Validated international definition of the thrombocytopenia, anasarca, fever, reticulin fibrosis, renal insufficiency, and organomegaly clinical subtype (TAFRO) of idiopathic multicentric Castleman disease
Yoshito Nishimura and Noriko Iwaki contributed equally to this study.
Abstract
Thrombocytopenia, anasarca, fever, reticulin fibrosis, renal insufficiency, and organomegaly (TAFRO) syndrome is a heterogeneous entity manifesting with a constellation of symptoms described above that can occur in the context of idiopathic multicentric Castleman disease (iMCD) as well as infectious diseases, malignancies, and rheumatologic disorders. So, iMCD-TAFRO is an aggressive subtype of iMCD with TAFRO syndrome and often hyper-vascularized lymph nodes. Since we proposed diagnostic criteria of iMCD-TAFRO in 2016, we have accumulated new insights on the disorder and additional cases have been reported worldwide. In this systematic review and cohort analysis, we established and validated a definition for iMCD-TAFRO. First, we searched PubMed and Japan Medical Abstracts Society databases using the keyword “TAFRO” to extract cases. Patients with possible systemic autoimmune diseases and hematologic malignancies were excluded. Our search identified 54 cases from 50 articles. We classified cases into three categories: (1) iMCD-TAFRO (TAFRO syndrome with lymph node histopathology consistent with iMCD), (2) possible iMCD-TAFRO (TAFRO syndrome with no lymph node biopsy performed and no other co-morbidities), and (3) TAFRO without iMCD or other co-morbidities (TAFRO syndrome with lymph node histopathology not consistent with iMCD or other comorbidities). Based on the findings, we propose an international definition requiring four clinical criteria (thrombocytopenia, anasarca, fever/hyperinflammatory status, organomegaly), renal dysfunction or characteristic bone marrow findings, and lymph node features consistent with iMCD. The definition was validated with an external cohort (the ACCELERATE Natural History Registry). The present international definition will facilitate a more precise and comprehensive approach to the diagnosis of iMCD-TAFRO.
1 INTRODUCTION
In 2010, Takai et al.1 first reported a series of cases exclusively in Japan with a constellation of non-specific clinical symptoms, including thrombocytopenia (T), anasarca (A), fever (F), reticulin fibrosis or renal insufficiency (R), and organomegaly (O). Since it was first described, a number of cases of TAFRO syndrome have been reported worldwide.2-10 This heterogeneous clinical entity can occur in the context of infectious diseases, malignancies, rheumatologic disorders, and idiopathic multicentric Castleman disease (iMCD). Multicentric Castleman disease (MCD) is a rare heterogenous systemic disorder characterized by systemic inflammation, multicentric lymphadenopathy with characteristic histopathological features, and organ dysfunction due to elevated pro-inflammatory cytokines including interleukin-6 (IL-6).11-16 Although MCD can be caused by uncontrolled human herpes virus 8 (HHV-8) infection in immunocompromised patients,17 approximately 50% of MCD cases are human immunodeficiency virus (HIV)-negative and HHV-8-negative MCD and have an unknown etiology (idiopathic MCD; iMCD).18, 19
We previously reported that patients with both iMCD and TAFRO had uniform clinical features and pathological findings with hyper-vascular proliferation in lymph nodes as well as myelofibrosis and megakaryocyte hyperplasia in bone marrow.13, 20 Thereafter, iMCD patients with TAFRO (iMCD-TAFRO) symptoms have been considered to have an aggressive clinical subtype of iMCD.19 The iMCD patients who do not meet the criteria for TAFRO often have elevated platelet counts, hypergammaglobulinemia, and a less aggressive course; these cases are described as iMCD not otherwise specified (iMCD-NOS). While anti-interleukin-6 therapy is recommended first-line for iMCD-TAFRO and iMCD-NOS patients, it is helpful to distinguish these subgroups as iMCD-TAFRO is typically more aggressive and additional therapies are often needed in patients who may not have sufficient time to wait for a clinical response.
