Risk factors for CKD stage II onset in a prospective cohort of homozygous sickle cell adults
Abstract
Prevalence of renal impairment is increasing with aging in sickle cell anemia (SCA) patients, and is responsible for a high morbidity and mortality. However, sickle cell nephropathy's natural course remains mostly unknown. We conducted a prospective observational cohort study aimed to identify risk factors for CKD stage II in a cohort of SCA patients. Baseline clinical and biological parameters were collected. Renal parameters were updated at each visit. Risk factors were analyzed using the Cox model. Five-hundred and thirty-five SCA patients were included with a median follow-up of 5.33 (IQR:2.10–8.13) years. Median age was 22 (IQR:19–30) years old. Glomerular hyperfiltration was detected in 299 (55.9%) patients, microalbuminuria and macroalbuminuria in 180 (34%) and 67 (12.7%) patients respectively. During follow up, CKD stage II onset was detected in 39 patients (7.3%). Risk factors for CKD stage II after adjustment on baseline eGFR and age were macroalbuminuria HR: 3.89 [95% CI: 1.61;9.43], diastolic blood pressure (DBP) above 70 mm Hg HR: 2.02 [1.02–3.971], LDH (for 100 IU/L increase) HR: 1.28 [1.12;1.48] and tricuspid regurgitation velocity >2.5 m/sec HR: 2.89 [1.20–6.99]. Multivariate analysis also found age as a strong independent risk factor with HR: (per year increase) 1.13 [1.09;1.16] and a 13.3-fold increase above 30 years (p < 0.001). Our results show a high incidence of CKD stage II with aging, with a strong significant risk increase after 30-years-old, and pinpoint baseline DBP, macroalbuminuria and increased LDH as independent risk factors raising the issue of optimal blood pressure targets for SCA patients.
1 INTRODUCTION
Sickle cell anemia (SCA) is one of the most frequent monogenic diseases worldwide with more than 300 000 births every year.1 Sickle cell anemia is due to an homozygous amino acid mutation of the Beta gene of haemoglobin (Hb), creating a pathological Hb called HbSS, which polymerizes in hypoxic or acidic conditions and induces rheological changes in the erythrocyte.2 These modifications cause vaso-occlusive crisis and chronic hemolysis leading to endothelial dysfunction and vasculopathy.3
Life expectancy has drastically increased in this population in occidental countries for the past fifty years, reaching a median age of 60 years old4 compared to 14 years old in the 1970s.5 With this increased life expectancy, SCA nephropathy is becoming a frequent complication of SCA leading to chronic renal failure.6 The prevalence of chronic kidney disease (CKD) stage II was reported to be around 12% in a pediatric population7 whereas in an adult population, CKD stage II and III were encountered in 8% of patients.8 End stage renal disease prevalence varies between series, from 1% to 11%.8-10 Surprisingly, the natural history of SCA nephropathy and the onset of CKD are currently mostly unknown. Indeed, several reports suggest that aging increases the prevalence of micro and macroalbuminuric patients in the SCA population.11 However, the classical view of glomerular hyperfiltration12 preceding microalbuminuria and macroalbuminuria, followed by a glomerular filtration rate (GFR) decrease is challenged by clinical observations.
A recent study13 reports that almost 40% of SCA patients experienced a fast eGFR decline (>3 ml/min/1.73 m2/year). The authors found that Hb genotype, proteinuria, higher platelets, reticulocytes count, higher systolic BP, lower Hb level and lower BMI were associated with rapid eGFR decline. However, studying the eGFR slope in a population with a high prevalence of glomerular hyperfiltration might not be as relevant as studying CKD stages in order to understand SCA nephropathy natural history. Indeed, decreasing eGFR to normal values in glomerular hyperfiltration SCA patients raises the issue whether a normalization is occurring rather than an impairment of renal function.
Therefore, the aim of our study was to assess the incidence CKD stage II in a cohort of adult SCA patients and to identify specific risk factors related to the first steps of chronic kidney disease.
