Optimization of idarubicin and cytarabine induction regimen with homoharringtonine for newly diagnosed acute myeloid leukemia patients based on the peripheral blast clearance rate: A single-arm, phase 2 trial (RJ-AML 2014)
Yunxiang Zhang, Xiaoyang Li and Xiangqin Weng contributed equally to this work.
These data were presented in part at the 58th Annual Meeting of the American Society of Hematology from December 3 to 6, 2016 (San Diego, CA, USA).
Funding information: National Natural Science Foundation of China, Grant/Award Number: 81970148; Shanghai Jiao Tong University School of Medicine Multi-Center Clinical Research Project, Grant/Award Number: DLY201513
Abstract
Individualized chemotherapy, which is at the forefront of acute myeloid leukemia (AML) treatment, has moderately improved outcomes over the past decade. Monitoring the peripheral blood blast burden during induction by flow cytometry has shown significant value in the evaluation of treatment responses. Our previous study reported the day 5 peripheral blast clearance rate (D5-PBCR) as an indicator of early treatment response, and D5-PBCR (+) patients showed poor outcomes. We performed the present phase 2 trial of early intervention in D5-PBCR (+) patients with homoharringtonine (HHT) introduced in the traditional induction regimen with anthracycline and cytarabine. The primary endpoint was complete remission (CR). This study enrolled 151 patients, 65 patients were D5-PBCR (+) and 55 patients completed induction with HHT addition. The overall CR rate after one course of induction was 84.4%, with 87.5% and 80.0% for the D5-PBCR (−) and D5-PBCR (+) groups, respectively. The incidence of grade 3/4 adverse events was comparable between the two groups. At the median follow-up of 53.1 months, median overall survival (OS) was not reached in the entire cohort, and median event-free survival (EFS) was 42.2 months. Neither the OS nor EFS showed significant differences between the D5-PBCR (−) and D5-PBCR (+) groups. Compared to historical data, significant improvements in both OS (p = .020) and EFS (p = .020) were observed in the D5-PBCR (+) group. In conclusion, optimization of induction chemotherapy with idarubicin and cytarabine according to D5-PBCR is feasible in patients with newly diagnosed AML. The addition of HHT demonstrated a good efficacy and safety profile.
1 INTRODUCTION
Anthracycline and cytarabine induction regimens have remained at the forefront of acute myeloid leukemia (AML) induction therapy for decades, but the complete remission (CR) rate and long-term survival are not satisfactory.1 Recent efforts have focused on the combination of traditional chemotherapeutic agents with novel agents, such as FLT3 inhibitors.2 However, these new pharmaceuticals have only been applied to certain AML subtypes, and their cost is too high. Thus, more universal and cost-effective therapies are urgent to treat patients with AML.
Homoharringtonine (HHT), also known as omacetaxine mepesuccinate, was originally identified more than 45 years ago3 as a novel plant-based alkaloid with antitumor properties against a variety of hematologic malignancies, including AML4 and chronic myeloid leukemia.5 A previous preclinical study reported that HHT is a protein synthesis inhibitor affecting leukemic cells, which potentiates the therapeutic efficacy of anthracycline and cytarabine.6 In China, HHT-based induction regimens have been widely used in the treatment of AML for more than 30 years because of their therapeutic efficacy and low cost.7
An open-label, randomized, controlled, phase 3 study performed in 17 institutions in China showed that the HHT 2 mg/m2/day on days 1–7, cytarabine 100 mg/m2/day on days 1–7, and daunorubicin 40 mg/m2/day on days 1–3 (HAD) regimen achieved better CR rate in young patients with newly diagnosed AML than the traditional daunorubicin and cytarabine (DA) regimen.8 However, induction with the three-drug regimen may benefit only some AML patients, while the remaining patients may be getting exposed to excessive chemotherapy. Thus, we need an approach to identify patients who are not sensitive to the traditional induction regimen and may benefit from the addition of HHT.