While elevated serum alkaline phosphatase (ALP) without hyperbilirubinemia and transaminase elevation or the lack of polyclonal hypergammaglobulinemia can also help to distinguish iMCD-TAFRO from iMCD-NOS, no specific disease markers have been found to date. Due to the lack of specific disease markers,21 poor understanding of pathophysiology, rapid clinical deterioration at the onset of disease, intense thrombocytopenia, and small volume lymphadenopathy making lymph node biopsy challenging, the diagnosis of iMCD-TAFRO has been extremely difficult.22 It is also imperative to exclude potential differential diagnoses that can have similar clinical presentations including fever of unknown origin, hyperinflammatory status, anasarca, and other common features (Figure 1). Although several diagnostic criteria have been proposed for iMCD and for TAFRO,13-15, 23 no consensus definition has been established for iMCD-TAFRO based on international data despite its aggressive clinical presentation and high mortality. The purpose of this study is to establish an up-to-date international definition of iMCD-TAFRO based on a comprehensive clinicopathological review of iMCD-TAFRO cases from the literature and a natural history registry.

2 METHODS
2.1 Literature search and selection criteria
We performed a systematic literature review of iMCD-TAFRO to extract data on clinical features including thrombocytopenia, anasarca, fever, renal dysfunction and organomegaly, laboratory data, lymph node size, and histopathological characteristics. To identify cases for this review, we searched for articles published in PubMed and Japan Medical Abstracts Society databases, which includes manuscripts published in Japanese journals, as of May 2019, with the term “TAFRO”. We also screened the reference lists of the retrieved articles to find any eligible cases. Duplicate publications and non-peer reviewed articles were excluded at the first stage by reviewing abstracts. Using the strategy, we extracted 65 case report or case series articles including 75 patients with iMCD-TAFRO. We excluded 21 cases for the following reasons: if cases showed decreases in both complement component 3 and 4 that could be suggestive of systemic lupus erythematosus (SLE, eight cases); if cases were diagnosed or associated with Sjögren syndrome (SjS, four cases); if patients had suspected POEMS syndrome with positive λ light chain restricted monoclonal protein or were diagnosed as diffuse large B-cell lymphoma (DLBCL) during the clinical courses (four cases); or if patients had any findings that were suggestive of other differential diagnoses including cytoplasmic anti-neutrophil cytoplasmic antibody, human herpesvirus 8-DNA or Epstein-Barr virus (EBV) in the bone marrow, extremely elevated immunoglobulin-G (IgG), and positive deposits of immunoglobulin-A and M (IgA, IgM) as well as C3 in the kidney biopsy specimen (five cases). As a result, 54 cases were included in this study (Figure 1). Two independent investigators (N.I., and Y.N.) performed the search and confirmed that the 54 cases had none of the exclusion criteria. We further classified the 54 cases into three categories; iMCD-TAFRO (Group one: cases with TAFRO syndrome with lymph node histopathology which is consistent with iMCD), TAFRO with possible iMCD (Group one: cases with TAFRO syndrome with no lymph node biopsy performed and no other co-morbidities), and TAFRO without iMCD or other co-morbidities (Group three: cases with TAFRO syndrome with lymph node histopathology not consistent with iMCD, but no other comorbidities identified). Serum alkaline phosphatase (ALP) values of the Japanese articles were converted to the International Federation of Clinical Chemistry and Laboratory Medicine values (global standard) from the Japan Society of Clinical Chemistry values by multiplying 0.35 as appropriate.13 We judged that the data were missing if any specific variables were not available in the articles.
2.2 Statistical analysis
We calculated summary statistics for different variables by tabulation including percentages. We used the Wilcoxon test to compare the continuous variables between
Group one and Group two. The Fisher's exact test was used to compare the categorical data between the two groups. The threshold for significance was defined as the p value <0.05. Due to the small number of cases in Group three, statistical analysis to compare the differences between Group one, Group two, and Group three were deferred. All statistical analyses were conducted with JMP Version 15.1 (SAS Institute, Cary, NC, USA).
