Association of clonal hematopoiesis mutations with clinical outcomes: A systematic review and meta-analysis
Funding information: Cancer Prevention and Research Institute of Texas, Grant/Award Numbers: CPRIT FP9178, CPRIT RR190077; National Cancer Institute, Grant/Award Numbers: NCI K08CA263313, NCI L30CA253796; National Institutes of Health, Grant/Award Number: P30 CA016672
Abstract
Clonal hematopoiesis (CH) mutations are common among individuals without known hematologic disease. CH mutations have been associated with numerous adverse clinical outcomes across many different studies. We systematically reviewed the available literature for clinical outcomes associated with CH mutations in patients without hematologic disease. We searched PubMed, EMBASE, and Scopus for eligible studies. Three investigators independently extracted the data, and each study was verified by a second author. Risk of bias was assessed using the Newcastle-Ottawa Scale. We identified 32 studies with 56 cohorts that examine the association between CH mutations and clinical outcomes. We conducted meta-analyses comparing outcomes among individuals with and without detectable CH mutations. We conducted meta-analyses for cardiovascular diseases (nine studies; HR = 1.61, 95% CI = 1.26–2.07, p = .0002), hematologic malignancies (seven studies; HR = 5.59, 95% CI = 3.31–9.45, p < .0001), therapy-related myeloid neoplasms (four studies; HR = 7.55, 95% CI = 4.3–13.57, p < .001), and death (nine studies; HR = 1.34, 95% CI = 1.2–1.5, p < .0001). The cardiovascular disease analysis was further stratified by variant allele fraction (VAF) and gene, which showed a statistically significant association only with a VAF of ≥ 10% (HR = 1.42, 95% CI = 1.24–1.62, p < .0001), as well as statistically significant associations for each gene examined with the largest magnitude of effect found for CH mutations in JAK2 (HR = 3.5, 95% CI = 1.84–6.68, p < .0001). Analysis of the association of CH mutations with hematologic malignancy demonstrated a numeric stepwise increase in risk with increasing VAF thresholds. This analysis strongly supports the association of CH mutations with a clinically meaningful increased risk of adverse clinical outcomes among individuals without hematologic disease, particularly with increasing VAF thresholds.
1 INTRODUCTION
Clonal hematopoiesis (CH) is characterized by acquired mutations in the hematopoietic system that provide a competitive advantage resulting in the clonal expansion of the mutated cell population.1 While CH was initially thought to occur primarily in the context of hematologic cancer, advances in gene sequencing technology have shown that clonal expansions can be found in healthy individuals.2, 3
Mutations in leukemia driver genes detected in otherwise healthy individuals are common.4, 5 When detected using traditional Massively Parallel Sequencing approaches, initially designed to sequence tumors and identify germline mutations, CH mutations are commonly found in over 10% of individuals aged 65 years or older, increasing in frequency to over 30% at age 85 years or older.6 However, when utilizing increasingly sensitive detection methods, CH mutations are ubiquitous beginning in middle age.7, 8
While these mutations may frequently signal a benign process, their clinical significance is not fully understood.4 CH has been associated with both hematologic and nonhematologic disease, including those related to chronic inflammatory states.9 In addition, CH mutations have been associated with cardiovascular disease (CVD) and all-cause mortality.6, 10 While the exact mechanisms are still being investigated, cells harboring CH-driver mutations can increase the expression of pro-inflammatory cytokine and chemokine genes, which contribute to the development of atherosclerosis and correspond to plaque burden.11, 12 Importantly, inhibition of CH-mediated signaling pathways has been shown to attenuate CVD risk.13, 14 CH mutations have also been associated with type 2 diabetes, obesity, numerous solid tumors, peripheral arterial disease, and severe COVID-19, among many others.15-18 The extent to which CH mutations are directly involved in the pathogenesis of this diverse array of human disease, potentially through their role in pro-inflammatory pathways, is unclear.19
The number of clinical outcomes associated with CH mutations is rapidly expanding. Further complicating interpretation of these disease-CH associations is the lack of consensus as to what constitutes a clinically significant CH mutation burden (i.e., variant allele fraction [VAF] or the proportion of the sequence reads that observed a specific variant of interest) in an otherwise healthy individual. A comprehensive evaluation of the literature to understand the association of CH mutations with clinical outcomes will address an important knowledge gap and guide future clinical and research efforts. Here, we undertake a systemic review and meta-analysis of the published literature to examine the association of CH mutations with clinical outcomes, including examination of disease risk according to CH mutation burden. We hypothesize that CH mutations are associated with adverse clinical outcomes in patients without hematologic disease.