2 METHODS
2.1 Populations of patients
We conducted a monocentric prospective observational study in Tenon Hospital sickle cell centre (Assistance Publique des Hôpitaux de Paris, France). From October 2006 to September 2017, all patients with a diagnosis of SCA, that is homozygous sickle cell disease (HbSS), followed in our centre were prospectively included in the cohort. Patients were managed in accordance with French guidelines14 and seen approximately every 6 months during a medical routine visit, or more often if judged necessary by the physician. The medical visit included a clinical examination and a routine blood and urine test. Patients with baseline impaired kidney function (eGFR < 90 ml/min/1.73 m2) and patients with no clinical revaluation during follow up were excluded from the analysis.
2.2 Data collection
During a routine visit, medical checkup including relevant and biological factors were collected: age (years), gender, systolic blood pressure (SBP) (mm Hg), diastolic blood pressure (DBP) (mm Hg), location of birth (French native or not), body mass index (kg/m2), history of acute or chronic SCA complication such as skin ulcers, acute chest syndrome, retinopathy, ongoing treatments such as hydroxycarbamide, ACEI (Angiotensin Converting Enzyme Inhibitor), part of a chronic transfusion program. Biological characteristics included blood and urine samples. Serum creatinine was measured using an enzymatic assay (μmol/L). Estimated glomerular filtration rate (eGFR) was calculated according to the CKD-EPI formula and expressed in ml/min/1.73 m2. Urine protein/creatinine ratio and urine albumin/creatinine ratio (ACR) were expressed in mg/mmol. Microalbuminuria and macroalbuminuria were defined as an ACR between 3 and 30 mg/mmol and more than 30 mg/mmol respectively. Hemolysis parameters included hemoglobin (g/dl), reticulocytes (109/L), platelets (109/L) leucocytes (109/L), ferritin (μg/L). Patients had cardiovascular evaluation with cardiac echography and tricuspid velocity regurgitation (TRV) measurement (meter/second). Patients were screened for chronic kidney disease at each medical visit and serum creatinine, eGFR and ACR were updated. The primary outcome was the time of first occurrence of CKD stage II defined by eGFR< 90 ml/min/1.73 m2 according to CKD EPI formula during the follow-up period.
2.3 Statistics
Quantitative variables are expressed as means (standard deviation) in the case of normal distribution or medians (interquartile range) otherwise. Categorical variables are expressed as numbers (percentage). Normality of distributions was assessed using histograms and QQ (quantile-quantile) plots. Cumulative incidence of CKD stage II was estimated using the Kaplan-Meier method. Survival distributions were compared using the logrank test.
Among the baseline characteristics, we assessed the predictors of first occurrence of CKD stage II during follow up using univariate and multivariate Cox's regression models. The proportional hazards assumption for each potential predictive factor was assessed by examining the Schoenfeld residuals plots and the log-linearity assumption for continuous prognostic factors were assessed, first using Martingale residual plots and second using restricted cubic spline functions. We then adjusted the HR results on predefined cofounding factors (age and eFGR at baseline).
Factors with a p value less than 0.05 in univariate analysis were introduced in a multivariable Cox regression model with backward stepwise selection using a 0.05 level for removing criteria.
We performed a sensitivity analysis to add robustness to our model and looked at predictors of first occurrence of eGFR < 80 ml/min/1.73 m2 using the Cox regression model with a univariate model and secondarily with an adjusted model (on age and baseline eGFR).
Statistical testing was conducted at the two-tailed α-level of 0.05. All statistical analyses were used using R software 3.5.1.
3 RESULTS
Six-hundred eighty-eight patients were eligible for this study. Twenty-eight patients (4%) were excluded because of an impaired baseline kidney function with CKD stage II, III and IV present in 17 (2.5%), 7 (1%) and 4 (0.6%) patients respectively. One-hundred twenty-five patients with no revaluation visit were also excluded (Figure 1). Overall, 535 patients were included for the analysis with a median follow-up period of 5.33 years (Interquartile Range: 2.10–8.13). Three hundred eighty-three (71.6%) patients were followed more than 2.5 years, 280 (52.3%) more than five years and 161 (30.1%) more than 7.5 years. Median time between two visits was 8.6 months (IQR: 6.03–13).