Three days of anthracyclines (e.g., idarubicin, 10–12 mg/m2, or daunorubicin 60–90 mg/m2) and 7 days of cytarabine (100–200 mg/m2; “3 + 7”) has been the standard induction therapy for the past decade.9, 10 As previously reported,11 we conducted an observational study in AML patients who were treated with a dose-reduced IA/DA induction regimen (idarubicin 8–10 mg/m2 on days 1–3 or daunorubicin 45–60 mg/m2 on days 1–3, and cytarabine 100 mg/m2 on days 1–7). By monitoring the peripheral blood blast burden during induction using multiparameter flow cytometry (MFC), we developed an effective evaluation method for early treatment response. We concluded that patients with a day 5 peripheral blast clearance rate ≥ 99.55% [D5-PBCR (−)] achieved a CR rate of 94.6% after one course of induction, and patients with D5-PBCR(+) showed poor treatment response and inferior long-term prognosis. Thus, D5-PBCR can be used as a sensitive marker to evaluate early treatment response and guide modifications in the induction regimen.
AML patients in China generally receive reduced-dose induction therapy due to restrictions posed by various factors, such as the economic situation of the patients, ward environment, drug choice, and difficulty in blood transfusion. A large-scale, real-world study including 1207 newly diagnosed young adult AML patients (aged 18–60 years) showed that only 10.6% of patients received idarubicin 12 mg/m2/d on days 1–3 for intensive induction chemotherapy in China. Meanwhile, 10 mg/m2/d for 3 days was the most widely used dose, which accounted for 65.2% of the AML patients.12
Based on the real-world data of Chinese patients and the findings of our previous study, we designed this clinical trial to address two research questions: (i) a reduced-intensity IA regimen achieves a similar efficacy in D5-PBCR (−) patients and (ii) the addition of HHT reversed unfavorable outcomes in D5-PRCR (+) patients. Herein, we report the response and survival results of this trial.
2 METHODS
2.1 Study design and participants
We conducted a single-center, single-arm, phase 2 clinical trial at Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine in Shanghai, China. Inclusion criteria were patients with untreated newly diagnosed AML as defined by the World Health Organization criteria13 with the presence of more than 0.5% blasts in the peripheral blood. Patients aged between 18 and 55 years were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 3 or less, and those aged between 56 and 60 years old were required to have an ECOG performance status of 2 or less. Eligible patients were also required to have a left ventricular ejection fraction of 50% or more, and normal organ function, including an aminotransferase level of less than two times the upper limit of normal, a total bilirubin level of less than 35 μmol/L, and a creatinine level of 150 μmol/L or less.
We excluded patients with acute promyelocytic leukemia or secondary AML arising from the myelodysplastic syndrome or myeloproliferative neoplasm, the presence of therapy-related AML, those who were intolerant or allergic to any of the drugs used in the trial, and those who could not understand or comply with the protocol (Appendix S1).
This study was approved by the Institutional Review Board of Ruijin Hospital and performed in accordance with the Declaration of Helsinki (reference number: clinical trial 2014-65). Written informed consent was obtained from all the patients enrolled in the study. The trial was registered in the Chinese Clinical Trial Registry (ChiCTR-OPC-15006085).
2.2 Procedures
All patients enrolled in the study received idarubicin 10 mg/m2 on days 1–3 and cytarabine 100 mg/m2 on days 1–7 (IA) induction regimen. Peripheral blood specimens were collected before and on day 5 of induction. The peripheral blood blast burden was determined by MFC, and D5-PBCR was calculated as previously reported.11 The standard operating protocol is provided in Appendix S2. D5-PRCR (+) patients received additional chemotherapy (HHT 2 mg/m2 on days 6–10). To ensure safety, patients with unstable hemodynamic parameters or severe cardiac or pulmonary diseases, such as acute heart failure, acute myocardial infarction, and respiratory failure, were excluded from the study.
