Muscle structural, energetic and functional benefits of endurance exercise training in sickle cell disease
†EXDRE collaborative study group:
Anoosha Habibi, M.D., Justine Gellen-Dautremer, M.D., Jean-Antoine Ribeil, M.D., Ph.D., Jean-Benoit Arlet, M.D., Ph.D., Sarah Mattioni, MD; Jugurtha Berkenou, Noémie Delrieux, M.D., François Lionnet, M.D., Jean-François Grenot, Arnauld Garcin, Ph.D., Gaetana Di Liberto, Ph.D.
Funding information: Association ar.mony; Société française de cardiologie
Abstract
Sickle cell disease (SCD) patients display skeletal muscle hypotrophy, altered oxidative capacity, exercise intolerance and poor quality of life. We previously demonstrated that moderate-intensity endurance training is beneficial for improving muscle function and quality of life of patients. The present study evaluated the effects of this moderate-intensity endurance training program on skeletal muscle structural and metabolic properties. Of the 40 randomized SCD patients, complete data sets were obtained from 33. The training group (n = 15) followed a personalized moderate-intensity endurance training program, while the non-training (n = 18) group maintained a normal lifestyle. Biopsies of the vastus lateralis muscle and submaximal incremental cycling tests were performed before and after the training program. Endurance training increased type I muscle fiber surface area (P = .038), oxidative enzyme activity [citrate synthase, P < .001; β-hydroxyacyl-CoA dehydrogenase, P = .009; type-I fiber cytochrome c oxidase, P = .042; respiratory chain complex IV, P = .017] and contents of respiratory chain complexes I (P = .049), III (P = .005), IV (P = .003) and V (P = .002). Respiratory frequency, respiratory exchange ratio, blood lactate concentration and rating of perceived exertion were all lower at a given submaximal power output after training vs non-training group (all P < .05). The muscle content of proteins involved in glucose transport and pH regulation were unchanged in the training group relative to the non-training group. The moderate-intensity endurance exercise program improved exercise capacity and muscle structural and oxidative properties. This trial was registered at www.clinicaltrials.gov as #NCT02571088.
1 INTRODUCTION
Sickle cell disease (SCD) is the most frequent worldwide genetic disease.1 This hemoglobinopathy results in synthesis of abnormal S hemoglobin (HbS) which may polymerize once deoxygenated, potentially resulting in the sickling of red blood cells (RBC). Sickle-shaped RBC are less deformable and adhere better to endothelium,1 the combination of which may cause sickle RBC entrapment in the microcirculation. Entrapment of RBC may progress to painful vaso-occlusive crises (VOC), one of the main complications of the disease,1 and may lead to failure of vital organs.2 Sickle RBC are also fragile, causing hemolysis, release of free hemoglobin into circulation and severe chronic anemia.1 SCD patients are often subjected to arterial desaturation3, 4 and arterial blood flow impairment due to the presence of free hemoglobin.5 Collectively, anemia, arterial desaturation and blood flow disorders result in hypoxemia, lower oxygen supply and potentially tissue hypoxia.6-8 Therefore, the low muscle tissue oxygen index observed at rest9 should not spare the active muscles where O2 and energy demands are particularly elevated during physical exercise or activity. Besides, skeletal muscle of SCD patients displays impaired oxidative capacities, characterized by lower citrate synthase (CS), β-hydroxyacyl-CoA dehydrogenase (β-HAD) and cytochrome c oxidase (COx) activities compared to healthy persons.6 This means that if oxygen supply to active skeletal muscles is reduced, the capacity of muscle to utilize oxygen for muscle energy metabolism is also reduced. More specifically, these metabolic defects impair adenosine triphosphate (ATP) production and lactate disposal10 from oxygen-dependent mitochondrial pathways, instead favoring non-oxidative glycolysis for energy supply and consequently lactate production. Because of the high energy demand required, physical exercise and activity exacerbate lactate production, inducing in SCD patients with restricted lactate removal capacities very early lactate accumulation when exercise power output increases.11, 12 However, lactate accumulation may be detrimental for patients. Indeed, increased blood lactate is accompanied by systemic acidosis10 which in turn favors Hb polymerization, sickling and potentially VOC.13 Skeletal muscle of SCD patients is also hypotrophic6 and that is detrimental for force production.14 Taken together, the metabolic and structural defects observed in skeletal muscles of SCD patients contribute to poor muscle function, exercise tolerance and quality of life in SCD patients.15 Therefore, any intervention that might reverse the aforementioned muscle structural, metabolic and functional dysfunctions observed in SCD patients should be considered as a potential therapeutic strategy.