3 RESULTS
3.1 Clinical and radiological features
Figure S1 describes our search and article selection strategy. Our systematic review identified 175 articles published between January 2013 and May 2019 about iMCD-TAFRO. Of the 75 patients identified, 21 patients were excluded from the study as noted above. Demographics as well as chief clinical and radiological features of the 54 patients are presented in Table S1 according to the following groups: cases with TAFRO syndrome and lymph node histopathology consistent with iMCD (Group one), cases with TAFRO syndrome with no lymph node biopsy performed and no other co-morbidities (Group two), and cases with TAFRO syndrome with lymph node histopathology that is not consistent with iMCD, but no other comorbidities identified (Group three). The median ages of patients in the three groups were 54 years, 66 years, and 47 years, respectively, with a slight male predominance overall. Asian patients were predominant but our cohorts included eight Caucasian and one Hispanic patients. Thrombocytopenia (T of TAFRO), defined as a platelet level of less than 100 × 103/μl, upon the pre-treatment nadir was prevalent in all cases. All the cases had some types of anasarca on computed tomography (CT) or fluorodeoxyglucose-positron emission tomography/CT (FDG-PET/CT) (A). Pleural effusion and ascites were more common than subcutaneous edema or pericardial fluid. Regarding fever and inflammation (F), all patients had either fever of more than 37.5°C or CRP of more than 2.0 mg/dl. Of the 47 patients with recorded minimum pre-treatment estimated glomerular filtration rate (eGFR), 27/35 (77.1%) in Group one, 8/10 (80.0%) in Group two, and 1/2 (50.0%) in Group three had eGFR less than 60 ml/min/1.73 m2. Of note, 11/41 (26.9%) in Group one, 6/11 (54.5%) in Group two and 1/2 (50.0%) in Group three underwent hemodialysis therapy (R) during their clinical courses. Reticulin fibrosis and megakaryocyte hyperplasia were found on bone marrow biopsy in about 80%–90% of the cases. All patients had some form of organomegaly (O), which we defined as hepatomegaly, splenomegaly, and/or small volume lymphadenopathy. Lymphadenopathy was present in all cases in Group one; hepatomegaly and splenomegaly were present in approximately half of the cases in Group one. Hepatomegaly was noted in only 2/11 (18.2%) of the cases in Group two. Only one patient in Group one had pulmonary involvement (bilateral parenchymal ground-glass opacities and interlobular septal thickening).
3.2 Laboratory findings
Chief laboratory findings of the included patients are summarized in Table S2. Serum immunoglobulin levels were in the low-ranges to mid-ranges in the recorded cases Groups one and two. All the recorded iMCD-TAFRO cases had serum IgG levels less than 2000 mg/dl. Elevated serum ALP was also noted in the patient population with the median ALP level of 209 U/L (range: 58.5–736 U/L) and 189 U/L (range: 78.1–503 U/L) in Group one and two, respectively. Despite the increase in ALP, serum transaminase levels remained generally normal. Only a small proportion of cases had highly elevated serum lactate dehydrogenase (LDH) levels with 21/25 (84.0%) and 9/10 (90.0%) in Group one and two having LDH ≤ 400 U/L. Regarding autoantibodies, some of the patients had positive antinuclear antibody (ANA), rheumatoid factor (RF), anti- Sjögren-syndrome-related antigen A (SS-A) antibody, and anti-Sjögren-syndrome-related antigen B (SS-B) antibody, although none of them satisfied classification criteria for systemic autoimmune diseases. It should be noted that a few patients with iMCD-TAFRO had strongly positive serum SS-A and/or SS-B levels. None of them had positive anti-double strand DNA (dsDNA) antibody, cytoplasmic-antineutrophil cytoplasmic antibody (C-ANCA), or perinuclear-antineutrophil cytoplasmic antibody (P-ANCA).
3.3 Biopsy Site of the iMCD-TAFRO patients
Data about biopsy, a critically important procedure to diagnose iMCD-TAFRO, are summarized in Table S3. Histological features of iMCD-TAFRO are shown in Figure S2. Nearly all of the patients had a bone marrow biopsy (48/54: 88.9%) and 79.6% had a lymph node biopsy (43/54). Other biopsy sites included kidney, liver, skin, intestine, adrenal gland, or pleura. Of the 52 cases with detailed records about biopsy sites, 46/52 (84.6%) received biopsy from more than one organ. Of note, lymph node biopsy is the only procedure that can confirm a diagnosis of iMCD.
