Nocturnal peripheral vasoconstriction predicts the frequency of severe acute pain episodes in children with sickle cell disease
Funding information: National Heart, Lung, and Blood Institute, Grant/Award Numbers: 1R01HL079937, 1R01HL25295, U01 HL117718; National Institute of Biomedical Imaging and Bioengineering, Grant/Award Number: EB001978
Abstract
The basic model of SCD physiology states that vaso-occlusion occurs when hemoglobin S-containing red blood cells (RBC) undergo sickling before they escape the capillary into a larger vessel. We have shown that mental stress, pain and cold, and events reported by patients to trigger SCD vaso-occlusive crisis (VOC), cause rapid and significant decrease in blood flow, reducing the likelihood that RBC could transit the microvasculature before sickling occurs. However, the critical link between decrease in microvascular blood flow and the incidence of future sickle VOC has never been established experimentally in humans. Using data from centrally adjudicated, overnight polysomnograms (PSG), previously collected in a prospective multi-center cohort sleep study, we analyzed the beat-to-beat amplitudes of vasoconstriction reported by the fingertip photoplethysmogram in 212 children and adolescents with SCD and developed an algorithm that detects vasoconstriction events and quantifies the magnitude (Mvasoc), duration, and frequency of vasoconstriction that reflect the individual's inherent peripheral vasoreactivity. The propensity to vasoconstrict, quantified by median Mvasoc, predicted the incidence rate of post-PSG severe acute vaso-occlusive pain events (P = .006) after accounting for age and hemoglobin. Indices of sleep-disordered breathing contributed to median Mvasoc but did not predict future pain rate. Median Mvasoc was not associated with vaso-occlusive pain events that occurred prior to each PSG. These results show that SCD individuals with high inherent propensity to vasoconstrict have more frequent severe acute pain events. Our empirical findings are consistent with the fundamental SCD hypothesis that decreased microvascular flow promotes microvascular occlusion.
1 INTRODUCTION
Sickle cell disease (SCD) is an inherited blood disorder that results from an amino acid substitution in the β chain of hemoglobin, producing sickle hemoglobin (HbS).1 So, HbS polymerizes a short time after it releases oxygen to tissues, causing the normally flexible red blood cells (RBC) to become rigid and take on a sickle-shape. Red blood cells need to bend in order to traverse the small diameter capillaries in the microvasculature. If the flow rate is slower than the time to polymer formation, the RBC are not able to exit from the narrow microvasculature into a larger vessel before this flexible-to-rigid transformation occurs, and they become lodged in the microvasculature, thereby obstructing blood flow. Thus, any factor that decreases microvascular flow could promote vaso-occlusion.2 The clinical manifestation of extensive obstruction of microvascular flow is a clinically perceptible painful vaso-occlusive crisis (VOC), the hallmark of SCD.
While much of the literature in SCD has focused on nitric oxide (NO)-mediated vascular dysfunction and cellular adhesion in the post-capillary venules as key factors that may decrease microvascular flow and promote vaso-occlusion, the role played by the sympathetic and parasympathetic nervous systems in causing vasoconstriction in the pre-capillary arteriole, and decreasing microvascular flow has only recently been considered.3 Our prior work has demonstrated that anticipatory pain,4 experimental mental stress,5 and cold exposure6 all cause significant global vasoconstriction in SCD and non-SCD controls and these responses vary significantly among all individuals.3 Sighs (deep breaths) trigger peripheral vasoconstriction frequently in SCD but rarely in non-SCD indivuals,7 and cold face stimulation reduces the sensitivity of the baroreflex control of peripheral vascular resistance more in non-transfused than transfused individuals with SCD or in controls.8 Taken together, these data indicate that individuals with SCD have hyperactive autonomic nervous system responses.