To assess the treatment response, a bone marrow evaluation was performed at the time of peripheral hematological recovery (absolute neutrophil count > 1 × 109/L and platelet count > 100 × 109/L) or by day 42 in the absence of optimal hematological recovery. Responses were evaluated according to the International Working Group Criteria for AML by the treating clinician.14 Minimal residual disease (MRD) was assessed based on leukemia-associated immunophenotypes (LAIPs) by MFC using a 10-color panel of bone marrow specimens collected at the time of response as previously described.15 Patients in partial remission (PR) received another induction cycle with the same drug and dosage. Patients in CR received at least two cycles of high-dose cytarabine (HiDAC, cytarabine 2 g/m2 every 12 hours on days 1–3) for consolidation chemotherapy. Patients with favorable cytogenetic/molecular risks received four courses of the HiDAC regimen. Patients with intermediate and unfavorable cytogenetic/molecular risks were advised of allogeneic hematopoietic stem-cell transplantation. Investigator-assessed adverse events (AEs) were reported according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03) during induction for all patients.
Bone marrow aspirate specimens obtained at diagnosis were screened for cytogenetic and molecular changes according to institutional standards as previously described.16 All patients received supportive care measures, including transfusions, antimicrobial agents (including CYP3A4 inhibitor antifungal agents), and growth factors, if needed, according to institutional standards.
2.3 Outcome
The primary endpoint of this phase 2 trial was a composite of CR and CR with incomplete hematologic recovery (CRi). Secondary endpoints were the 3-year overall survival (OS, the time from study entry to death from any cause), 3-year event-free survival (EFS, the time from study entry to treatment failure, disease relapse, or death from any cause), early mortality (the incidence of death within 1 month after induction), MRD, drug toxicity, and safety. For treatment efficacy analysis, a comparison to historical data was conducted. The historical data consisted of 44 cases with D5-PBCR evaluation from our previous observational study11 and 59 consecutive cases of AML diagnosed in our hospital from September 2014 to January 2015. All patients received induction therapy with idarubicin (10 mg/m2 on days 1–3) and cytarabine (100 mg/m2 on days 1–7).
Patient characteristics, including morphology, cytogenetics, immunophenotype, molecular and cytogenetic features, hemogram of D5-PBCR, and clinical outcomes, were also described and analyzed.
2.4 Statistical analysis
The clinical trial was designed using the single-arm, Simon two-stage model. We hypothesized that the CR rate may increase by 15% with the addition of HHT (from 56% to 71%). Thirty-six patients were accrued in the first stage of the analysis and if more than 20 CR or CRi were observed, 19 additional patients were included. Therefore, the sample size of D5-PRCR (+) patients who needed HHT was 55. Based on our historical data, the rate of D5-PRCR (+) in patients with AML was 45%. Assume that 20% of patients may lost to follow-up, at least 146 eligible patients were needed.
Efficacy and safety analyses were performed according to protocols in all the patients who received at least one dose of the treatment.
Categorical variables are summarized as frequencies and percentages. Continuous variables were summarized as the median and interquartile range (IQR). Pearsons chi-square and Fisher's exact tests were used for univariate analyses of categorical variables, and the Wilcoxon rank test was used to analyze continuous variables. The log-rank test and Cox regression analysis were used to analyze the differences between the groups with respect to OS and EFS. All exploratory subgroup analyses were performed post hoc. Statistical analysis was performed using the SPSS statistical software package (version 22.0) and R (version 4.0.3).