3.4 Definition for iMCD-TAFRO
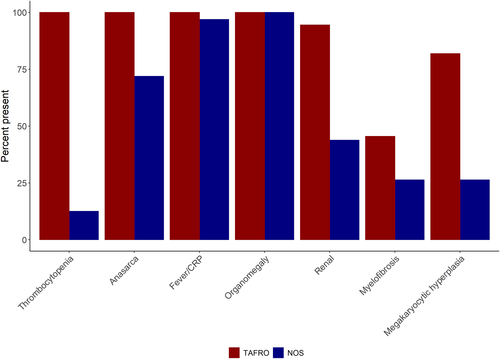
Based on the context of previous research21 and the findings of the current study, we propose an international definition for iMCD-TAFRO (Table 1). This definition requires at least four clinical criteria (thrombocytopenia, anasarca, fever/hyperinflammatory status, and organomegaly [hepatomegaly, splenomegaly, +/− small volume lymphadenopathy], or TAFO), renal dysfunction or pathological feature in bone marrow, such as reticulin fibrosis (R) or megakaryocyte hyperplasia as well as pathological criteria (iMCD histopathological features in lymph node) and exclusion criteria. Tables 2 and 3 summarize and compare iMCD-TAFRO/TAFRO syndrome criteria and iMCD criteria proposed to date, respectively. To evaluate the applicability of this definition of iMCD-TAFRO in an independent cohort, we interrogated the patient-powered arm of the ACCELERATE Natural History Registry27 to identify the proportion of iMCD patients who would meet the proposed definition (TAFRO + iMCD-consistent lymph node histopathology according to an expert panel + exclusion of overlapping conditions as well as additional clinical and pathological criteria); Table 4 and Figure 2. Among the 68 pathology-reviewed, expert-confirmed cases of iMCD in ACCELERATE that primarily come from the United States, 36 cases would meet the proposed criteria for iMCD-TAFRO. Importantly, all iMCD patients with thrombocytopenia also had anasarca. In this cohort, there were four patients with thrombocytopenia but did not meet TAFRO criteria. Two met TARO criteria but not F criteria, though both had elevated ESR, suggesting inflammation, and two met TAFO criteria but not R criteria, though neither had a bone marrow report to confirm the presence of reticulin fibrosis or hyperplasia. Given that these are real-world data, it is possible that all four of these patients may have met TAFRO if the necessary criteria were measured at the time of diagnosis. Additional clinical criteria of elevated alkaline phosphatase (mean: 171.1 (SD: 99.7) U/L) and low to normal gammaglobulin/IgG levels (mean gammaglobulin: 1.54 (0.94) g/dl; mean IgG: 1286 (958.6) mg/dl) were also confirmed in this cohort. Though mildly elevated LDH (<2x upper limit of normal) was considered as additional clinical criteria and there was a higher relative proportion of iMCD-TAFRO patients with elevated LDH, it was less than 25%, so it was not included in the final definition. The interrogation of this independent cohort suggests that the above definition which was primarily established based on data from Japan is appropriate for identifying iMCD-TAFRO patients internationally.
|
| Exclusion criteria - Must rule out the following diseases |
|
- Abbreviations: CRP, C-reactive protein; CT, computed tomography; eGFR, estimated glomerular filtration rate; HHV-8, human herpesvirus-8.
| Inclusion criteria | Exclusion criteria | |||
|---|---|---|---|---|
| Required histopathological criteria | Required clinical criteria | Other criteria | ||
| Iwaki et al. for iMCD-TAFRO (2016)13 | (Mandatory)
|
(Mandatory)
|
(Need 1 or more)
|
|
| Masaki et al. for TAFRO syndrome (2020)23 |
|
(Mandatory)
|
(Need two or more)
|
|
| Present definition of iMCD-TAFRO | (Mandatory for definite diagnosis)
|
(Mandatory)
|
(At least one of the following required)
|
|
- † indicates fever of unknown etiology above 37.5°C and/or serum CRP ≥ 2 mg/dL.