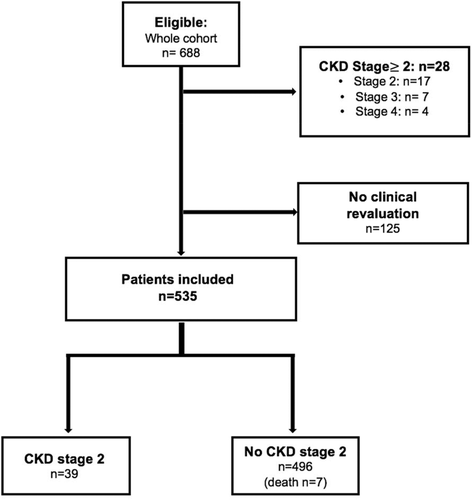
As shown in Table 1, median age was 22 years old (IQR: 19–30) with 314 (58.7%) women. At baseline, 20 patients had arterial hypertension (defined as SBP> 140 mm Hg and/or DBP> 90 mm Hg), and 29 (5.4%) patients were treated by ACEI. Glomerular hyperfiltration (defined as an eGFR> 130 ml/min/1.73 m2) was detected in 299 (55.9%) patients. In our population, median ACR was low: 2.77 (IQR:1.20–9.20) mg/mmol with 282 (53.3%) patients without albuminuria. Microalbuminuria and macroalbuminuria were detected in 180 (34.0%) and 67 (12.7%) patients respectively. Mean hemoglobin was 8.84 (+/−1.29) g/dl, with 139 (26.0%) patients under hydroxycarbamide and 55 (10.3%) patients undergoing a chronic transfusion program at the time of inclusion.
| Whole population (n=535) | N | CKD stage 2 during follow-up (n=39) | No CKD stage 2 during follow up (n=496) | |
|---|---|---|---|---|
| Age (years) | 22.0 [19.0;30.0] | 535 | 35.0 [26.0;41.0] | 22.0 [19.0;29.0] |
| Men | 221 (41.3%) | 535 | 15 (38.5%) | 206 (41.5%) |
| Born in France | 248 (48.2%) | 515 | 7 (17.9%) | 241 (50.6%) |
| BMI (kg/m2) | 20.8 (3.18) | 532 | 22.3 (3.63) | 20.7 (3.11) |
| Blood pressure | ||||
| SBP (mm Hg) | 114 (13.4) | 527 | 118 (15.5) | 114 (13.2) |
| SBP >120 mm Hg | 146 (27.7%) | 527 | 16 (41.0%) | 23 (59.0%) |
| DBP (mm Hg) | 65.6 (9.60) | 527 | 71.0 (10.7) | 65.2 (9.39) |
| DBP >70 mm Hg | 140 (26.5%) | 527 | 24 (61.5%) | 15 (38.5%) |
| MAP (mm Hg) | 8.18 (0.99) | 527 | 8.67 (1.15) | 8.14 (0.96) |
| TRV (m/s) | 2.39 (0.24) | 451 | 2.53 (0.29) | 2.38 (0.23) |
| Biological parameters | ||||
| LDH (IU/L) | 438 (177) | 520 | 484 (268) | 434 (168) |
| Bilirubin (mg/L) | 45.0 [30.0;64.0] | 518 | 43.0 [29.5;61.0] | 45.0 [30.0;64.0] |
| Reticulocytes (109/L) | 301 (129) | 524 | 311 (130) | 300 (129) |
| Leucocytes (109/L) | 9.65 (3.06) | 535 | 9.11 (2.73) | 9.69 (3.08) |
| Hb (g/dl) | 8.84 (1.29) | 534 | 8.58 (1.32) | 8.86 (1.28) |
| Platelets (109/L) | 423 (165) | 533 | 382 (164) | 426 (165) |
| Ferritin (mg/L) | 169 [76.0;419] | 496 | 266 [75.5;454] | 162 [76.0;416] |
| Baseline renal parameters | ||||
| Serum creatinine (μmol/L) | 54.8 (11.6) | 535 | 68.4 (12.2) | 53.7 (10.9) |
| eGFR ml/min/1.73m2 | 131 (15.6) | 535 | 109 (13.8) | 133 (14.3) |
| Proteinuria (mg/mmol) | 10.0 [0.00;25.0] | 529 | 17.6 [0.25;76.8] | 10.0 [0.00;23.0] |
| No albuminuria | 282 (53.3%) | 529 | 13 (35.1%) | 269 (54.7%) |
| Microalbuminuria | 180 (34.0%) | 529 | 13 (35.1%) | 167 (33.9%) |
| Macroalbuminuria | 67 (12.7%) | 529 | 11 (29.7%) | 56 (11.4%) |