3 RESULTS
3.1 Patients and baseline characteristics
Patients were enrolled between February 2015 and July 2017. We screened 156 patients, aged 18–60 years, of which five were excluded and 151 were included in the RJ-AML 2014 trial (Figure S1). All patients received the IA induction regimen, and on induction day 5, peripheral blood specimens were collected to analyze residual blasts/leukemic cells by MFC. Of the 151 patients, 65 (43.0%) had D5-PBCR (+). Table 1 shows the demographics, as well as the laboratory and genetic characteristics of the intention-to-treat (ITT) cohort, according to the D5-PBCR results. Baseline characteristics, including age, sex, white blood cell count, hemoglobin level, platelet count, blast count in bone marrow, and cytogenetic parameters, were similar between the two groups. Compared to historical data, patients in the Ruijin-acute myeloid leukemia-2014 (RJ-AML 2014) trial showed similar baseline characteristics (Table S1).
| Characteristics | All patients (n = 151) | D5-PBCR (−) (n = 86) | D5-PBCR (+) (n = 65) | p |
|---|---|---|---|---|
| Age, years | 42 (29–51) | 41.5 (29–51) | 42 (29–51) | .82 |
| Sex | .12 | |||
| Male | 75 (49.7%) | 38 (44.2%) | 37 (56.9%) | |
| Female | 76 (50.3%) | 48 (55.8%) | 28 (43.1%) | |
| White blood cell count, ×109/L | 17.5 (4.3–65.6) | 25.3 (5.8–68.8) | 11.3 (3.7–47.7) | .07 |
| Hemoglobin, g/L | 93 (76–110) | 94 (75–113) | 93 (76–110) | .95 |
| Platelets, ×109/L | 46 (21–85) | 42 (19–84) | 55 (22–89) | .22 |
| Blasts in bone marrow, % | 68 (43–84) | 70.5 (53–85) | 62 (36–83) | .06 |
| Cytogenetics | .06 | |||
| Favorable | 25 (16.6%) | 15 (17.4%) | 10 (15.4%) | |
| Intermediate | 105 (69.5%) | 64 (74.5%) | 41 (63.1%) | |
| Unfavorable | 21 (13.9%) | 7 (8.1%) | 14 (21.5%) | |
| Mutation status | ||||
| NPM1 | 32 (21.2%) | 18 (20.9%) | 14 (21.5%) | .93 |
| FLT3–ITD | 23 (15.2%) | 14 (16.3%) | 9 (13.8%) | .68 |
| CEBPAbi | 32 (21.2%) | 23 (26.7%) | 9 (13.8%) | .06 |
| Treatment response after one cycle of inductiona | ||||
| Composite CR, no./total (%) | 114/135 (84.4%) | 70/80 (87.5%) | 44/55 (80.0%) | .24 |
| CR | 104 (77.0%) | 64 (80.0%) | 40 (72.7%) | |
| CRi | 10 (7.4%) | 6 (7.5%) | 4 (7.3%) | |
| Partial remission, no./total (%) | 4/135 (3.0%) | 2/80 (2.5%) | 2/55 (3.6%) | .7 |
| Patients with MRD < 0.1%, No./total (%) | 67/108 (62.0%) | 40/68 (58.8%) | 27/40 (67.5%) | .37 |
| Early Death, No./total (%) | 4/135 (3.0%) | 3/80 (3.8%) | 1/55 (1.8%) | .52 |
| CR after one cycle of induction,b no./total (%) | ||||
| Favorable | 36/38 (94.7%) | 24/26 (92.3%) | 12/12 (100%) | .32 |
| Intermediate | 60/67 (89.6%) | 35/39 (89.7%) | 25/28 (89.3%) | .95 |
| Unfavorable | 18/30 (60.0%) | 11/15 (73.3%) | 7/15 (46.7%) | .14 |
- Note: Data are n (%) or median (IQR), unless otherwise stated.
- Abbreviations: CEBPAbi, CEBPA biallelic mutation; CR, complete remission; CRi, complete remission with incomplete hematologic recovery; MRD, minimal residual disease.
- a A total of 135 patients completed one cycle of induction. LAIPs were identified in 68 patients in D5-PBCR (−) group, and in 40 patients in D5-PBCR (+) group.
- b Cytogenetic and molecular risk stratification according to the NCCN guideline 2015.
