Primary central nervous system erythroid sarcoma with NFIA-CBFA2T3 translocation: A rare but distinct clinicopathologic entity
To the Editor:
Pure erythroid leukemia (PEL) is a rare, aggressive acute leukemia that commonly presents with bone marrow involvement and pancytopenia and is associated with secondary disease (following prior cytotoxic chemotherapy or an antecedent myeloid neoplasm), complex karyotype, and TP53 abnormalities.1, 2 Extramedullary sarcomatous presentations are exceedingly rare. Here, we describe a unique case of primary central nervous system (CNS) erythroid sarcoma with an NFIA-CBFA2T3 translocation with no evidence of bone marrow (BM) or peripheral blood involvement.
A 3-year-old male presented to the emergency department with rapidly progressing upper extremity flaccid paralysis and an inability to walk. Complete blood count (CBC) was normal. Imaging of the brain and spine showed extensive nodular leptomeningeal enhancement, multiple extra-axial intracranial masses involving the pineal, right intraventricular, and left anterior pontine regions (Figure 1A,B), a C2-C6 mass causing spinal cord compression, and a T2-T3 non-compressive lesion. The patient was taken emergently for C2-C6 laminectomy and tumor debulking.
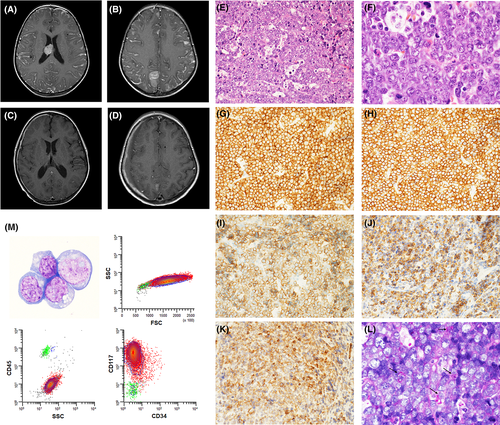
The H&E sections of the tumor mass (Figure 1E,F) showed sheets of intermediate size cells with oval to irregular nuclei, prominent nucleoli, and small amounts of cytoplasm. Frequent tingible-body macrophages and mitotic figures were present. Extensive immunohistochemical analysis showed that the cells were positive only for CD43, CD117 (Figure 1G,H), and c-MYC and were negative for CD34, MPO, lysozyme, CD61, CD3, CD20, PAX-5, CD10, CD30, and TdT as well as for markers of muscle, germ cell, neuroendocrine, neural, neuroectodermal, and epithelial differentiation. INI-1 was retained. In situ hybridization for EBV-encoded RNA (EBER) was negative. The Ki-67 proliferative index was >95%. Based on the pathologic findings, a hematopoietic malignancy was suspected, but the lesion could not be definitively classified. A BM biopsy showed a normocellular marrow with maturing trilineage hematopoiesis, no dysplasia, and no increase in blasts. Cytogenetic analysis on the BM showed a normal karyotype. A lumbar puncture was performed, and the cerebrospinal fluid preparations showed large-sized cells with round to oval nuclei, prominent nucleoli, and small to moderate amounts of basophilic, occasionally vacuolated cytoplasm (Figure 1M). Flow cytometry on the cerebrospinal fluid revealed an abnormal population of cells with increased forward scatter and bright positivity for CD117, which was negative for CD45, CD34, CD13, CD15, CD16, CD33, CD38, HLA-DR, CD11b, CD14, CD64, CD19, CD2, CD4, CD7, and CD56.
Targeted next generation sequencing using a 648-gene panel and whole transcriptome RNA sequencing (Tempus XT; Tempus, Chicago, IL) revealed an NFIA-CBFA2T3 rearrangement as well as a pathogenic variant in LZTR1 (c.180C > A; p.C60*; 40.9% variant allele frequency) and variants of unknown significance in SMARCA4, CTCF, KMT2A, HIST1H1E, and SLC26A3. After identification of the NFIA-CBFA2T3 translocation, additional immunohistochemical stains were performed (Figure 1I,K). Tumor cells were positive for CD71, E-cadherin, and glycophorin A. A PAS stain (Figure 1L) showed coarse granular cytoplasmic positivity in scattered cells. A final diagnosis of de novo primary CNS erythroid sarcoma was rendered. At the time of publication, the patient had completed five of six planned cycles of chemotherapy. An MRI after four cycles showed improvement in the intraventricular and leptomeningeal disease (Figure 1C,D).