| History of SCA complication | ||||
| Skin ulcers | 68 (12.7%) | 535 | 11 (28.2%) | 57 (11.5%) |
| Retinopathy | 276 (57.4%) | 530 | 31 (83.8%) | 245 (55.2%) |
| Acute chest syndrome | 309 (58.3%) | 530 | 20 (52.6%) | 289 (58.7%) |
| Treatment | ||||
| ACEI | 29 (5.43%) | 534 | 7 (17.9%) | 22 (4.44%) |
| Hydroxycarbamide | 139 (26.0%) | 535 | 10 (25.6%) | 129 (26.0%) |
| Transfusion program | 55 (10.3%) | 535 | 6 (15.4%) | 49 (9.88%) |
- Note: Categorical variables are expressed as number (%); continuous variables are expressed as mean (+/−standard deviation) or median [first quartile-third quartile] depending on the distribution.
- Abbreviations: ACEI, angiotensin converting enzyme inhibitor; CKD, chronic kidney disease; DBP, diastolic blood pressure; LDH, lactate dehydrogenase; MAP, mean arterial pressure; SBP, systolic blood pressure; SCA, sickle cell anemia; TRV, tricuspid regurgitation velocity.
During follow up, eGFR decrease below 90 ml/min/1.73 m2 was detected in 39 patients (7.3%) with a mean eGFR decline of 7.4 (+/−18.05) ml/min/year in this group. Of note, mean eGFR decline was statistically higher in this group than the rest of the cohort (1.97 ml/min/year, p < 0.001). Among patients with baseline glomerular hyperfiltration (eGFR> 130 ml/min/1.73 m2), four patients experienced CKD stage II during follow up with a mean eGFR decrease of 8.4 (+/−1.4) ml/min/year, whereas mean eGFR decrease was 1.73 (+/−5.5) ml/min/year in the rest of the subgroup. Last, among the 15 patients with a baseline eGFR between 90 and 95 ml/min/1.73 m2, seven patients experienced CKD stage II, whereas eGFR remained stable in the eight other cases. The eGFR evolution of patients in CKD stage II group is shown in Figure S1.
During follow-up seven patients died. Causes of death were: sudden death (n = 2), acute chest syndrome (n = 2), acute right heart failure secondary to pulmonary hypertension (n = 1), infectious complications (n = 2). Among this subgroup, baseline mean eGFR was 135.6 (+/−9.8) ml/min/1.73 m2 and at last follow up, mean eGFR was 130.7 (+/−10.6) ml/min/1.73 m2, with no history of renal failure.
As shown in Table 2, history of skin ulcer and higher BMI were associated with a greater risk for CKD stage II (HR: 2.26 [95% CI: 1.12–4.54] and HR: 1.15 [1.06–1.25] respectively), whereas being born in France was associated with a decreased risk (HR: 0.21 [0.09;0.48]). However, these associations did not remain statistically significant after adjustment on age and baseline eGFR.