3.2 Efficacy
Of the 65 D5-PBCR (+) patients, 9 (13.8%) were unfit for the addition of HHT due to severe infection or unstable hemodynamic parameters, and 1 patient was lost to follow-up. Of the 86 D5-PBCR (−) patients, 4 discontinued the IA regimen on day 6 or 7 of induction due to grade 3/4 infection, and 2 patients were lost to follow-up. A total of 135 patients who completed one cycle of induction were divided into two groups based on the D5-PBCR results and were included for further analysis. The cutoff date for this analysis was December 1, 2020.
The composite CR rate after one course of induction (CR1) for all patients was 84.4% (114/135), including 104 (77.0%) who had a CR and 10 (7.4%) who had a CRi. PR was achieved in 3% of all patients. The overall CR rate for all patients was 92.6% after two courses of induction. The CR1 rate for the D5-PBCR (−) group was 87.5% and that for the D5-PBCR (+) group was 80.0%. For patients with favorable and intermediate risk, the CR rates after one cycle of induction were 94.7% and 89.6%, respectively, whereas the CR1 rate was only 46.7% in D5-PBCR (+) patients with unfavorable risk (Table 1). For patients with CR1, LAIPs were identified in 68 and 40 patients in D5-PBCR (−) and D5-PBCR (+) groups, respectively. MRD-negative (MRD < 0.1%) status was achieved in 58.8% of patients in the D5-PBCR (−) group and 67.5% of patients in the D5-PBCR (+) group.
The median follow-up in this trial was 53.1 months (IQR 45.4–59.8). The median OS was not reached in the entire cohort (95% CI 47.2 to not reached; Figure 1A), with a 3-year survival of 61.7% (95% CI 54.0–70.6%). The median OS, which showed no difference between the D5-PBCR (−) and D5-PBCR (+) groups, was also not reached [D5-PBCR (−): 95% CI 32.2 to not reached, D5-PBCR (+): 95% CI 46.7 to not reached; Figure 1C]. The median EFS was 42.2 months (95% CI 21.0 to not reached; Figure 1B), with a 3-year survival of 52.4% (95% CI 44.5–61.5%). The median EFS also showed no difference between the two groups [D5-PBCR (−): 42.2, 95% CI 20.2 to not reached, D5-PBCR (+): 48.4, 95% CI 19.7 to not reached; Figure 1D].
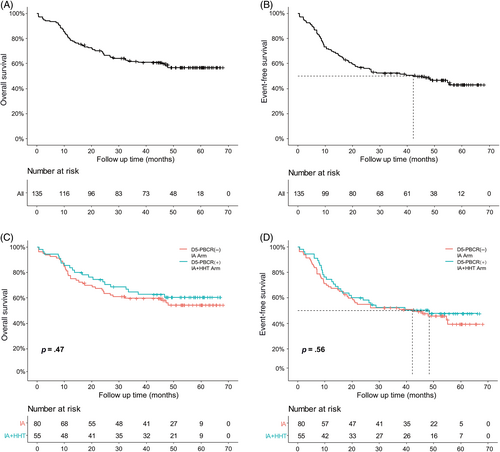
3.3 Safety
Four patients died before day 28 and therefore, could not undergo response assessment. These deaths were attributable to infections or bleeding complications, and the early mortality rate was similar between the IA and IA + HHT groups (Table 1). The incidences of nonhematologic toxicities, including hepatic, renal, cardiac, and gastrointestinal events, were not significantly different (p > .05). The profile of grade 3/4 AEs was comparable between the groups. The most frequent attributable AEs (possibly, probably, or definitely related to HHT) included bleeding, increased alanine aminotransferase and aspartate aminotransferase levels, anorexia, and nausea (Table 2).