2 METHODS
This systematic review was performed and reported in accordance with the meta-analysis of observational studies in epidemiology (MOOSE)20 statement and followed the enhancing the quality and transparency of health research (EQUATOR) Reporting Guidelines.
2.1 Search strategy
We systematically searched for articles indexed in PubMed, EMBASE, and Scopus (inception to July 9, 2020). The concepts used to search these databases were “clonal AND (hematopoiesis OR (mosaic* AND blood)).” The reference lists of recent reviews and included studies were screened for additional references. Authors were contacted directly when full-texts or supplementary data were not readily accessible.
2.2 Study selection, inclusion, and exclusion criteria
We included studies meeting the following criteria: 1) population-based (e.g., cohort, case–control), 2) in humans, 3) available in English (articles were not required to be published in an English language journal but an English language copy for initial screening was required), 4) an original research article (e.g., not a conference proceeding), 5) among individuals without known active hematologic disease at determination of CH mutation status, 6) that reported a measure of risk (e.g., odds ratio [OR], hazard ratio [HR], risk ratio) for a health outcome (e.g., survival or a disease outcome such as diabetes, stroke, malignancy, etc.) among individuals with and without CH mutations determined using sequencing data, 7) where analyses minimally accounted for age as a confounder (e.g., adjustment, stratification, matching).
All stages of screening were performed in Covidence,21 an online systematic review management software. Three authors (MKN, MRT, KTN) jointly participated in each step of screening and full-text review. For each article, any two of these authors conducted screening and full-text review. Disagreement was resolved by the third author that had not participated in the initial screening or full-text review of that article.
2.3 Data extraction
Data on the characteristics of each included study were extracted independently by one author (MKN, MRT, or KTN), and verified by a second author (MKN or KTN), using an adapted Cochrane data extraction template (http://cccrg.cochrane.org/author-resources). Extracted data included first author, cohort name(s) or description, cohort location(s), genes examined, disease outcome, follow-up time, sample size, effect estimate type (e.g., HR, OR), effect estimate, 95% confidence interval [CI], p-value, and adjustment covariates. We additionally, a priori, extracted summary statistic data according to different VAF thresholds to define the exposure (e.g., VAF ≥10%) and specific gene associations when available. We extracted data from all studies regardless of overlap with other included publications. When individual studies reported results in multiple cohorts, where possible, we extracted summary data for each cohort. If a study reported results for a given clinical outcome only by individual genes without a combined gene analysis, we extracted the reported association with the smallest standard error. We utilized the Newcastle-Ottawa Scale for assessing the quality of nonrandomized studies in meta-analyses, which rates studies based on the quality of selection (e.g., of cases and controls; score range 0–4), comparability (e.g., of the exposed and unexposed groups; score range 0–2), and ascertainment of the exposure or outcome (for case–control and cohort studies, respectively; score range 0–3), where a higher numeric rating indicates a better quality score.22
2.4 Statistical analysis
We conducted meta-analyses to calculate summary statistic HRs and 95% CIs for identified health outcomes among individuals with and without detectable CH mutations. Ratios of rates, including ORs and HRs, were considered equivalent measures of risk.23, 24 If 95% CIs or p-values were not reported, we calculated them from the existing summary data, where possible, to allow for inclusion in the meta-analysis.
We synthesized effect estimates of clinical outcome categories using a random-effects meta-analysis model if three or more independent studies reported on that outcome category. Random-effects meta-analytic models were selected a priori as a conservative approach given expected variability in methods used to identify CH mutations and diversity of study populations and phenotypes. Where there were multiple summary statistics in the literature for a clinical outcome in the same cohort (e.g., CVD) we utilized the summary statistic with the smallest standard error in the meta-analysis. When studies reported more than one analysis model, we chose the model accounting for the largest number of confounding factors.
The presence of small-study effects was not evaluated as no individual meta-analysis had at least 10 included studies.25 All effect estimates and corresponding 95% CIs were log-transformed for analysis. All analyses were conducted using R version 4.0.0. Tests were considered significant if the two-sided p-value was less than .05.