Monitoring vasoconstriction during sleep offers an excellent, relatively well controlled opportunity to study the sympathetic nervous system activity, which causes vasoconstriction.9 Transient arousals from sleep represent the strongest form of sympathetic stimulus that occurs naturally and frequently in all humans.10 These fluctuations in sympathetic output lead to corresponding fluctuations in cardiovascular activity and peripheral blood flow.9 In the general population, the cardiovascular responses to these arousals include brief, but potent increases in respiratory effort, heart rate and blood pressure.11 Blasi et al.12 showed that the arousal-induced reductions in parasympathetic nervous system activity, which opposes the sympathetic nervous system, are transient and strongly coupled with respiration. However, the sympathetic effects last significantly longer and may accumulate if repetitive arousals occur in close succession. Periodic limb movements, which happen commonly in children with SCD,13 also trigger sympathetic nervous system (SNS) responses, leading to transient increases in heart rate and blood pressure during sleep.14 Given the strong biological premise that deoxygenation of HbS is the basis of the pathogenesis of SCD, our group and others have tested the hypothesis that nocturnal hypoxia is associated with an increase in the incidence rate of severe vaso-occlusive episodes.15 We showed that experimental hypoxia caused parasympathetic withdrawal only in SCD individuals, but did not decrease perfusion in SCD or control individuals.7 Furthermore, we showed in the rigorous multi-center prospective NIH-funded Sleep and Asthma Cohort (SAC) study in children with SCD, that there was no relationship between nocturnal decreased oxygen saturation or other standard sleep-related parameters, and increased incidence rate of severe vaso-occlusive pain eposides.16
In this report, we analyzed the photoplethysmography signal recorded during a standard sleep study, along with the clinical laboratory data and rate of severe sickle pain crisis obtained as part of the SAC study.16 This was to test the hypothesis that the magnitude of the peripheral vasoconstriction that occurred naturally during sleep would predict frequency of severe vaso-occlusive pain episodes. In doing so, we tested in humans the corollary of the Eaton-Hofrichter hypothesis2 that decreased microvascular perfusion relative to the delay time for HbS polymerization, in this case due to greater levels of peripheral vasoconstriction, would result in an increased likelihood of vaso-occlusion and thus higher incidence of VOC. To test the direction of causality, we also determined whether the rate of severe vaso-occlusive pain episodes prior to the sleep study influences the nocturnal vasoconstriction parameters.
2 METHODS
2.1 Sleep and asthma cohort description
The Sleep and Asthma Cohort (SAC) study16 enrolled 252 children who were homozygous for sickle cell hemoglobin (HbSS) or compound heterozygous for sickle β0 thalassemia (HbSβ0) from three clinical centers. Participants receiving regular blood transfusion therapy, on continuous positive airway pressure support, with chronic lung disease other than asthma, smoking cigarettes, or HIV-positivity were excluded. Overnight sleep studies (polysomnograms: PSG) were obtained on all participants in the SAC. Deidentified digital recordings of all channels from each PSG and clinical datasets from 212 participants were selected for our analyses based on the quality of the polysomnogram recording and completeness of the records on pain events. Further details about the recruitment and consent process in the SAC are given in Willen et al.16
2.2 Data analysis
The information used for the detection and quantification of peripheral vasoconstriction episodes during sleep was derived from two channels of the polysomnogram recordings: (a) the sequences of R-peaks extracted from the electrocardiogram (ECG), from which we determined the R-to-R interval (RRI) on a beat-to-beat basis over the entire sleep duration; and (b) the finger photoplethysmogram (PPG) waveform acquired from the non-oxygen sensitive component of the optical signal from the pulse oximetry sensor (Masimo SET [v2], Irvine, CA). From each PPG pulse, we derived the peak-to-trough amplitude (PPGa), which reflects the pulsatile change in blood volume caused by arteriolar blood flow in the fingertip.17, 18 The PPGa is largely modulated by the autonomic nervous system on a beat-to-beat basis.19 Thus, the relative changes in PPGa were used to quantify the degree of peripheral vasoconstriction/vasodilation at any given time.
The PPG recording in each polysomnogram was visually examined to exclude segments with obvious artifacts due to motion, interruptions to the sleep study, signal clipping or other abrupt alterations in gain of the channel that made the signal quality inadequate for analysis. Using the algorithm that we developed (supplemental materials), the magnitude of each vasoconstriction event detected during the overnight recording of the PPGa signal was calculated.