| HR [95% CI] | p value | adjusted HR* | p value | multivariate | p value | |
|---|---|---|---|---|---|---|
| Men | 0.92 [0.48;1.76] | 0.802 | 1.85 [0.83;3.22] | 0.151 | ||
| BMI (kg/m2) | 1.15 [1.06;1.25] | 0.001 | 1.04 [0.95;1.15] | 0.386 | ||
| Born in France | 0.21 [0.09;0.48] | <0.001 | 0.51 [0.21;1.27] | 0.148 | ||
| Age (per year) | 1.13 [1.09;1.16] | <0.001 | - | - | 1.05 [1.01–1.1] | 0.023 |
| Age (y/o) | ||||||
| <20 | - | - | - | - | ||
| 20–30 | 2.70 [0.74–9.83] | 0.132 | - | - | ||
| 30–40 | 13.30 [3.87–45.63] | <0.001 | - | - | ||
| >40 | 43.56 [11.93–59.09] | <0.001 | - | - | ||
| Blood Pressure | ||||||
| SBP (for 10 mm Hg) | 1.02 [1.00;1.04] | 0.017 | 1.02 [0.82;1.27] | 0.851 | ||
| SBP>120 mm Hg | 1.66 [0.88;3.15] | 0.119 | 0.83 [0.424–1.623] | 0.585 | ||
| DBP (for 10 mm Hg) | 1.51 [1.22;1.86] | <0.001 | 1.26 [0.95;1.67] | 0.116 | ||
| DBP>70 mm Hg | 3.76 [1.97;7.17] | <0.001 | 2.02 [1.02–3.971] | 0.042 | 2.12 [1.41–2.82] | 0.037 |
| MAP (for 10 mm Hg) | 1.44 [1.18;1.77] | <0.001 | 1.16 [0.88;1.53] | 0.288 | ||
| TRV >2.5 m/sec | 3.23 [1.20–6.99] | 0.001 | 2.89 [1.20–6.99] | 0.019 | ||
| Biological Parameters | ||||||
| LDH (for 100 IU/L) | 1.15 [1.00;1.32] | 0.049 | 1.28 [1.12;1.48] | <0.001 | 1.30 [1.10–1.56] | 0.003 |
| Bilirubin (mg/L) | 0.93 [0.84;1.04] | 0.231 | 1.01 [1.00;1.02] | 0.174 |
||
| Reticulocytes (109/L) | 0.96 [0.75;1.22] | 0.725 | 1.00 [1.00;1.00] | 0.151 | ||
| Hb (g/dl) | 0.89 [0.69;1.13] | 0.332 | 0.92 [0.73;1.17] | 0.4924 | ||
| Leucocytes(109/L) | 0.93 [0.83;1.05] | 0.227 | 1.07 [0.95;1.20] | 0.285 | ||
| Platelets (109/L) | 0.80 [0.64;1.00] | 0.053 | 1.00 [1.00;1.00] | 0.252 | ||
| Ferritin (for 100 mg/L) | 1.02 [0.99;1.04] | 0.172 | 1.00 [1.00;1.00] | 0.054 | ||
| Baseline renal parameters | ||||||
| eGFR (ml/min/1.73 m2) | 0.91 [0.90;0.93] | <0.001 | - | - | 0.91 [0.88–0.95] | <0.001 |
| Glomerular hyperfiltration (eGFR>130 ml/min/1.73 m2) | 0.09 [0.03;0.25] | <0.001 | - | - | ||
| No albuminuria | Ref. | - | - | - | ||
| Microalbuminuria | 1.48 [0.68;3.19] | 0.320 | 1.11 [0.46;2.72] | 0.814 | ||
| Macroalbuminuria | 4.20 [1.88;9.38] | <0.001 | 3.89 [1.61;9.43] | 0.003 | 3.99 [2.99–4.97] | 0.006 |
| Proteinuria (100 mg/mmol) | 1.32 [1.18;1.48] | <0.001 | 1.30 [1.16;1.46] | <0.001 | ||
| History of SCA complication | ||||||
| Skin ulcer | 2.26 [1.12;4.54] | 0.022 | 1.64 [0.81;3.34] | 0.171 | ||
| Retinopathy | 2.98 [1.24;7.17] | 0.015 | 1.73 [0.70;4.25] | 0.235 | ||
| Acute chest syndrome | 0.84 [0.44;1.58] | 0.582 | 1.08 [0.56;2.08] | 0.815 | ||
| Treatment | ||||||
| Hydroxycarbamide | 1.27 [0.62;2.60] | 0.521 | 0.79 [0.38;1.65] | 0.53 | ||
| ACEI | 5.39 [2.37;12.3] | <0.001 | 2.83 [1.18;6.76] | 0.019 | ||
| Transfusion program | 1.64 [0.68;3.91] | 0.269 | 1.82 [0.75;4.41] | 0.186 | ||
- Abbreviations: 95% CI, 95% confident Interval; ACEI, angiotensin converting enzyme inhibitor; adjusted HR*, hazard ratio adjusted on age and baseline eGFR; CKD, chronic kidney disease; DBP, diastolic blood pressure; HR, hazard ratio; LDH, lactate dehydrogenase; MAP, mean arterial pressure; multivariate, multivariate model using stepwise regression; SBP, systolic blood pressure; SCA, sickle cell anemia; TRV, tricuspid velocity regurgitation.