3 RESULTS
3.1 Search results
Our study selection process is summarized in Figure S1. After duplicate removal, we screened 5101 unique entries. Of these, 173 underwent full-text review with 32 studies ultimately included in the systematic review (see Figure S1 for reasons for exclusion). The 32 studies included in our systematic review, with individual cohorts and outcomes summarized separately where data were available, are presented in Table S1. Studies were published from 2014 to 2021 primarily in North American and European populations and reported on a variety of clinical associations, including, most commonly, CVD, hematologic cancer, therapy-related hematologic neoplasms, and death. Follow-up ranged from a median of 1.4 to 13.1 years, where reported, with cohort sizes ranging from 39 to 36 660 individuals. The number and proportion of CH mutations by gene in each study are summarized in Table S2.
3.2 Quality of evidence
Assessment of study quality using the Newcastle-Ottawa Scale22 is presented in Table S3. Among 32 studies included, 20 were of good quality, 6 of fair quality, and 6 of poor quality. Most studies scored high on the comparability scale due to extensive adjustment for potential confounders, such as age and comorbidities. All included studies used objective measures (i.e., gene sequencing) to ascertain exposure, which was defined as the presence of identifiable CH mutations above a certain VAF threshold. Study quality was most notably limited by short follow-up time in cohort studies.
3.3 Cardiovascular disease
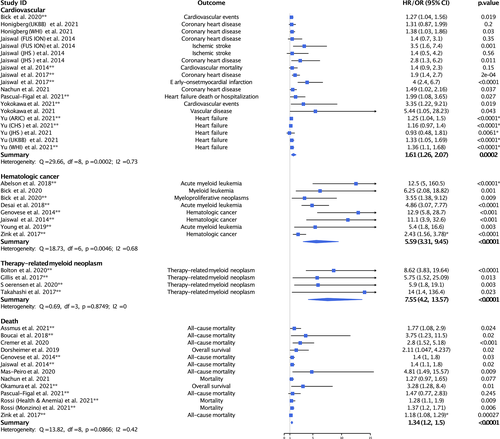
Meta-analyses and forest plots are presented in Figure 1 with study details presented in Table S1. After accounting for studies with overlapping cohorts, our meta-analysis of nine studies or cohorts showed that individuals with CH mutations had a statistically significantly increased risk of CVD (HR = 1.61, 95% CI = 1.26–2.07, p = .0002; I2 = 73%, p = .0002). We additionally meta-analyzed data examining the association of CH mutations with atherosclerotic CVD (HR = 1.85, 95% CI = 1.39–2.47, p < .001; I2 = 63.2%, p = .012) and coronary heart disease (HR = 1.57, 95% CI = 1.37–1.81, p < .001; I2 = 0%, p = .42) and found statistically significantly increased risk (see Table S1 for specification of studies included in each meta-analysis). Atherosclerotic CVD included coronary heart disease, early-onset myocardial infarction, and ischemic stroke. Coronary heart disease included studies specifically reporting on coronary heart disease.
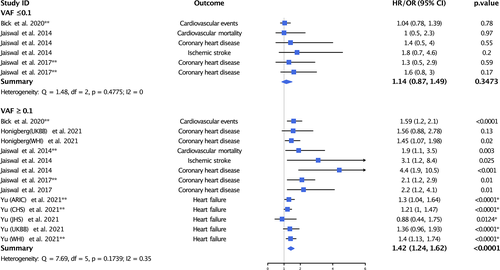
To better understand the impact of VAF on CVD, we extracted and meta-analyzed data on the association of CH mutations with CVD according to CH mutation VAF (Figure 2). When examining the association of CH mutations with a VAF of 10% or less, we did not find a statistically significant association between CH mutations and CVD (HR = 1.14, 95% CI = 0.87–1.49, p = .3473; I2 = 0%, p = .4775). Conversely, when examining the association of CH mutations with a VAF of 10% or more we found a statistically significant association between CH mutations and increased risk of CVD (HR = 1.42, 95% CI = 1.24–1.62, p < .0001; I2 = 35%, p = .1739).
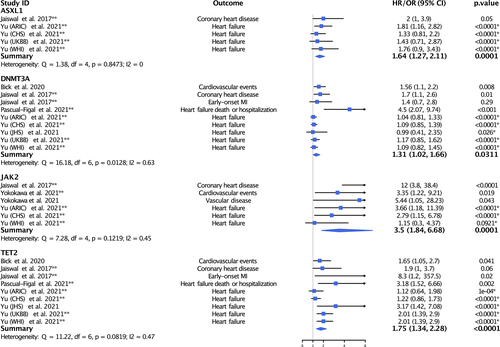
We next examined the association of CH mutations with CV outcomes stratified by gene (Figure 3). We observed statistically significant associations for each gene examined with the largest magnitude of effect found for CH mutations in JAK2 (HR = 3.5, 95% CI = 1.84–6.68, p < .0001; I2 = 45%, p = .1219).