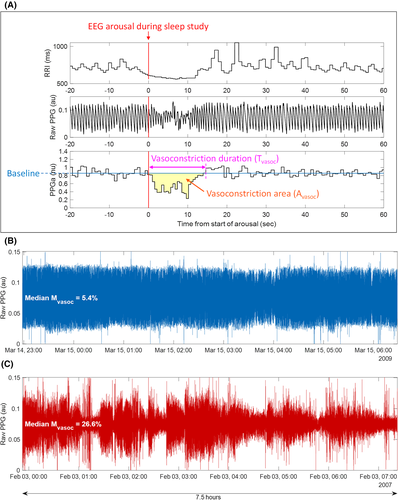
Figure 1A displays an example of such a vasoconstriction episode, immediately following an arousal from sleep (shown by the red vertical line at time zero). As is the case with most arousals, there is an increase in heart rate indicated by a decrease in R-to-R interval (Figure 1A, top tracing). For each identified vasoconstriction episode, the algorithm generated the following quantitative descriptors: (a) vasoconstriction duration (Tvasoc), for example, the time from the onset to the end of vasoconstriction; (b) vasoconstriction “area” (Avasoc), computed by integrating the difference between the PPGa and the pre-vasoconstriction baseline over Tvasoc; and (c) vasoconstriction magnitude (Mvasoc). Note, Mvasoc is calculated by dividing Avasoc by Tvasoc for that particular vasoconstriction episode and represents the average reduction in PPGa over Tvasoc. For each participant, the algorithm also computed the frequency of vasoconstrictions per hour (fvasoc). Thus, the median values of these extracted descriptors over the total duration of sleep for each participant represent the basal vasoreactivity and characterize the nocturnal vasoconstriction phenotype of each participant
Autonomic balance based on heart rate variability was determined by computing the following standard indices for each individual based on the electrocardiogram obtained during the polysomnogram: (a) the root mean square differences of successive R-R intervals (RMSSD), which assesses parasympathetic (vagal) modulation of heart rate; and (b) the ratio of low-frequency power to high-frequency power of heart rate variability (LHRRRI), which represents the balance between sympathetic and vagal control of heart rate.20 We also used other standard sleep study indices available from the centrally adjudicated polysomnograms in the SAC clinical datasets.16 These included the full night obstructive apnea-hypopnea index (OAHI = number of apneas or hypopneas per hour of sleep), arousal index (AI = number of arousals per hour of sleep), and the number of periodic limb movements per hour of sleep (PLMI = periodic limb movement index) as potential predictors of post-PSG pain rate.
Severe pain episodes, as defined in the SAC study,16 were used to calculate pain rates. Post-PSG pain rate for any given participant was calculated as the number of SCD-associated pain episodes per year severe enough to require hospital admission and treatment with opioids that occurred between the date of the PSG and the end of follow-up (Tpost-psg, years), divided by the duration of follow-up (Tpost-psg). Pre-PSG pain rate for the same participant was calculated as the number of severe SCD-associated pain episodes per year, as defined above, occurring between birth or Tpost-psg years before PSG, whichever is smaller, and the date of the PSG (Tpre-psg, years), divided by Tpre-psg. More details on the severe pain episode definition and quality control in the SAC study16 are provided in the supplemental methods.
2.3 Statistical analyses
2.3.1 Predictors of the post-PSG pain rate
A larger number of participants had zero or very low pain-rates, resulting in a strongly positive skewing of the frequency distribution of the pain rate data (Table 1). Therefore, we employed negative binomial regression to determine if the vasoconstriction parameters Tvasoc, Avasoc, Mvasoc and fvasoc were predictive of the post-PSG pain rate. The final negative binomial regression models for post-PSG pain rate also included the sleep parameters (OAHI, AI and PLMI), the baseline heart rate variability parameters (RMSSD and LHRRRI) as well as age at the time of the PSG, sex, hemoglobin, white blood cell count, and reticulocyte count.
| Characteristics | Median (inter quartile range) |
|---|---|
| Number of participants | 212 |
| Age at PSG (years) | 10.4 (14.7) |
| Male (%) | 50.5 |
| Total follow-up time after PSG (years) | 5.0 (9.7) |
| Hemoglobin (g/dL) | 8.1 (5.8) |
| White blood cell count (×109/L) | 11.8 (19.8) |
| Reticulocytes (%), N = 211 | 10.9 (27.1) |
| Platelet count (×103) (μL−1), N = 189 | 420 (862) |
| Obstructive apnea hypopnea index (events/h) | 0.6 (37.3) |
| Arousal index (events/h) | 7.9 (22.1) |
| Periodic limb movement index (events/h) | 0.6 (7.1) |
| Nocturnal hemoglobin oxygen saturation (%) | 96.4 (4.4) |
| Oxygen desaturation index, 3% (events/h) | 2.3 (5.7) |
| Pre-PSG pain rate (events/y) | 0.34 (15.9) |
| Post-PSG pain rate (events/y) | 0.34 (7.2) |
2.3.2 Predictors of inherent nocturnal vasoconstriction
We also determined if the pain rate prior to the sleep study (pre-PSG pain rate) affected the magnitude of vasoconstriction. As the group distribution of median Mvasoc was normal, a multiple linear regression was used to determine the association between Mvasoc and pre-PSG pain rate. The final multivariable models for predicting Mvasoc also included age, sex and hemoglobin, each of the sleep parameters (OAHI, AI and PLMI) and the baseline heart rate variability parameters (RMSSD and LHRRRI) as covariates.