- Abbreviations: ALP, alkaline phosphatase; AOSD, adult-onset Still disease; BM, bone marrow; Covid-19-CSS, Covid-19 cytokine storm syndrome; CRP, C-reactive protein; CT, computed tomography; EBV, Epstein-Barr virus; eGFR, estimated glomerular filtration rate; HHV-8, human herpesvirus-8; HIV, human immunodeficiency virus; IgG, immunoglobulin G; JIA, Juvenile idiopathic arthritis; LN, lymph node; ML; malignant lymphoma; MM, multiple myeloma; RA, rheumatoid arthritis; SjS, Sjögren syndrome; SLE, systemic lupus erythematosus; TB, tuberculosis; TTP/HUS, thrombotic thrombocytopenic purpura/hemolytic uremic syndrome.
| Inclusion criteria | Exclusion criteria | ||
|---|---|---|---|
| Major criteria | Minor criteria | ||
| Fajgenbaum et al. (2017)15 | (Mandatory)
|
(Need two or more of 11 with at least one laboratory criterion) Laboratory
Clinical
|
|
| Fujimoto et al. for CD (2018)26 | (Mandatory)
|
N/A |
|
- Abbreviations: CD, Castleman disease; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HHV-8, human herpesvirus-8; HIV, human immunodeficiency virus; IgG4-RD, IgG4-related diseases; LN, lymph node; UCD, unicentric Castleman disease.
| iMCD patients in ACCELERATE, who are confirmed by an expert panel as meeting iMCD diagnostic criteria (pathology consistent, multicentric lymphadenopathy, clinical criteria, and exclusionary criteria)a | (N = 68) | ||
|---|---|---|---|
| TAFRO, N (%) | NOS, N (%) | Combined | |
| Newly proposed TAFRO criteria met (TAFRO) vs not met (NOS), N (%) | 36 (52.9) | 32 (47.1) | 68 |
| Required TAFRO criteria that were met, N (%) | |||
| At least T (thrombocytopenia), N (%)b | 36 (100) | 4 (12.5)a | 40 (58.8) |
| At least A (anasarca/fluid accumulation), N (%) | 36 (100) | 23 (71.9) | 59 (86.8) |
| At least F (fever or elevated CRP), N (%) | 36 (100) | 26 (81.3) | 62 (91.2) |
| At least O (organomegaly: hepatomegaly, splenomegaly, or lymphadenopathy), N% | 36 (100) | 32 (100) | 68 (100) |
| At least T+A, N (%) | 36 (100) | 4 (12.5) | 40 (58.8) |
| At least T+F, N (%) | 36 (100) | 2 (6.3) | 41 (60.3) |
| At least A+F, N (%) | 36 (100) | 19 (59.4) | 55 (80.9) |
| Additional Clinical Criteria, N (%) | |||
| Renal dysfunction (elevated creatinine or decreased eGFR) | |||
| Present, N (%) | 34 (94.4) | 14 (43.8) | 48 (70.6) |
| Absent, N (%) | 2 (5.6) | 18 (56.3) | 20 (29.4) |
| Not recorded, N | 0 | 0 | 0 |
| Fibrosis of bone marrowc | |||
| Present, N (%) | 15 (45.5) | 5 (26.3) | 20 (38.5) |
| Absent, N (%) | 18 (54.5) | 14 (73.7) | 32 (61.5) |
| Not recorded, N | 3 | 13 | 16 |
| Megakaryocytic hyperplasia of bone marrowc | |||
| Present, N (%) | 27 (81.8) | 5 (26.3) | 32 (61.5) |
| Absent, N (%) | 6 (18.2) | 14 (73.7) | 20 (38.5) |
| Not recorded, N | 3 | 13 | 16 |
| At least one of R (renal dysfunction, fibrosis of bone marrow, megakaryocytic hyperplasia of bone marrow), N (%) | |||
| Present, N (%) | 36 (100.0) | 17 (53.1) | 53 (77.9) |
| Absent, N (%) | 0 (0.0) | 15 (46.9) | 15 (22.1) |
| Not recorded, N | 0 | 0 | 0 |
| Additional laboratory parametersd | |||
| Gammaglobulin (g/dl, ref: <1.7) | |||
| N | 14 | 14 | 28 |
| Mean (SD) | 1.54 (0.94) | 2.87 (2.08) | 2.20 (1.72) |
| >Upper limits normal, N (%) | 4 (28.6) | 8 (57.1) | 12 (42.9) |
| Immunoglobulin G (mg/dl, ref: <1700) | |||
| N | 29 | 24 | 53 |
| Mean (SD) | 1285.7 (958.6) | 2898.6 (2015.5) | 2916.0 (1717.1) |
| >Upper limits normal, N (%) | 6 (20.7) | 16 (66.7) | 22 (41.5) |
| Alkaline phosphatase (U/L, ref: <147) | |||
| N | 36 | 30 | 66 |
| Mean (SD) | 171.1 (99.7) | 133.0 (93.1) | 153.8 (97.9) |
| >Upper limits normal, N (%) | 16 (44.4) | 7 (23.3) | 23 (34.8) |
| Lactate dehydrogenase (U/L, ref: <400) | |||
| N | 34 | 23 | 57 |
| Mean (SD) | 358.9 (327.2) | 200.7 (160.4) | 295.1 (281.6) |
| >Upper limits normal, N (%) | 8 (23.5) | 3 (13.0) | 11 (19.3) |
- Abbreviations: ART, ACCELERATE Registry Team; CAS, Certification and Access Subcommittee; CRP, C-reactive protein; NOS, not otherwise specified.