3.4 Hematologic cancer
We meta-analyzed data from seven studies examining the association of CH mutations with the risk of hematologic malignancy (Figure 1). We found that individuals with CH mutations had a large and statistically significantly increased risk of hematologic cancer (HR = 5.59, 95% CI = 3.31–9.45, p < .0001; I2 = 68%, p = .005).
We additionally examined the association of CH mutations with hematologic malignancy according to the VAF threshold used to define CH mutations (Figure 4). While there were not sufficient studies using the same VAF threshold category to generate summary effects, we observed a stepwise numeric trend of increasing risk of hematologic cancer with increasing VAF threshold ranging from a VAF of 0.5% or greater (HR = 2.4, 95% CI = 1.0–6.1, p = .06) to a VAF of 10% or greater (HR = 49, 95% CI = 21–120, p < .001).

There were a limited number of studies examining the association of CH mutations with hematologic malignancy stratified by gene (Figure 4), which did not demonstrate clear gene-specific patterns.
3.5 Therapy-related myeloid neoplasm
We next meta-analyzed data from four studies examining the association of CH mutations with the risk of therapy-related myeloid neoplasm (Figure 1). We found that CH mutations conferred a large and statistically significantly increased risk of therapy-related myeloid neoplasm (HR = 7.55, 95% CI = 4.2–13.57, p < .0001; I2 = 0%, p = .875).
3.6 Death
We conducted a meta-analysis of nine studies or cohorts examining the risk of death among individuals found to carry CH mutations (Figure 1). We found that CH mutations conferred a large and statistically significantly increased risk of death (HR = 1.34, 95% CI = 1.2–1.5, p < .0001; I2 = 42%, p = .087).
3.7 Other results
Results for extracted data that were not included in the meta-analyses are presented in Table S4.2, 15-18, 26-34 Reasons for exclusion from meta-analysis included insufficient reporting of summary data (e.g., no effect estimate) or lack of sufficient related outcomes to form a group of three or more studies. Notably, two studies17, 26 indicated an association between CH mutations and solid malignancy and two studies indicated an association between CH mutations and metabolic disease, including obesity15 and type 2 diabetes.18
4 DISCUSSION
In our systemic review and meta-analysis of 32 studies, we found that individuals with CH mutations had a statistically significant increased risk of CVD, particularly atherosclerotic CVD, hematologic cancer, therapy-related myeloid neoplasms, and death. In addition, published studies strongly support that the clinical relevance of CH mutations is greater with higher VAF thresholds. This comprehensive analysis of published studies strongly supports the association of CH mutations with a clinically meaningful increased risk of adverse hematologic and nonhematologic clinical outcomes and can be used to prioritize future investigations.
CVD was the most common clinical disease outcome associated with CH mutations. Among cardiovascular outcomes examined, the largest magnitude of effect was observed between CH mutations and atherosclerotic CVD. This finding is important from a public health perspective as CVD is the leading cause of death in the elderly, but a significant proportion of patients with CVD lack established risk factors (e.g. diabetes, dyslipidemia, and hypertension), or have just one risk factor.35, 36 There is also increasing evidence that most middle-aged individuals at low risk for CVD based on traditional risk factors exhibit subclinical atherosclerosis.37, 38 Therefore, it is reasonable to conclude that there are other, not yet identified, age-dependent risk factors contributing to the development of CVD, of which CH mutations may be a meaningful contributor.