De novo pure erythroid sarcoma has been previously described in six pediatric patients. Five of six reported cases have shown orbital or CNS involvement,3 suggesting that erythroid sarcoma should be considered in the differential diagnosis of “small round blue cell” lesions involving the brain or orbit in pediatric patients. Whereas stains for CD45, CD34, and MPO are frequently negative in PEL/erythroid sarcoma, CD43 is especially helpful in establishing hematopoietic lineage, particularly in cases that might also express CD99 and in the rare cases, such as ours, that lack BM involvement at diagnosis.
Our case represents the second report of primary CNS erythroid sarcoma with NFIA-CBFA2T3 and the first with no morphologic or cytogenetic evidence of BM involvement. A recent report by Liu et al.3 described a strikingly similar pediatric patient with CNS erythroid sarcoma with a t(1;16)(p31;q24) NFIA-CBFA2T3 fusion who showed only cytogenetic evidence of BM involvement, and was also considered to have primary CNS disease. Including our case, the NFIA-CBFA2T3 rearrangement has now been reported in five cases of PEL/erythroid sarcoma,3, 4 all in young children, suggesting that this translocation should be considered a recurrent genetic abnormality in PEL/erythroid sarcoma.
The NFIA-CBFA2T3 fusion is believed to cause transcriptional repression of NFIA target genes.4 NFIA is a member of the NF1 family of transcription factors and has been shown to function as a regulator of hematopoietic progenitor cell fate, suppressing granulocytic differentiation and at the same time being required for erythroid differentiation.3 CBFA2T3 (also known as RUNX1T3) is part of the ETO or MTG family of transcriptional regulators, along with CBFA2T1 (RUNX1T1) and CBFA2T2, which act to repress gene transcription through interaction with transcription factors.3 While t(8;21)(q22;q22.1) RUNX1T1-RUNX1 fusions define a specific subtype AML within the WHO classification, t(16;21)(q24;q22) CBFA2T3-RUNX1 translocations have also been reported as a recurrent abnormality in AML.5
Molecular testing in this case identified a loss of function mutation in LZTR1. Germline loss of function LZTR1 mutations occur in Noonan Syndrome, and somatic mutations have been reported in multiple tumor types.6 Loss of LZTR1-mediated ubiquitination of RAS has been shown to activate RAS/MAPK signaling.6 Liu et al.3 identified multiple gene mutations in their case, in addition to an NFIA-CBFA2T3 translocation, including an activating mutation in EPOR, which encodes the erythropoietin receptor. As a receptor tyrosine kinase, EPOR signals through the RAS/MAPK pathway, among others. Together, these two cases suggest that activation of RAS/MAPK signaling plays a role in the development of PEL/erythroid sarcoma with an NFIA-CBFA2T3 translocation. Furthermore, these cases suggest that de novo primary CNS erythroid sarcoma with t(1;16)(p31;q24) NFIA-CBFA2T3 may represent a rare but distinct clinicopathologic entity. Whether primary CNS erythroid sarcoma with NFIA-CBFA2T3 or cases of PEL with this translocation are associated with different outcomes than PEL occurring in adults in the context of complex karyotypes and TP53 abnormalities remains uncertain, and additional studies are required to address this question. Increased awareness of this entity is critical to avoid diagnostic delay and/or misdiagnosis in pediatric patients who may present with primary CNS disease without overt BM involvement.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Yevgeniy Linnik and Emily F. Mason designed the study and wrote the manuscript. Ian Dryden and David R. Head contributed to pathologic review and manuscript preparation. Devang Pastakia provided clinical care and contributed to manuscript preparation.