The statistical significance was defined as P < .05. All statistical analyses were carried out using JMP Pro 14 software (SAS Institute Inc., Cary, NC).
3 RESULTS
3.1 Participant characteristics
Summary statistics for the baseline characteristics of the 212 participants analyzed in this study are presented in Table 1. The median duration from the date of polysomnogram to the end of follow-up was 4.99 years (interquartile range: 1.69 years). Participants are children with sickle cell disease from the Sleep and Asthma Cohort study who underwent a centrally adjudicated overnight polysomnography and were followed prospectively to determine the number of acute severe vaso-occlusive pain episodes requiring hospitalizations.
3.1.1 The magnitude of vasoconstriction predicts post-PSG pain rate
Table 2 shows the values of the vasoconstriction parameters derived from the 212 polysomnogram studies. The mean number of vasoconstriction events per hour during each polysomnogram was 36.4 (range 21.3 to 46.6), with a mean duration of 13.3 seconds (range 6.5 to 21.8). The frequency of vasoconstriction events increased with lower hemoglobin, while their duration increased with age and was higher in males. The primary vasoconstriction parameter, Mvasoc had a mean of 14.5% (4.5% to 26.9%), decreased with age, and was higher in males. However, the effects of age and gender were not large, even though they were statistically significant. None of the r2 values were greater than 0.1 (not shown).
| Parameters | Mean b (SD) | Parameter estimate (p value) a | ||
|---|---|---|---|---|
| Age | Sex (male) | Hemoglobin | ||
| Mvasoc (%) | 14.5 (4.06) | −0.14 (p = .0272) | 0.97b (p < .001) | 0.02 (p = .947) |
| Tvasoc (s) | 13.3 (2.83) | 0.19 (p < .001) | 0.38 (p = .041) | 0.10 (p = .530) |
| Avasoc (%∙ s) | 194.4 (75.0)b | 0.001 (p = .741) | 0.04 (p < .001) | 0.004 (p = .663) |
| fvasoc (events/h) | 36.4 (4.6) | 0.10 (p = .172) | −0.21 (p = .507) | −0.77 (p = .004) |
| RMSSD (ms) | 55.7 (37.8)b | 0.014 (p = .002) | 0.01 (p = .481) | 0.01 (p = .477) |
| LHRRRI (unitless) | 0.87 (0.78)b | 0.005 (p = .313) | 0.02 (p = .461) | −0.10 (p = .597) |
- a Multiple linear regression analysis. Bolded values indicate statistical significance.
- b Not-normally distributed parameters, log-transformed prior to performing statistical analysis.
The median Mvasoc during the polysomnogram was significantly associated with the incidence rate of VOC-related pain episodes after adjusting for age and hemoglobin (P = .0056), as shown in Table 3 (model 1). White cell and reticulocyte counts were not significant in any of the models. The incidence rate ratio (IRR) range was 3.29, implying that the participant with the highest median Mvasoc had a predicted pain rate that was more than three times higher than that of the participant with the lowest median Mvasoc (IRR 3.29, 95% CI 1.42-7.63). The known effects of age and hemoglobin on SCD pain21 were also significant in the model. In contrast, the other parameters quantifying vasoconstriction (Tvasoc, fvasoc and Avasoc) were not associated with post-PSG pain rate (Model 2-4; Table S2), nor were any of the indicators of sleep disturbance (OAHI, AI, and PLMI) (Model 5-7; Table S2). Similarly, none of the measures of baseline autonomic function based on heart-rate variability (RMSSD and LHRRRI) predicted post-PSG pain rate (Model 8-9; Table S2).