In healthy persons, it is well-established that endurance exercise training is effective in improving type I muscle fiber cross-sectional area and oxidative enzyme activities.16, 17 Similar improvements have been reported in pathological populations such as fascioscapulohumeral muscular dystrophy18 and chronic obstructive pulmonary disease (for oxidative enzyme activities only).19 Endurance training also increases the muscle content of proteins involved in the transport of glucose (GLUT4) and lactate [monocarboxylate transporters 1 (MCT1) and 4 (MCT4)], ameliorating both muscle energy substrate supply and pH regulation.20 Indeed, lactate transport via MCT1 and MCT4 is coupled (in a 1:1 ratio) with a proton (H+ ion) so that muscle lactate transport also participates in muscle pH regulation.21, 22 Recently, the feasibility and the safety of a moderate-intensity endurance exercise training program were demonstrated in SCD children but benefits of this home-based training program were mitigated.23 The training program used in the present study for adult SCD patients without severe chronic complications increased patients' exercise capacity, muscle microvascular density and quality of life.24
The main goal of the present study was to evaluate the effects of a moderate-intensity endurance training program on skeletal muscle structural and metabolic properties, as well as on their functional consequences. Specifically, we hypothesized that moderate-intensity endurance training would (a) reduce muscle hypotrophy, (b) improve muscle oxidative capacity, (c) increase muscle content of proteins involved in energy substrate transport (glucose and lactate) and pH regulation and (d) ameliorate physical ability/functional capacity of patients.
2 METHODS
2.1 Study population
Forty adult homozygous SCD patients without severe chronic complications were included in the present study. Of the 40 SCD patients, 33 (83%) completed this study [34 ± 10 years old, 18 (55%) men and 15 (45%) women]. Seven patients were not analyzed, comprising five patients in the training group (one lost to follow-up, two could not balance training program and work schedule, one pregnancy, one appendicitis surgery) and two patients in the non-training group (one protocol violation and one lost to follow-up).24 Patients' baseline characteristics [reported separately24 but repeated here for reader convenience] were similar between groups (Table 1). The experiment was approved by the local ethics committee and conformed to the standards set by the Declaration of Helsinki for human studies. This study took place at Henri Mondor University Hospital (APHP), Créteil (France) and was directed by the University Hospital of Saint-Etienne (France). We excluded from the study patients that had (a) experienced a vaso-occlusive episode in the 6 weeks preceding the protocol, (b) transfused for less than 3 months or (c) been receiving cardiac antiarrhythmic therapy or medical treatment modifying the activity of the ANS: beta-blockers, sympathomimetic, atropine or patients that had (d) AIDS, (e) any illness, particularly unresolved inflammatory illnesses, in the previous month, (f) any hemoglobinopathy other than SCD, (g) cardiac arrhythmia, (h) a pacemaker or (i) type I or II diabetes. Patients who were morbidly obese (BMI above 35), pregnant, postpartum or breastfeeding and patients that either did no adhere to the protocol or did not meet the inclusion criteria were also excluded.