There is compelling evidence of a direct causal relationship between CH mutations and CVD. In large population data sets, the frequency of cardiovascular events and the burden of atherosclerosis seen on imaging correspond to CH mutation clone size.12, 39 In animal models, mechanistic ties between CH mutation burden and accelerated atherosclerosis11, 12 and heart failure32, 33 have been demonstrated. RNA sequencing of cells bearing inactivating TET2 mutations, one of the most commonly implicated genes in CH, shows increased expression of pro-inflammatory mediators implicated in the pathogenesis of atherosclerosis, such as interleukin-1-beta (IL-1β) and interleukin-6 (IL-6).12, 42 IL-1β has been shown to mediate CH-associated atherosclerosis and ischemia.43, 44 In addition, anti-IL-1β monoclonal antibody therapy in patients with prior myocardial infarction results in lower recurrent events in randomized studies.13 IL-6, which is induced by IL-1, has been causally linked with CVD in multiple large-scale genetic and biomarker studies,45, 46 and IL-6 signaling deficiency has been shown to attenuate CVD risk among individuals with CH.14
Our meta-analysis suggests that CH mutations in JAK2 might be particularly important in mediating CVD risk. This is plausible from a physiologic standpoint. Clonal mutations in JAK2 have been shown to contribute to thrombosis and vascular inflammation by increasing formation of pro-thrombotic neutrophil extracellular traps, facilitating vessel wall attachments via β1/β2 integrin activation, and exacerbating vasoconstriction.47-49 JAK2 mutations have also been shown to accelerate complex plaque formation and delay clearance of apoptotic cells, contributing to plaque instability and rupture risk.48, 49 While the mechanisms by which CH mutations in TET2 and JAK2 contribute to CVD have been most studied, there likely exist other, independent pathways by which CH mutations increase CVD risk.
Our analysis also showed that the association between CH mutations and CVD may be limited to CH mutations with a VAF ≥10%. VAF is a measure of the mutation burden and refers to the proportion of the sequence reads that observed a specific variant of interest. While the intrinsic error rate of next-generation sequencing has commonly restricted analyses to VAF of 2% or higher,50 the use of newer sequencing technology and bioinformatic methods allows for detection of clonal mutations of increasingly lower frequencies.50, 51 More recent studies suggest that CH mutations are ubiquitous in all adults when sufficiently deep sequencing methods are utilized.3, 8, 50 However, the clinical relevance of such small clones remains unclear. In our meta-analysis, we examined VAF in CVD given the high volume of publications for this phenotype. Unfortunately, the published literature is limited to studies using a 10% VAF cut-off to define categories. Therefore, while we can conclude that CH mutations with a VAF of 10% or greater are likely more clinically significant than those with a VAF of 10% or less, we are unable to determine a more exact clinically relevant VAF threshold, as findings in the VAF ≥10% group may be completely driven by the presence of larger clones (e.g. VAF ≥20%). More investigation regarding clinically relevant VAF thresholds is needed.
In our meta-analysis, we found more than 6-fold increased risk of hematologic cancer among individuals harboring CH mutations. Acute myeloid leukemia (AML) was the most frequently observed malignancy.51-53 This is not surprising, as CH among individuals without hematologic cancer is largely secondary to leukemia driver mutations and is thus considered a neoplasia precursor state.4 However, while the presence of multiple clones is a hallmark of cancer, it is not considered neoplastic by itself.6 Patients with myelodysplastic syndromes typically have mutations in two or more driver genes, while the median number of mutations in de novo AML is five.18, 54 In contrast, most individuals with CH mutations have only one leukemia driver gene mutation (when using VAF of 2% or greater to define CH).18 Progression to overt neoplasia requires acquisition of secondary mutations, which explains how patients with identifiable CH mutations can remain clinically stable for years.6, 55 Accordingly, while the presence of CH mutations carries a significantly increased risk of hematologic malignancy, the absolute risk remains relatively small.18, 56 However, we found that the risk of hematologic cancer is numerically greater with increasing VAF thresholds, which may help guide screening and prevention strategies.