| PREDICTORS OF POST-PSG PAIN RATE | |||
|---|---|---|---|
| IRR* | 95% CI | P value | |
| Model 1. Mvasoc as the predictor of post-PSG pain rate (negative binomial regression) | |||
| Mvasoc (magnitude of vasoconstriction, %) | 3.29 | 1.42-7.63 | .006 |
| Age at PSG (y) | 3.17 | 1.85-5.44 | <.0001 |
| Hemoglobin (g/dL) | 2.47 | 1.21-5.03 | .013 |
| PREDICTORS OF MEDIAN Mvasoc | |||
| Parameter estimate | SE | P value | |
| Model 2. Pre-PSG pain rate as the predictor of Mvasoc (multiple linear regression)Pmodel < .001 | |||
| Pre-PSG pain rate (events/y) | 0.28 | 0.17 | .114 |
| Age at PSG (y) | −0.15 | 0.06 | .017 |
| Hemoglobin (g/dL) | −0.04 | 0.23 | .846 |
| Sex (male = 1, female = 0) | 0.97 | 0.27 | <.001 |
| Model 3. Pre-PSG AI as the predictor of Mvasoc (multiple linear regression)Pmodel < .001 | |||
| Pre-PSG pain rate (events/y) | 0.26 | 0.17 | .171 |
| Age at PSG (y) | −0.16 | 0.06 | .014 |
| Hemoglobin (g/dL) | −0.06 | 0.22 | .800 |
| Sex (male = 1, female = 0) | 0.88 | 0.27 | .001 |
| Arousal indexa (AI, events/h) | 5.04 | 1.85 | .007 |
| Model 4. Pre-PSG OAHI as the predictor of Mvasoc (multiple linear regression) Pmodel < .0001 | |||
| Pre-PSG pain rate (events/y) | 0.28 | 0.17 | .107 |
| Age at PSG (y) | −0.16 | 0.06 | .014 |
| Hemoglobin (g/dL) | 0.08 | 0.23 | .736 |
| Sex (male = 1, female = 0) | 0.92 | 0.26 | < .001 |
| Obstructive apnea hypopnea indexa (OAHI, events/h) | 0.89 | 0.31 | .004 |
| Model 5. Pre-PSG PLMI as the predictor of Mvasoc (multiple linear regression) Pmodel < .001 | |||
| Pre-PSG pain rate (events/y) | 0.24 | 0.17 | .107 |
| Age at PSG (y) | −0.12 | 0.06 | .061 |
| Hemoglobin (g/dL) | −0.07 | 0.23 | .766 |
| Sex (male = 1, female = 0) | 0.88 | 0.27 | .001 |
| Periodic limb movement indexa (PLMI, events/h) | 0.98 | 0.41 | .018 |
- a Indicates log-transformed variables. Bolded P values indicate statistical significance. Pmodel is the P value of the F-test of the overall significance of the regression model.
Figure 1B,C provides a striking illustration of the association between vasoconstriction reflected by median Mvasoc and incidence of pain. The raw PPG signal from one representative participant with a low pain rate of 0.55 events/year (Figure 1B) is compared to the corresponding signal from a participant with a high pain rate of 1.62 events/year (Figure 1C). The median Mvasoc was 5.4% in the participants in Figure 1B, while 26.6% in participants in Figure 1C. Furthermore, the pattern of larger and longer vasoconstriction events is visibly different in Figure 1C with higher median Mvasoc and higher associated pain rate demonstrating how Mvasoc reflects the pattern of vasoconstriction. The values of median Mvasoc adjusted for age and sex for participants with post-PSG pain rates above (high) or below (low) the 60th, 70th, 80th and 90th percentiles can be found in Figure 1 of supplemental materials, providing a graphic representation of the statistical findings obtained through negative binomial regression.
Of the 212 participants in this study, 72 were noted in the SAC study as having been prescribed hydroxyurea at the time of the polysomnogram or in the period after. The initial analysis included hydroxyurea in the model and showed that hydroxyurea contributed significantly to the model (P < .0001) but was associated with an increase in post-PSG pain rate, a result opposite to the well documented effect of hydroxyurea on SCD crisis rate. When we further examined the data, 30.6% of the 72 participants prescribed hydroxyurea had greater than 1.5 pain episodes per year, compared to only 10% of the 140 participants who were never prescribed hydroxyurea, indicating that hydroxyurea was non-randomly prescribed to participants with more pain. Because of this bias and the lack of any information regarding hydroxyurea intake by patients, we did not include hydroxyurea as a variable in the primary analysis of the 212 participants presented above (model 1; Table 3). Interestingly, when we separately analyzed the data from the 72 participants prescribed hydroxyurea, the incidence rate ratio (IRR) range for Mvasoc prediction of pain rate was 5.12 (95% CI 1.42-7.63), which was significant (P = .0014) and higher than that found in the analysis of all 212 participants. Details and further discussion of the hydroxyurea analysis are presented in supplemental materials (Table S1).