Age appeared as a strong factor associated with an increased risk of kidney function impairment with a HR of 1.13 [1.09;1.16] per year increase (Table 2, Figure 2(A),(B)). As shown in Table 2 and Figure 2, CKD stage II risk increased by 13.3 and 43.6-fold in patients above 30 and 40 years of age, respectively, compared to patients below 20 years of age.
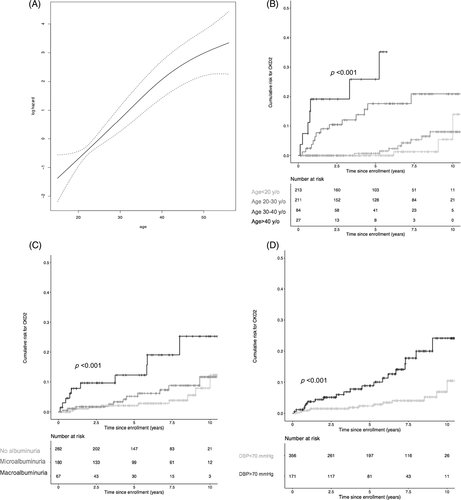
Other risk factors associated with CKD stage II onset were: baseline eGFR HR: 0.91 [0.90–0.93], LDH (for 100 IU/L) HR: 1.15 [1.00;1.32], proteinuria (for 100 mg/mmol) HR: 1.32 [1.18–1.48] and macroalbuminuria HR: 4.20 [1.88–9.38]. As shown in Table 2 and Figure 2(C), whereas CKD stage II onset was associated with macroalbuminuria, no statistical association was found with microalbuminuria. Of notice, 70.3% of patients in the CKD stage II group had no baseline macroalbuminuria and 35.1% no albuminuria.
Baseline DBP was also a strong risk factor for CKD impairment HR: 1.51 for 10 mm Hg increase [95% CI: 1.22;1.86], with a HR of 3.76 [1.97;7.17] (p < 0.001) for patients with baseline DBP above 70 mm Hg (Table 2 and Figure 2(D)). Note, SBP was also associated with an increased risk in univariate analysis, but did not remain statistically significant after adjustment.
More patients with tricuspid regurgitation velocity (TRV) > 2.5 m/sec at baseline developed CKD stage II during follow-up, with a HR of 4.54 [1.91–10.78] in univariate analysis.
As shown Table 2, after adjustment on age and eGFR at baseline, remaining significant risk factors associated with CKD II were: LDH HR:1.28 [1.12;1.48], macroalbuminuria HR: 3.89 [1.61;9.43], proteinuria (for 100 mg/mmol) HR: 2.83 [1.18;6.76], diastolic blood pressure above 70 mm Hg HR: 2.02 [1.02–3.971]. Of notice, sensitivity analysis looking at risk factors for eGFR < 80 ml/min/1.73 m2 (see Table S1) showed similar results with LDH HR: 1.30 [1.11–1.54], macroalbuminuria HR: 7.46 [2.40–23.24], proteinuria (for 100 mg/mmol) HR: 1.34 [1.20–1.49], DBP > 70 mm Hg HR: 3.08 [1.33–7.13] and TRV>2.5 m/sec 2.84 [1.18–6.83] associated with kidney function impairment.