| Variables | All n = 32 | Non-training patients n = 18 | Training patients n = 15 | P value |
|---|---|---|---|---|
| Age, years | 34.1 (1.7) | 33.6 (2.1) | 34.7 (2.9) | .738 |
| Males, n | 18 (54.5%) | 10 (55.6%) | 8 (53.3%) | .898 |
| BMI, kg/m2 | 22.0 (0.5) | 22.2 (0.6) | 21.8 (0.7) | .682 |
| Hemoglobin, g/dL | 9.2 [8.3; 9.7] | 9.2 [8.2; 9.7] | 9.3 [8.6; 10.4] | .677 |
| Hematocrit, % | 27.4 (0.7) | 27.9 (0.9) | 26.8 (1.2) | .253 |
| Hemoglobin S, % | 83.4 [76.3; 87.8] | 84.8 [76.1; 88.6] | 80.3 [77.3; 87.3] | .518 |
| Hemoglobin F, % | 5.2 [2.9; 11.3] | 5.2 [3.0; 6.9] | 5.9 [3.2; 12.0] | .459 |
- Note: Results are expressed as mean (SEM), n (% of population) or median [IQR].
2.2 Study design
Included SCD patients had a biopsy of the vastus lateralis muscle and performed a submaximal incremental cycling test (SIT) as previously described.24, 25 Then patients were randomly assigned either to a training (n = 20) or non-training control (n = 20) group. Subsequently, patients of the training group completed an 8-week endurance exercise training program while patients of the non-training group were asked to maintain their usual lifestyle. After this 8-week period, the measurements performed before randomization were repeated.
2.3 Muscle biopsies
Muscle biopsies were performed using the percutaneous technique26 and were obtained from the vastus lateralis. After shaving and asepsis (10% iso-Betadine, MEDA Pharma, Paris, France), local anesthesia of cutaneous and subcutaneous tissues was performed using 2% lidocaine (AstraZeneca, Rueil-Malmaison, France). Then a small incision (not exceeding 8 mm) was made, cutting skin and muscular aponeurosis. Subsequently, a Weil-Blakesley forceps (Lawton, Tittlingen, Germany) was inserted and approximately 150 to 200 mg of muscle was extracted. Hemostasis was then ensured by 5 minutes of compression. Access to the muscle was then closed by sterile strips. Part of the biopsy sample that contained well-identified fascicles were oriented under a stereo microscope and mounted in cryomount (Histolab, Göteborg, Sweden), then frozen in isopentane (Chevron Phillips Chemicals International, Overijse, Belgium) and finally stored in liquid nitrogen until histochemical and immunohistochemical analyses were performed. The remainders of the samples were frozen and stored in liquid nitrogen until further biochemical analysis was performed. The post-training period muscle biopsy was performed 2 to 3 cm proximal to the pre-training biopsy.
2.4 Submaximal cardiopulmonary exercise test on cycle ergometer
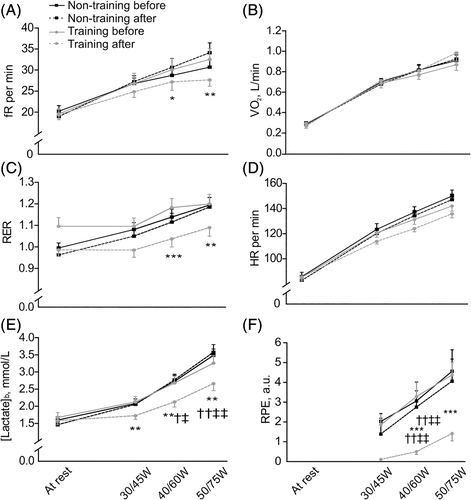
SITs were performed on a cycle ergometer (Ergoselect Confort, General Electric, WA) and started at a workload of 20 or 30 W (W) and increased every 2 minutes by 10 or 15 W for females and males, respectively. Cardiopulmonary parameters were continuously recorded (ErgoCard, Medisoft, Sorinnes, Belgium) while blood lactate concentration ([lactate]b) (Lactate Scout+, EKF diagnostics, Cardiff, UK) was assessed every minute. Approaching the end of each stage, rating of perceived exertion (RPE) was recorded. Exercise was stopped as soon as a [lactate]b ≥ 4 mmol/L was recorded. [lactate]b and the cut-off concentration of 4 mmol/L were chosen because (a) the close association between blood acidosis and [lactate]b is well documented10, 27-29 suggesting that [lactate]b is as a good biomarker/surrogate for blood acid/base status changes, (b) [lactate]b can be measured within seconds (while pH measurements