| Adverse event | All Patients (n = 135) | D5-PBCR (−) IA Arm (n = 80) | D5-PBCR (+) IA + HHT Arm (n = 55) | p |
|---|---|---|---|---|
| Adverse Events (grade ≥ 3 by NCI CTCAE v4.03) | ||||
| Bleeding | 24 (17.8%) | 11 (13.8%) | 13 (23.6%) | .14 |
| Cardiac | 10 (7.4%) | 7 (8.8%) | 3 (5.5%) | .36 |
| Gastrointestinal | 15 (11.1%) | 7 (8.8%) | 8 (14.5%) | .29 |
| Hepatobiliary | 10 (7.4%) | 4 (5.0%) | 6 (10.9%) | .17 |
| Infection | 56 (41.5%) | 38 (47.5%) | 18 (32.7%) | .09 |
| Respiratory | 13 (9.6%) | 9 (11.3%) | 4 (7.3%) | .44 |
| Dermatologic | 4 (3.0%) | 2 (2.5%) | 2 (3.6%) | .54 |
| Hematologic recovery | ||||
| Time to recovery, days | ||||
| ANC > 500/μL | 22 (20–24) | 21 (20–23) | 22 (20–24) | .08 |
| PLT > 20 000/μL | 21 (19–23) | 20 (18–22) | 23 (20–25) | .001 |
| Transfusion requirements, units | ||||
| Red blood cells suspension | 8 (4–12) | 8 (4–12) | 8 (4–13) | .93 |
| Platelets | 6 (4–8) | 6 (4–8) | 5 (4–7) | .98 |
- Note: Data are n (%) or median (IQR).
- Abbreviations: ANC, absolute neutrophil count; PLT, platelet count.
The median time to reach absolute neutrophil count recovery of 0.5 × 109/L in the responsive patients was 22 days (IQR, 20–24), and the median time to platelet recovery of 20 × 109/L was 21 days (IQR, 19–23) in the entire cohort. The duration of neutropenia was similar between the two treatment arms, whereas the median duration of thrombocytopenia was prolonged in the IA + HHT group (23 vs. 20 days, p = .001).
3.4 Comparative analysis with historical data
Compared to historical data, the CR1 rate for the entire cohort increased from 72.8% to 84.4% (p = .028). Furthermore, the CR1 rate in D5-PBCR (+) patients increased with the addition of HHT (80.0% vs. 62.7%, p = 0.049), and the overall CR rate also improved (90.9% vs. 76.5%, p = .043). The CR rate in D5-PBCR (−) patients was similar between the historical data and the phase 2 trial (Table S2).
Overall, the comparison of the median OS (phase 2: not reached, 95% CI 47.2 to not reached; historical data: 39.7, 95% CI 22.3 to not reached; p = .028) and EFS (phase 2: 42.2, 95% CI 21.0 to not reached; historical data: 15.0, 95% CI 11.4–39.1; p = 0.025) between the phase 2 trial and historical data revealed a significant difference in long-term survival (Figure S2). In subgroup analysis, the comparison of OS and EFS in the D5-PBCR (−) patients showed no significant differences between the phase 2 trial and historical data (Figure 2A,C). For D5-PBCR (+) patients, there were significant differences in both the median OS (phase 2: not reached, 95% CI 46.7 to not reached; historical data: 26.8, 95% CI 13.9 to not reached; p = .020; Figure 2B) and median EFS (phase 2: 48.4, 95% CI 19.7 to not reached; historical data: 12.4, 95% CI 9.5–33.9; p = 0.020; Figure 2D) by the addition of HHT.