Among the 39 patients reaching the primary outcome with an eGFR <90 ml/min/1.73 m2, six patients had a transient eFGR <90 ml/min/1.73 m2 during follow-up whereas 33 had a persistent decline in eGFR <90 ml/min/1.73 m2. Among these six patients only one had a stable eGFR (128 ml/min/1.73 m2 at baseline and 121 ml/min/1.73 m2 at the end of follow up) while the five others had a mean loss of 10 ml/min/1.73 m2 during the study. When performing analysis on the 33 patients with persistent eGFR decline, similar risk factors were found (Table S2).
In the multivariate analysis, independent risk factors associated with CKD stage II onset were macroalbuminuria HR: 3.34 [95% CI: 1.34–8.35], DBP (above 70 mm Hg HR: 2.12 [95% CI: 1.41–2.82], LDH HR:1.30 [95% CI: 1.13–1.49], age (for one year increase) HR:1.05 [1.01–1.10] and baseline eGFR HR: 0.91 [95% CI: 0.89–0.95] (Table 2).
4 DISCUSSION
Our data show that the incidence of CKD stage II in a young adult SCA population (median age 22 years old) with a median follow up of 5.3 years is up to 7.3%. Moreover, in our study macroalbuminuria, increased LDH, increased DBP above 70 mm Hg, and age (particularly above 30 years old) were independent risk factors associated with CKD stage II onset.
To our knowledge this study is the largest clinical cohort looking at eGFR decline and its related risk factors in an SCA population. This study provides an insight of SCA nephropathy natural course and pinpoints different risk factors for CKD stage II onset.
Gosmanova et al.8 reported in an adult SCA populations an incidence of at least 4.1% of CKD stage II after a 5 years follow-up using CKD EPI eGFR. Our finding of an incidence of 7.3% of CKD stage II seems to be in the same range, despite a younger mean age in our cohort (31 vs. 25 years old in our population). Because no measured GFR were available, we used the CKD-EPI equation as it was reported to be more accurate than the MDRD equation to estimate GFR in this population.15, 16 We ruled out the eGFR decline slope as an outcome, and considered the onset of CKD stage II as a more clinically relevant outcome. As a matter of fact, the mean annual eGFR decline in the CKD stage II group was indeed greater compared to the rest of the cohort (7.46 ml/min/1.73 m2 vs. 1.97 ml/min/1.73 m2, p < 0.001). Thus, CKD stage II endpoint identifies patients with a fast eGFR decline. In order to rule out potential bias, we also performed a sensitivity analysis analyzing risk factor for eGFR < 80 ml/min/1.73 m2 and found similar risk factors (Table S1). Furthermore, among the 39 patients experiencing CKD stage II during follow up, only 15 patients had baseline eGFR between 90 and 95 ml/min/1.73 m2 and seven experienced CKD stage II during follow up, thus reasonably ruling out the age parameter included in the CKD EPI equation as a main bias. We chose not to consider mortality as a competitive risk for stage II chronic kidney disease because only seven patients (1.3%) died during follow-up and their last mean eGFR value was 130.8 ml/min/1.73 m2 (+/−9.3) (i.e., far above the threshold of 90 ml/min/1.73 m2).
Aging was a strong independent risk factor for CKD stage II, backing up the need to better understand SCA nephropathy natural history with the significant increase in life expectancy in this population.1, 2 Indeed, as previously reported by Serjeant et al. CKD prevalence reaches up to 85% of patients after 60 years old.4 Gosmanova et al. found that after 5 years of follow up, the overall prevalence of CKD had increased of 42% with a mean age of 31 years old at the beginning of following.8 Kidney function impairment is associated with particularly high mortality in SCA patients1, 9, 10 raising the question of when to start treatments, such as ACEIs or hydroxyurea to help prevent CKD progression. Our results showed a linear risk for CKD stage II aging, with a particularly higher risk after 30 years old (Figure 2(A),(B)). Early treatment, around the age of 25 to 30 years could help protect kidney function impairment, with further prospective studies with longer follow-up needed to prove if efficient.