- a ACCELERATE is an international natural history registry of Castleman disease (CD) patients of all subtypes who enroll directly via a web-based portal, in which they electronically provide consent and Health Insurance Portability and Accountability Act authorization to the ACCELERATE Registry Team (ART). The ART collects and extracts all medical records into the study database. Each case undergoes Certification and Access Subcommittee (CAS) review and grading. CAS-approved grade three or higher are those who are considered likely to have CD.
- b Due to the nature of real-world data, thrombocytopenia required at least two platelet measurements of <100 k/μl to meet thrombocytopenia in this cohort.
- c If a bone marrow biopsy was performed and myelofibrosis or megakaryocytic hyperplasia was not documented in the pathology report, then it is considered to be absent. It is possible that a reticulin stain was not performed or a description of megakaryocytic hyperplasia was not included though it may have been present.
- d Closest value to date of diagnosis within +/− 90 days.
4 DISCUSSION
In the present study, we systematically analyzed and reviewed the clinical, radiological, and laboratory features of 54 published cases either with iMCD-TAFRO, possible iMCD with TAFRO syndrome without lymph node biopsy and other identified co-morbidities, and TAFRO syndrome without having evidence of iMCD on lymph node biopsy without comorbidities, reported between January 2013 and May 2019.
Clinically, our data on international cases support that the characteristic features of iMCD-TAFRO are thrombocytopenia, anasarca, fever and hyperinflammatory status, renal insufficiency, and organomegaly. Clinical criteria need to be sensitive enough not to miss possible iMCD-TAFRO cases. As all patients had pre-treatment platelet levels ≤100 × 103/μl, we adopted the cut-off serum platelet value of 100 × 103/μl as one of the clinical criteria to improve the sensitivity. The cut-off value is compatible with the latest criteria proposed by Masaki et al.23 in 2020. Because all the included cases had some sort of fluid accumulation recognized on imaging studies, anasarca should remain one of the required clinical criteria as in our previous diagnostic criteria. Fever and hyperinflammatory status, which are common first presentations of iMCD-TAFRO, were prevalent in our cases as previously reported22, 27 and all cases satisfied the cut-off value of body temperature ≥ 37.5°C and/or CRP ≥ 2.0 mg/dl which we incorporated into the criteria in this update. Renal insufficiency, a condition that was noted as one of the minor criteria in the criteria proposed by us in 2016 as well as by Masaki et al.,15, 23 continues to be important although it was not uniformly present at presentation. Our data suggested that approximately 75% of iMCD-TAFRO cases had mild to moderate renal impairment on presentation; a considerable number of patients required hemodialysis. Though renal dysfunction would have likely developed if given sufficient time in each of these patients, we decided to make renal dysfunction as an optional feature so as to not slow down timely identification of iMCD-TAFRO. However, presence of renal dysfunction is strongly supportive/confirmatory of TAFRO. To determine renal dysfunction, we recommend findings of eGFR≤ 60 ml/min/1.73 m2, creatinine >1.1 mg/dl (female) or >1.3 mg/dl (male), or renal failure necessitating hemodialysis. The combined feature of organomegaly including lymphadenopathy, hepatomegaly, and splenomegaly occurred in all iMCD-TAFRO patients in both cohorts. These data support requiring thrombocytopenia (T), anasarca (A), fever or hyperinflammatory status (F), renal dysfunction (R;or pathological feature in bone marrow), and organomegaly (O; at least small volume lymphadenopathy) for the diagnosis of definite iMCD-TAFRO.