would require minutes), enabling a rapid decision on when to terminate exercise,25 (c) acidosis may rapidly develop since [lactate]b quickly accumulates when exercise intensity is further increased beyond 4 mmol/L30 and (d) complications may occur if blood acidosis progresses.13 Exercise was followed by 2 minutes of active recovery and 8 minutes of passive recovery. This protocol was used to identify pulmonary, circulatory, metabolic and psychologic parameters at different stages/absolute power outputs and/or at the first lactate threshold (LT1, which is delineated by the first inflection point of [lactate]b vs work rate curve) as previously described.25 LT1 indicates the transition from moderate to intense (or heavy) exercise.31 Because LT1 occurs at very low exercise intensities12, 24, 25, 32 that correspond to tasks of everyday living (including home activities)33, 34 in patients with SCD, LT1 may be a good maker of autonomy and quality of life of SCD patients.15 Investigated parameters at LT1 included respiratory rate (fR), minute ventilation (V˙E), oxygen uptake (V˙O2), respiratory exchange ratio (RER), heart rate (HR), [lactate]b and RPE.
2.5 Endurance exercise training program
On the cycle ergometer, patients in the training group performed 3 moderate-intensity endurance exercise training sessions per week for 8 weeks. The training workload was adjusted to target [lactate]b of 2.5 mmol/L. Each session consisted of (a) 5 minutes of warm-up (at 70% of the training workload), (b) 30 minutes of constant-load endurance exercise (at 100% of the training workload), (c) 5 minutes of cool-down (at 70% of the training workload) and then (d) 5 minutes of light stretching. Particular attention was paid to hydration of patients. To that purpose, patients were encouraged to drink regularly and water was provided ad libitum. A physician was present for clinical observation of patients at every training session. Blood pressure, peripheral oxygen saturation, [lactate]b and RPE were measured during each training session. If necessary, the exercise workload was modified between sessions so that [lactate]b during training approximately matched the target value of 2.5 mmol/L as previously described.24, 25
2.6 Muscle analysis
2.6.1 Muscle fiber type and morphometric analyses
Fiber type determination and morphometric analysis were performed by immunofluorescence on two serial cross-sections (Figure 1A) as described by Meunier and colleagues.35 Anti-laminin-α1 (Sigma, Saint-Quentin-Fallavier, France) was conjugated to Alexa-Fluor 488 (Invitrogen, Cergy-Pontoise, France) to identify the extracellular matrix. Additionally, monoclonal antibodies against myosin heavy chain-I (MHC-I) (BA-D5, DSHB) or myosin heavy-chain-IIA (MHC-IIA) (N2.261, Enzo life Sciences) were conjugated to Alexa-Fluor 546 (Invitrogen, Cergy-Pontoise, France) for fiber type determination. Images were captured with a high-resolution cooled digital DP-72 camera coupled to a BX-51 microscope (Olympus, Rungis, France) at a resolution of 0.64 μm/pixel. For each patient and condition, approximately 3 representative fields of 120 fibers were selected. Using the image processing software Visilog-6.9 (Noesis, Gif-sur-Yvette, France), contractile type (I, I-IIa, IIa, IIa-IIx or IIx) and muscle fiber cross-sectional area by type were determined. For cross-sectional area, only three fiber types were considered (I, IIa and IIx). Type I-IIa and IIA-IIx fibers were integrated into type I and IIx fibers, respectively.