Prevalence of macroalbuminuria was significantly higher in the CKD stage II group compared to the rest of the cohort (29.7% vs. 11.4%, p = 0.017), pointing out macroalbuminuria as an independent risk factor for CKD stage II progression (HR=3.98 [2.90–4.96], p = 0.006). However, to our surprise, 70.3% of patients in the CKD stage II group had no baseline macroalbuminuria. Previous cross sectional studies have shown an association between proteinuria or nephrotic syndrome and chronic renal failure,7, 8, 11 suggesting structural histological lesions such as glomerular focal sclerosis or membranous proliferative glomerulonephritis.17 However, given the specific finding that glomerulomegaly is a hallmark of SCA nephropathy, macroalbuminuria may also occur in patients with no or little other pathological lesions, and thus may be mainly related to an increased glomerular pressure and/or an increased renal blood flow.18 This latter hypothesis could explain the high prevalence of patients with hyperfiltration with macroalbuminuria and the significant albuminuria decrease under ACEI previously reported.18, 19 Conversely, we may assume that in CKD stage II macroalbuminuric patients witness glomerular histological lesions accounting for CKD progression, though renal lesions encountered in SCA nephropathy also include focal areas of necrosis, edema, interstitial inflammation, fibrosis, tubular atrophy, and hemosiderin deposits.17 Such non-glomerular histological lesions likely explain that 35.1% of patients who experienced CKD stage II had no albuminuria at baseline or during follow up. Microalbuminuria to the contrary was not associated with an increased risk for eGFR decline (Figure 2(C)).
Our data also show that increased baseline LDH was associated with the onset of CKD stage II, pointing out intravascular chronic hemolysis as an additional risk factor. Interestingly, chronic hemolysis is currently considered to be associated both with hyperfiltration and albuminuria,12, 20 with recent studies suggesting that hydroxycarbamide could be efficient at decreasing both.21 Though in our study hydroxycarbamide did not show any benefit on kidney function protection, the design of our observational study cannot address this specific issue which deserves an interventional randomized trial.
Our finding that DBP is a strong determinant of CKD progression with a 2.12-fold increase for patients with a value above 70 mm Hg (Figure 2(D)) is consistent with the view that “normal” blood pressure value targets in SCA patients should be lower than values defined for the general population.22 Indeed, as previously reported, values that would be considered as normal in healthy individuals should be considered at risk for cardiovascular complications such as stroke and pulmonary hypertension, and for increased mortality in patients with SCA.23 The pathophysiology of normal or low blood pressure in this population with often low systemic vascular resistance is mostly unknown and currently related to chronic hemolysis.3 A rise in blood pressure may suggest either an activation of the renin angiotensin system and/or NO deficiency related to endothelial dysfunction especially in kidneys but also in heart and large vessels as suggested by the finding of an increased tricuspid regurgitation velocity in CKD stage 2 group.
Limitations of the study: Accuracy of blood pressure values could be a matter of debate. Indeed, baseline blood pressure values are the result of only one measurement and not a mean of several measurements which could lead to a non-differential measurement bias. Therefore, identifying blood pressure targets in this population should require a more accurate methodology in future studies.
To conclude, identification of macroalbuminuria, diastolic blood pressure above 70 mm Hg, high hemolytic biomarkers and aging as independent risk factors for CKD progression in SCA population raises the issue of the adequate monitoring and preemptive treatment in high risk patients, especially above 30 years of age. Future interventional studies in SCA patients at high risk for CKD progression targeting optimal blood pressure using ACEI or other antihypertensive agents in addition to hydroxycarbamide are needed.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Acquirement of data is explained in the methods section. All data are available for checking if needed.