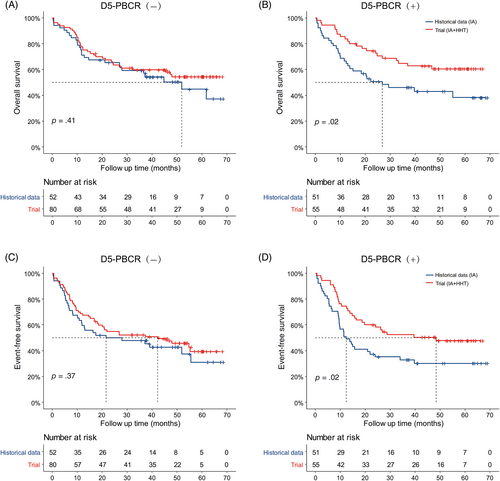
4 DISCUSSION
The goal of this phase 2 trial was to modify the traditional 3 + 7 induction regimen according to the D5-PBCR of patients, which is a parameter for evaluating the early treatment response. Idarubicin at 10 mg/m2/day yielded a composite CR of 87.5% in the D5-PBCR (−) group. The addition of HHT to the induction regimen in D5-PBCR (+) patients achieved a promising composite CR of 80% after one course, which represented an increase of nearly 18% compared to the historical data. Our data also showed that long-term survival can be improved by this treatment strategy.
Despite many efforts to improve treatment outcomes over the past few decades, the outcomes for AML patients remain unsatisfactory. After the administration of anthracycline for 3 days and cytarabine for 7 days (commonly referred to as the “3 + 7” regimen), CR was achieved in 60%–80% of younger adults.1, 17 Traditional concepts state that increasing the dose of chemotherapeutic drugs can improve efficacy, and many efforts have been made in the past few decades in this area. For instance, the EORTC-GIMEMA AML-12 trial demonstrated that HiDAC achieved higher remission and survival rates than standard-dose cytarabine in patients younger than 46 years.18 The dose escalation of daunorubicin from 45 mg/m2 to 90 mg/m2 per day as part of the induction regimen resulted in a higher CR and an improved OS (median, 23.7 vs. 15.7 months).19 However, in the UK NCRI AML17 trial,20 daunorubicin at 90 mg/m2 showed no benefits compared to 60 mg/m2 dosage in any group or subgroup. Meanwhile, a multicenter, nonrandomized, clinical controlled study in China12 compared the efficacy and safety of the IA regimen, in which idarubicin at 8, 10, or 12 mg/m2 was administered as the induction regimen for adult patients. It was reported that in favorable- and intermediate-risk groups, idarubicin at 10 mg/m2 was clinically superior to 8 and 12 mg/m2, suggesting that there are differences in “standard treatment” for different AML subgroups.
Several large-scale studies,17, 21, 22 together with the National Comprehensive Cancer Network23 and the European LeukemiaNet (ELN)10 guidelines, recommend the IA regimen with idarubicin dose at 12 mg/m2/day for 3 days. However, real-world data show that the therapeutic dose of idarubicin, limited by many factors, was even lower than 10 mg/m2/day for 3 days in more than half of the Chinese patients. Because of the bone marrow suppression associated with HHT, two large-scale trials using HHT for induction treatment applied reduced dose of anthracyclines.8, 24 To provide more clinical data for the precise use of idarubicin and to explore whether the dose of chemotherapy can be reduced in the D5-PBCR (−) group, the initial dose of idarubicin was set to 2 mg/m2/day lower than that recommended in the guidelines. Our results showed that the dose-reduced IA regimen achieved a composite CR rate of 87.5% in the D5-PBCR (−) group. Furthermore, the CR rates were 92.3% and 89.7% in the favorable and intermediate groups, respectively, which was not inferior to previous studies that used idarubicin at 12 mg/m2.21, 22
Further improvement of the response rate of newly diagnosed AML patients to induction chemotherapy has always been a challenge. Several trials have assessed the combination of different chemotherapeutic drugs with different mechanisms of action to the “3 + 7” regimen to improve treatment outcomes. For instance, the Polish Adult Leukemia Group study25 showed that the addition of cladribine to the standard induction regimen increased the CR rate and improved the survival of adult patients with AML. Another study from the Dutch-Belgian hemato-oncology cooperative group (HOVON) and Swiss group for clinical cancer research (SAKK) reported that clofarabine improved survival in the ELN 2010 intermediate group.26
It can be concluded from the above data that the response rate and survival can be improved to a certain extent by the addition of a third chemotherapeutic drug. However, therapeutic benefits were evident in only some patients, while the remaining patients were exposed to excessive chemotherapeutic agents, which could cause toxic effects, including prolonged hospitalization and delayed neutrophil/platelet recovery. In our study, the addition of HHT increased the CR rate and improved both OS and EFS in the D5-PBCR (+) group compared to the historical data. We also observed that after the addition of HHT, the proportion of patients with MRD negativity after morphologic CR increased by more than 10%, indicating that the addition of HHT to the IA regimen can produce a deeper remission.
