etoposide, and smoustin.
- g Conditioning with carmustine, etoposide, cytarabine, and melphalan.
This study is limited by its retrospective nature, limited sample size due to the rarity of this disease, missing data for certain laboratory values and missing treatment-related data, heterogeneous follow-up, and data obtained over a long period over which treatments have changed. Despite these limitations, the findings in our study are important to enhance understanding of this rare disease and in providing our experience in treatment with contemporary regimens, especially ASCT where experience is limited. The favorable outcomes seen with ASCT in this cohort and in previous reports suggest that transplant may have a role in MCD, including patients with MCD associated with POEMS.
In summary, in this paper, we described the clinical characteristics, treatments, and outcomes of patients with MCD over a 15-year period, during which ASCT was utilized in a subset of patients. MCD should be recognized as a heterogeneous disease, demanding an individualized treatment approach at present.8, 13 With improvement in patient selection and outcomes with transplantation over the recent years, ASCT may be considered a major treatment option in patients with MCD, especially those with the POEMS-variant. Future studies are needed to clarify the optimal timing and ideal candidates for ASCT in MCD.
PATIENT CONSENT STATEMENT
All patients authorized use of their electronic medical records for research purposes.
CONFLICT OF INTEREST
P.K. is a principal investigator of research studies for which Mayo Clinic has received funding from AbbVie, Takeda, Sanofi, Janssen, Karyopharm, Glaxo SmithKline, Regeneron Pharmaceuticals, Ichnos Sciences, and Amgen. He has served on the Medical advisory board meetings of Sanofi, Pharmacyclics, BeiGene, Cellectar, GSK, X4, and Karyopharm. A.D. received research funding from Celgene, Millennium Pharmaceuticals, Pfizer, and Janssen and received a travel grant from Pfizer. M.A.G. served as a consultant for Millennium Pharmaceuticals and received honoraria from Celgene, Millennium Pharmaceuticals, Onyx Pharmaceuticals, Novartis, GlaxoSmithKline, Prothena, Ionis Pharmaceuticals, and Amgen. M.Q.L. received research funding from Celgene. N.L. serves on an advisory board for Takeda Pharmaceuticals. S.K.K. served as a consultant for Celgene, Millennium Pharmaceuticals, Onyx Pharmaceuticals, Janssen, and Bristol-Myers Squibb and received research funding from Celgene, Millennium Pharmaceuticals, Novartis, Onyx Pharmaceuticals, AbbVie, Janssen, and Bristol-Myers Squibb. E.M. received honorarium from Janssen and consultation fee from Protego (fee paid to institution). The remaining authors declare no competing financial interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