3.1.2 Determinants of the vasoconstriction magnitude
To establish if prior history of VOC pain increased vasoconstriction, we determined the effect of pre-PSG pain rate and other covariates on median Mvasoc. As seen in Table 3, all models (2–4) demonstrated that prior pain had no effect on median Mvasoc, even after adjusting for other covariates. However, younger participants and males had larger median Mvasoc (model 2; Table 3), and hemoglobin had no effect.
Importantly, each of the indices quantifying sympathetic stimuli characteristic of sleep-disordered breathing (OAHI, AI and PLMI) was strongly predictive of median Mvasoc (model 3-5; Table 3), even though they did not independently predict post-PSG pain rate (model 5-7, Table S2). There was no association between the baseline measures of cardiac autonomic function and median Mvasoc (model 5-6; Table S3).
4 DISCUSSION
Over four decades ago, Eaton and Hofrichter postulated that the relation between retention of RBC in the microvasculature as a consequence of decreased regional blood flow and time to flexible-to-rigid transformation of the HbS-containing RBC, is the fundamental mechanism of vaso-occlusive crisis.2 Thus, two basic factors determine whether vaso-occlusion occurs: (a) the delay time between deoxygenation and polymerization of HbS; and (b) how fast the RBC are moving through the microvasculature.22 If the microvascular blood flow decreases significantly, whether from adhesion in the postcapillary venules or constriction of pre-capillary arterioles, such that the time to transit the capillary network and to reach a vessel larger than the RBC diameter is longer than the time to HbS polymerization, the RBC become rigid in small vessels and lodge, thereby occluding flow. The vast majority, if not all, of the proposed mechanisms for SCD pathology to this day involve delay time to HbS polymerization or microvascular transit time as the final common path to VOC.
In the course of a number of studies in humans using peripheral perfusion as a biomarker to understand the triggers of VOC, we have shown that cold,8 mental stress,5 respiration,7 and pain itself,4, 23 all factors mentioned by patients to precede VOC, cause significant peripheral vasoconstriction, and that each study participant, both SCD and controls, seems to have a characteristic baseline vasoconstriction pattern that was observed over several experimental visits.6 We suspected that individuals with high inherent vasoconstriction would have more frequent VOC2.
The present data based on the amplitude of the PPG signal from a commonly used clinical sensor provides a noninvasive means of quantifying the magnitude of sympathetically mediated constriction of the arterioles upstream of the capillary, thereby enabling the tracking of periods when microvascular blood flow is significantly reduced. The median value of the vasoconstriction magnitude parameter, Mvasoc, measured over several hours during sleep clearly reflects the magnitude and duration of vasoconstriction, as seen when comparing Figure 1B (low Mvasoc) to Figure 1C (high Mvasoc). Importantly, based on a standard definition of severe SCD VOC events that was adopted by the SAC, vasoconstriction magnitude quantified by median Mvasoc strongly predicts subsequent severe VOC rate. Obviously, individuals with SCD experience many more VOC events that are less severe and do not result in hospitalization. This information was not available in the SAC dataset. Nevertheless, it is clear that sympathetic nervous system-mediated vasoconstriction predicts future frequency of severe VOC events. Furthermore, the fact that Mvasoc is strongly related to incidence of VOC over a median of 5 years after the polysomnogram is consistent with the vasoconstriction magnitude being a characteristic of the individual that persists over time.
An implicit assumption in our model is that high propensity to peripheral vasoconstriction leads to high frequency of future VOC episodes. Could past VOC-related pain occurring before the polysomnogram increase peripheral vasoconstriction instead? When we analyzed the relationship between median Mvasoc and the rate of severe pain events measured in the period preceding the polysomnogram, we found no association between pre-PSG pain rate and median Mvasoc (Table 3), providing strong support for the direction of causality, and suggesting that high vasoconstriction magnitude predisposes to frequent VOC, and not the other way around. Taken together, these data suggest that autonomic-mediated vasoreactivity plays a major role in promoting VOC in SCD and provides the first experimental evidence in humans validating the Eaton-Hofrichter model2 on a macro scale.