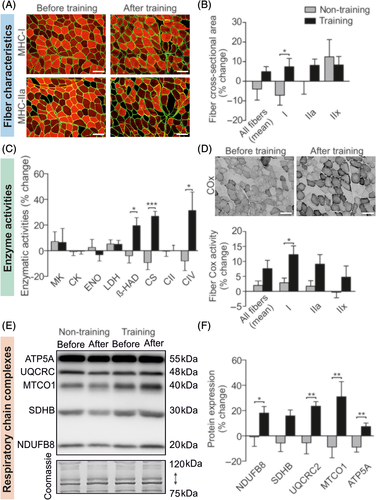
2.6.2 Enzyme activity
Approximately 20 mg of muscle were homogenized in 400 μL of buffer (Manitol 225 mM, tris HCL 10 mM, saccharose 75 mM, EDTA 0.1 mM) with a tissue homogenizer (Ultra-Turrax T8 IKA-Werke, Staufen, Germany). Samples were centrifuged (20 minutes, 650 g, 4°C) and the supernatant was carefully collected. The pellet was re-homogenized in 2/3 of the previous buffer and centrifuged a second time (20 minutes, 650 g, 4°C) with the previously-collected supernatant. The protein concentration was determined with bovine serum albumin (BSA) as a standard (Pierce BCA Protein Assay Kit, Thermo Scientific, Waltham, MA) and the supernatants were stored at −80°C until subsequent analyses were performed. Maximal activities of myokinase (MK), creatine kinase (CK), enolase (ENO), lactate dehydrogenase (LDH), CS, β-HAD and second (CII) and fourth (CIV) respiratory chain complexes (UI/mg of protein) were determined by absorbance with a multi-label microplate reader (CLARIOstar, BMG LABTECH GmbH, Germany).
2.6.3 Histoenzymology
COx activity was determined histochemically on serial transverse sections. Slices for COx assessment were incubated for 120 minutes at 37°C in 0.05 M phosphate buffer (pH 7.3) containing 20 mg of 3,3-diaminobenzidine tetrahydrochloride (Sigma-Aldrich), 140 mg of cytochrome c (Sigma Biochemical, Poole, UK), 3 g of saccharose (Carlo Erba Reactive-SDS) and 4 mL of catalase (Sigma-Aldrich) solution. After incubation, slices were rinsed three times in distilled water and dehydrated in three different alcohol baths. Measurement of COx optical density was performed using Visilog-6.9 software. COx and MHC images were then matched to analyze the COx activity per fiber type (I, IIa, IIx). As with the fiber cross-sectional area, type I-IIa and IIa-IIx fibers were integrated into type I and IIx fibers, respectively.
The double COx/SDH (succinate dehydrogenase) labeling/staining was also assessed to visualize muscle fibers with mitochondrial dysfunction (blue fiber) [COx-deficient (COx-) and SDH-normal (SDH+)]. Slices were first incubated in the COx medium for 120 minutes at 37°C and then rinsed in distilled water. Slices were then incubated in SDH medium for 120 minutes at 37°C in 0.2 M phosphate buffer (pH 7.0) containing 20 mg of nitroblue tetrazolium. After incubation, slices were rinsed three times in distilled water and dehydrated in three different alcohol baths. Fibers with normal COx activity produced a brown reaction product whereas fibers with reduced or absent COx activity produced the SDH blue reaction product (COx-/SDH+).