The analysis of laboratory data also indicated that iMCD-TAFRO cases often had additional criteria that can be observed but are not required. We found elevated ALP without other transaminase increases and rarely had marked hypergammaglobulinemia or high LDH levels, which were consistent with previous reports.22, 28 Also, moderately elevated serum interleukin 6 (IL-6) levels were noted in iMCD-TAFRO patients. Due to the Covid-19 pandemic and an evolving concept of Covid-19 cytokine storm syndrome (Covid-19-CSS) characterized by undue immune response,29 there has been a growing interest regarding cytokine-driven diseases.30, 31 In addition to a direct role of IL-6, recent data support a role for the sIL-6R:sgp130 buffering system in these syndromes. Although elevated serum IL-6 levels may not be diagnostic for iMCD-TAFRO and the etiology is unknown, it is important to recognize that iMCD-TAFRO patients often have hypercytokinemia. Despite the exclusion of possible complications of rheumatologic disorders, our results showed that some iMCD-TAFRO patients still had positive ANA, RF, and anti SS-A and -B antibodies. Among the 21 excluded cases, eight had significant decreases in complement components, a characteristic finding of SLE, despite the authors diagnosing them as iMCD-TAFRO. Moreover, four of the 21 cases had history of or coexisting SjS. These findings highlight that existing iMCD-TAFRO case reports might include patients with undiagnosed autoimmune disorders as suggested previously.32-36 Thus, clinicians need to carefully exclude those disorders, and the diagnostic criteria need to be defined to avoid misdiagnosis of autoimmune disorders as iMCD-TAFRO.
Previously, we reported that atrophic germinal centers combined with interfollicular vascular proliferation might be characteristic histopathological findings of iMCD-TAFRO on lymph node biopsy.2, 13 Despite the necessity of lymph node biopsy and its usefulness to diagnose iMCD-TAFRO, some cases may have severe thrombocytopenia and small volume lymphadenopathy,38 which could make a lymph node biopsy difficult or contraindicated. For this reason, the diagnostic criteria proposed by Masaki et al.23 did not mandate the histopathological analysis for the diagnosis of TAFRO syndrome. Our review showed that lymph node biopsy was performed in approximately 80% of the cases included in the present study. Given that iMCD cannot be diagnosed without histopathological evidence in lymph node tissue, lymph node biopsy needs to be done whenever possible. Though kidney biopsy would not be able to demonstrate histopathologic features consistent with iMCD-like lymph node tissue, kidney biopsy was performed in 20.4% of the cases, and there has been an increasing number of reports describing kidney biopsy in iMCD-TAFRO.38 A recent case series of seven iMCD-TAFRO patients reported that all patients had endotheliopathy with mesangiolysis and a double contour of the basal membrane with renal biopsy.39, 40 Furthermore, Mizuno and colleagues suggested that, unlike lymph node biopsy, the findings seen in the kidney specimen might persist even after the introduction of immunosuppressive treatment in their case series.40 In the meantime, due to the lack of data, renal pathology findings in iMCD-TAFRO need to be further examined to assess their consistency with lymph node findings. Bone marrow biopsy may also have diagnostic utility, considering the extent of thrombocytopenia observed in our study (the median pre-treatment platelet: 37 × 103/μl) and that approximately 90% of the cases had either reticulin fibrosis or increased megakaryocytes in the bone marrow specimen. Bone marrow biopsy is feasible without additional platelet transfusion in most cases and may provide useful information to exclude other differential diagnoses such as hidden hematologic malignancy and infectious diseases.41-43 Given that prior definitions have defined the R in TAFRO as either renal dysfunction or reticulin fibrosis, we recommend defining R as either renal dysfunction or reticulin fibrosis (or other bone marrow features) and looking for one of these features as required of iMCD-TAFRO. A previous study reported that adrenomegaly or adrenal ischemia on CT scan could be early signs of TAFRO syndrome,44 although it is not clear if these signs are found in those with iMCD-TAFRO. Unfortunately, only one patient in our study had an adrenal biopsy. Further research is needed on the specificity