We have previously shown that experimentally induced hypoxia causes loss of parasympathetic modulation of heart rate in SCD individuals, but not in controls. Importantly, transient hypoxia does not decrease microvascular flow in SCD individuals.7 Consistent with this finding, Willen et al.16 showed no significant association between low mean nocturnal SpO2 and incidence of VOC in the SAC participants studied here. On the other hand, we5, 7, 8, 24, 25 and others26-29 have shown significant dysautonomia in SCD. Therefore, we examined measures of cardiac ANS balance (LHR, RMSSD) but found no association with VOC rate (modesl 8-9 Table S2). In contrast, parasympathetic modulation of heart rate (RMSSD) was negatively correlated with OAHI (Table S4), while the sympathetic stimuli, OAHI, AI, and PLMI were all associated with increased median Mvasoc (models 3-5; Table 3). These findings show that sympathetic stimuli during sleep cause vasoconstriction as measured by Mvasoc and are consistent with existing knowledge about the detrimental effects of sleep-disordered breathing on autonomic and cardiovascular function.9, 30 These data raise the possibility that the net sympathetic nervous system activity quantified by Mvasoc derived from PPG signals may be the common path that links sleep-disordered breathing and cardiovascular disease.31, 32
The PPG signal is recorded ubiquitously in sleep studies for monitoring SpO2 and pulse rate, but changes in the magnitude of the PPG are ignored. Essentially, every wearable device that reports cardiac activity uses the PPG signal to measure heart rate but does not utilize the changes in PPG amplitude. Adaptation of these devices to measure Mvasoc augurs well for future large-scale SCD population studies, in particular to determine if transient increases in Mvasoc predict VOC in the immediate future. In fact, the recognition that the vasoreactivity detected by the PPG signal represents an easily accessible and readily quantifiable biomarker of sympathetic nervous system activity and tissue perfusion likely has implications for the study of human disease far beyond SCD.
It is important to emphasize that we do not argue that the distinct episodes of vasoconstriction we observed during sleep would predict immediate transition into VOC. Instead, the current data add neural-mediated vasoconstriction to the list of events than can increase transit time and the likelihood of more frequent severe VOC. We believe that the magnitude of the decrease in PPGa depicted in Figure 1 represents the collective effects of all inputs causing vasoconstriction, including modulation of the sensitivity of the vessel to constriction by nitric oxide or endothelin-1, the resistance to outflow by cellular adhesion in the postcapillary venules and the sympathetic nervous system triggered vasoconstriction, all of which contribute to the increase in microvascular transit time that promotes vaso-occlusion. Furthermore, the data showing that the vasoconstriction status at one point in time predicts the frequency of VOC over the next year or more suggest that the propensity to vasoconstrict measured by median Mvasoc is an inherent, or at least lasting, characteristic of each individual. And, that those who have more intense, more frequent, and more prolonged vasoconstriction events are more likely to have more frequent severe VOC.
The present data reveal for the first time that peripheral vasoconstriction strength, as a surrogate for microvascular transit time, predicts increased likelihood of severe sickle pain crisis frequency. The fact that individuals have intrinsic vasoreactivity phenotypes that can be identified by analysis of the PPG suggests that vasoreactivity is a biophysical biomarker for sympathetic balance that may have a role in predicting clinical outcomes in SCD as well as other sympathetic nervous system-related vascular disorders. Autonomic balance and vasoreactivity likely contribute to the known variability in the incidence rate of severe VOC pain and represent a plausible target for future therapeutic intervention.
ACKNOWLEDGEMENTS
The authors would also like to acknowledge Martine Torres, Ph.D. for her critical reading of the manuscript and editorial assistance. The authors acknowledge funding from NHLBI U01 HL117718 (TC, MK), 1R01HL25295 (CR), 1R01HL079937 (MD), and from NIBIB P41 EB001978 (MK).
CONFLICT OF INTEREST
The Aauthors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
TC conceived the project and approach; PC, MK developed the key parameters and analysis approach; PC, MK analyzed the data; PC, MK, TC interpreted the data; YJ, PC, MK, CR, MD developed the data extraction approach; MD was the lead PI on the SAC study, YJ extracted and assembled the data; PC, MK, TC wrote the paper; PC, MK,TC, CR, MD edited the paper.
Open Research
DATA AVAILABILITY STATEMENT
Readers may contact Dr. Thomas Coates ([email protected]) or Dr. Michael Khoo ([email protected]) to discuss access to the primary data.