Our meta-analysis of four studies showed that patients with CH mutations exposed to oncologic therapy had a more than 7-fold increased risk of developing a therapy-related hematologic neoplasm. Patients with CH mutations detected at the time of primary cancer diagnosis were more likely to develop therapy-related myeloid neoplasms following cytotoxic chemotherapy than patients without CH mutations.57, 58 Furthermore, the presence of CH mutations during autologous stem cell transplantation carried increased risk of developing therapy-related myeloid neoplasms and has been associated with inferior overall survival.59 Considering that clonal mutations are frequently detected at the time of primary cancer diagnosis, this information could be used to identify high-risk patients prior to therapy.59, 60 Interestingly, both donor-derived and preexisting CH have been shown to adversely impact outcomes of hematopoietic stem cell transplantation.60, 61
Several notable studies have examined the association of CH mutations and therapy-related hematologic neoplasms but did not meet criteria for inclusion in our manuscript. Reasons for exclusion included that they evaluated CH mutations among individuals with active hematologic disease and/or did not report summary analyses minimally adjusted for age. Gibson et al. performed targeted sequencing on cryopreserved autologous stem-cell products from 401 individuals who underwent autologous stem cell transplant (ASCT) for non-Hodgkin lymphoma.62 They found that 29.9% of individuals had detectable CH mutations at ASCT, which was associated with a statistically significantly increased 10-year cumulative incidence of therapy-related hematologic neoplasm (14.1% vs. 4.3%) and decreased 10-year overall survival (30.4% vs. 60.9%). Mouhieddine et al. similarly undertook targeted sequencing in stem cell products from 629 individuals with multiple myeloma who underwent ASCT; detecting CH mutations in 21.6% of patients.63 Individuals with CH mutations prior to ACST were not at an increased risk of therapy-related hematologic neoplasm (p = .4), but had statistically significantly worse overall survival and progression-free survival in an age-stratified analysis. Wudhikarn et al. performed targeted sequencing from pre-ASCT bone marrow mononuclear cell DNA, excluding plasma cells, in 101 patients with multiple myeloma and found CH mutations in 23%.64 No differences in second primary malignancies (30.4% vs. 15.3%; p = .13), OS (100.2 months vs. 135.6 months; p = .27), or event-free survival (36.4 months vs. 36.4 months, p = .34) were observed among those with and without CH mutations. More research is needed to understand how oncologic therapies interact with existing CH mutations and whether they can increase the risk of adverse outcomes, including late complications such as CVD and secondary leukemias.
Several clinical outcomes were excluded from our meta-analysis due to insufficient summary data or too few related clinical outcomes to combine. While supported by a more limited numbers of studies, statistically significant and clinically relevant associations with CH mutations have been observed for a diverse array of clinical outcomes, including solid tumors, psychiatric disease, pulmonary disease, peripheral arterial disease, melanoma, type 2 diabetes, obesity, and severe COVID-19.2, 15-18, 26, 28, 29 While most mechanistic data for the association of CH mutations with clinical phenotypes is focused on the pathogenesis of CVD, it is possible that the proposed pro-inflammatory processes contribute to a wider range of adverse associations.19, 64
4.1 Limitations
Our study has limitations. First, the studies included were observational, thus the findings could be the result of shared genetic and environmental risk factors or residual confounding. While all included studies adjusted their analyses for age, other potential confounding factors were not universally adjusted for that could contribute to the development of CH mutations and the examined outcomes, such as smoking. Overall, most of the included studies were judged to have a low risk of bias based on the assessment of study quality. Second, many of the cohorts come from tertiary medical centers and may not be representative of the general population. However, almost all studies selected the exposed and nonexposed cohorts from the same population and the frequency of CH mutations among these patients was not increased when compared to healthy adults. Third, we observed statistically significant heterogeneity in some of our meta-analyses. Methods of sequencing and identification of CH mutations were not uniform among the studies included in our meta-analyses and studies differed in their selection of patient populations and identification of outcomes. Notably, we did not observe heterogeneity when limiting our analysis to coronary heart disease alone, in our VAF analysis, or for most gene-specific analyses. This suggests that our grouping of disease phenotypes, studies with diverse methodology, and gene-specific effects may have contributed to our observed heterogeneity. While a priori specified the use of random-effects models to account for differences between studies, the presence of heterogeneity limits the interpretation of our results.
5 CONCLUSION
In conclusion, there is a strong and growing evidence base for the association of CH mutations with a broad range of both hematologic and nonhematologic outcomes. Considering rapid technological advances and decreasing cost of sequencing, screening for CH mutations has the potential to be an important tool to prognosticate cardiovascular risk, secondary malignancy risk, and other associated sequelae. A better understanding of clinically relevant CH mutation burden is needed as select individuals may benefit from multidisciplinary management including cardiovascular prevention in high-risk CH carrier clinics.65 Strategies to reduce the clonal burden may also have the potential to reduce the risk of malignancy, CVD, and other CH-associated conditions. Ultimately, more research is needed to broadly examine the association of CH mutations with diverse clinical phenotypes and to better define the practical clinical implications of CH mutations.
ACKNOWLEDGMENTS
This study was supported in part by the National Institutes of Health (Cancer Center Support Grant P30 CA016672). Kevin T. Nead and Mackenzie R. Wehner are Cancer Prevention Research Institute of Texas (CPRIT) Scholars in Cancer Research. Kevin T. Nead is supported by CPRIT RR190077, NCI L30CA253796, and NCI K08CA263313. Mackenzie R. Wehner is supported by CPRIT FP9178.
CONFLICT OF INTEREST
The authors have nothing to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