of these findings and the utility of this procedure for the diagnosis of iMCD-TAFRO. Taken together, while lymph node biopsy may not be essential for diagnosing TAFRO syndrome, which is a broad category including iMCD-TAFRO, lymph node histopathological findings are crucial for diagnosis of iMCD-TAFRO. Thus, we propose that a definite diagnosis of iMCD-TAFRO should be made only in the presence of lymph node histopathological analysis consistent with histopathologic features of the International iMCD Diagnostic Criteria15 to secure sufficient specificity and prevent misdiagnosis. While several different criteria have been proposed for the diagnosis of iMCD, iMCD-TAFRO, and TAFRO syndrome as shown in Tables 2 and 3, our present definition have been based on rigorous reviews of data from patients of different ethnicities and international discussions to establish a precise iMCD-TAFRO diagnostic definition. Ultimately, we hope to identify appropriate treatment strategies for patients with iMCD-TAFRO, who often have poor clinical outcomes and require second or third line therapies with immunosuppressants, cytotoxic agents, or monoclonal antibodies.22
Our study has a few limitations that need to be considered. First, as we analyzed the data of published case reports, there might be publication bias leading to overestimation of the prevalence of clinical symptoms and laboratory data as clinically significant cases may have been more likely to be published. Second, the small number of cases and missing data in a few of the included cases may lessen the precision of our analysis. Third, the data reported from the patient-powered arm of the ACCELERATE Natural History Registry likely does not reflect the proportion of iMCD-TAFRO to iMCD-NOS in the general population. Any patient who has received a pathology report suggestive of Castleman disease can self-enroll in the natural history registry. Previously, it was reported that ACCELERATE is skewed towards more symptomatic and less easily treated patients, who are more likely to seek out resources online.26 Accordingly, ACCELERATE is likely biased towards a higher iMCD-TAFRO population than the general iMCD population, as these patients typically have a more severe and unpredictable clinical course and may be more likely to seek out resources. More research is needed to determine the relative proportions of iMCD-TAFRO vs iMCD-NOS and whether iMCD-NOS should be further sub-classified. Finally, some of the patients considered to have iMCD-TAFRO by the studies' authors in the literature review may have actually had another condition given the challenges of diagnosing iMCD.
Despite the limitations, our systematic review and the validated international definition of iMCD-TAFRO represents an important attempt to improve the way we diagnose this rare and challenging disease entity. Despite the rarity of iMCD-TAFRO, data from the ACCELERATE Natural History Registry (NCT02817997) was used as a validation set. Our validated international definition, highlighting the necessity of histopathological analysis, should help to clear up a possible confusion between TAFRO syndrome and iMCD-TAFRO, and to facilitate a precise and comprehensive approach to diagnosis of iMCD-TAFRO in accordance with the international, evidence-based consensus diagnostic criteria for iMCD.
CONFLICT OF INTEREST
David C. Fajgenbaum has received grant funding from EUSA Pharma and Janssen Pharmaceuticals as well as study drug from Pfizer for a clinical trial. He has two provisional patents pending for the diagnosis and treatment of Castleman disease. The remaining authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Yoshito Nishimura and Noriko Iwaki equally contributed to the study to write the first draft of the manuscript and analyzed the data. David C. Fajgenbaum revised the manuscript. Sheila K. Pierson interrogated the ACCELERATE Natural History Registry to identify the applicability of the proposed definition. Mitsuhiro Kawano, Naoya Nakamura, Koji Izutsu, Kengo Takeuchi, and Midori Filiz Nishimura analyzed the data and helped the revision. Yoshinobu Maeda, Fumio Otsuka, Kazuyuki Yoshizaki, Eric Oksenhendler, Frits van Rhee and Yasuharu Sato designed and supervised the research. All authors reviewed and approved the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated and analyzed during the current study are available from the corresponding authors on reasonable request.