In the subgroup analysis, the improvement in efficacy was mainly attributed to the benefits in patients with favorable and intermediate cytogenetic/molecular risk. Unfortunately, for D5-PBCR (+) patients with unfavorable risk, the addition of HHT resulted in no therapeutic effect. In clinical practice, for high-risk patients, the combination of FLT3 inhibitor27, 28 or novel drugs such as CPX-35129 and PANDA30 is recommended. To better understand the mechanism of HHT, our group conducted some research and revealed that HHT can bind to NF-κB repressing factor and attenuate the transactivation activity of p65 on the MYC gene.31 Another study demonstrated that HHT can modulate the DNA epigenome in AML with FLT3 gene mutations.32 With gradual progress in basic research, we will continue to understand the mechanism of HHT over time and the obtained information can be used more widely and accurately in the future.
The toxicity in our study was comparable to that observed in previous studies using the high-dose DA/IA induction regimen.21, 22 As expected, the bone marrow was exposed to excessive chemotherapeutic drugs, which resulted in a longer platelet recovery time; however, neutrophil recovery was unaffected, in patients treated with HHT.
We acknowledge that there are several limitations to this phase 2 trial. As a single-arm trial, data on the effectiveness of HHT in D5-PBCR (+) patients need to be further verified. In addition, given the study size, subgroup analyses based on cytogenetic/molecular features were not performed. Therefore, further studies exploring these features are needed in the future. However, the results of this study are sufficiently promising to pursue a phase 3 randomized trial, and we are now recruiting patients (register number: ChiCTR-OIC-16007764).
In summary, this study is the first trial of early induction intervention based on the peripheral blast clearance rate in patients with AML. Our results indicated that the addition of HHT appears to mostly benefit intermediate-risk patients, whereas more potent drugs are needed for high-risk patients. IA + HHT is a well-tolerated regimen for young patients with AML. A multicenter, randomized, phase 3 study is now ongoing to assess the efficacy of this induction regimen as a new chemotherapeutic approach for AML patients, with the goal of improving outcomes.
ACKNOWLEDGMENTS
This study was supported by grants from the Shanghai Jiao Tong University School of Medicine Multi-Center Clinical Research Project Grant DLY201513 and the National Natural Science Foundation of China Grant 81970148. Hangzhou Min Sheng Pharmaceutical Group Co. Ltd provided homoharringtonine for this study. The authors declare no competing financial interests. We thank the patients, their families, and their caregivers; co-investigators, collaborators, and members of the study team involved in the trial. Our thanks also go to all international colleagues working on AML.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
AUTHOR CONTRIBUTION
QSC, JML, and JH designed the study. QSC, JML, and YXZ coordinated the study. XQW designed and validated the peripheral blast clearance rate monitoring. YS and JW did the flow cytometry tests and collected the data. YXZ and XYL gathered the data and administered the trial. YC, YS, YZ, HJZ, JHY, YFM, LNW, and MW recruited patients and collected data. YXZ, XYL, and QSC validated and interpreted the data. YXZ, XYL, and JH did the statistical analysis. YXZ, XYL, and QSC wrote the paper and all authors reviewed and approved the final manuscript.
CLINICAL TRIAL REGISTRATION
The trial was registered in the Chinese Clinical Trial Registry (ChiCTR-OPC-15006085).
PATIENT CONSENT STATEMENT
We obtained written informed consent from all patients enrolled in the study.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