2.6.4 Western blots
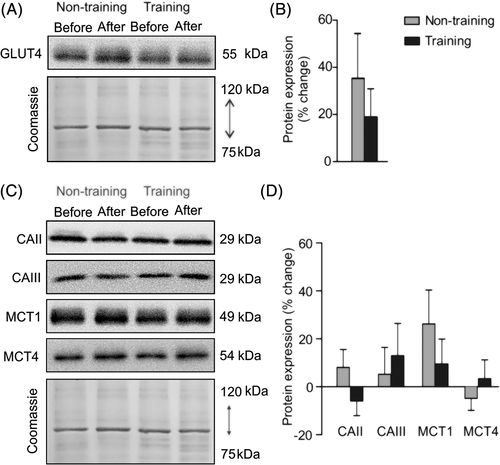
Approximately 20 mg of muscle was homogenized with a Polytron homogenizer (PT 1200 E, Kinematica, Switzerland) in an ice-cold buffer containing 20 mM Tris, pH 7.0, 270 mM sucrose, 5 mM EGTA, 1 mM EDTA, 1% Triton X-100, 1 mM sodium orthovanadate, 50 mM sodium-glycerophosphate, 5 mM sodium pyrophosphate, 50 mM sodium fluoride, 1 mM DTT and a protease inhibitor cocktail containing 1 mM EDTA (Roche Applied Science, Vilvoorde, Belgium). The samples were centrifuged for 10 minutes at 10000 g at 4°C. Supernatants were collected and used for protein concentration assay with BSA as a standard (Pierce BCA Protein Assay Kit, Thermo Scientific) and subsequent Western blot analysis. Protein samples of 10 μg were combined with Laemmli sample buffer and warmed for 5 minutes at 95°C (except for total oxphos warmed for 3 minutes at 55°C). Proteins were separated by SDS-PAGE (10%-12% resolving gels) for 2 hours at a constant intensity of 40 mA and transferred on polyvinylidene fluoride membranes at 80 V for 2.5 hours. After blocking for 1 hour in Tris-buffered saline plus 0.1% Tween 20 (TBST) containing 5% non-fat dry milk, membranes were incubated at 4°C overnight with one of the following antibodies: total oxphos complexes (ab110413, Abcam, 1:1000) against [complex I (CI) subunit NDUFB8, CII subunit SDHB, complex III (CIII) subunit UQCRC2, CIV subunit MTCO1 and complex V (CV) subunit ATP5A], GLUT4 (MA5-17176, Thermo Fisher Scientific, 1:1000), CAII (carbonic anhydrase II, BIRBORB10230, VWR, 1:250), CAIII (carbonic anhydrase III, BSBTPA1439, VWR, 1:1000), MCT1 (AB3538P, Merck Millipore, 1:1000) and MCT4 (AB3316P, Merck Millipore, 1:1000). Membranes were washed three times for 10 minutes in TBST, then incubated for 1 hour at room temperature with a secondary antibody conjugated to horseradish peroxidase-conjugated antibody raised against rabbit (A6154; Sigma-Aldrich) or mouse (610-1319; Rockland Immunochemicals, Limerick, PA). Three additional washes were performed before chemiluminescence detection with the ECL-Plus Western blot kit (Advansta, Menlo Park, CA). Pictures were taken with a GBox (Syngene, Cambridge, United Kingdom) using GeneSnap software. ImageJ software (National Institutes of Health, Bethesda, MD) was used for quantifications. Specific signals were normalized against total loaded protein determined by Coomassie blue staining.
2.7 Outcomes
The primary and secondary outcomes have previously been reported24 and were the changes (from baseline to the end of the 8-week intervention period) in the power output at (a) 4 mmol/L [lactate]b and (b) LT1, determined during SITs, respectively. After 8 weeks, mean power output at 4 mmol/L [lactate]b and LT1 were improved for training patients but not for non-training patients.24 The present study focused on the analysis of other secondary endpoints that were the (a) changes in fiber type distribution, (b) cross-sectional surface area of type I, IIa and IIx muscle fibers, (c) muscle enzyme activities and (d) muscle content of respiratory chain complexes and proteins involved in glucose and lactate transport and pH regulation. The changes in integrative physiologic and metabolic parameters were also considered.
2.8 Statistical analysis
Descriptive results are presented as mean (SE of the mean, SEM) or median [inter-quartile range, IQR]. The relative mean change between groups (non-training vs training) was assessed using T-tests for independent groups (normal data) or Mann-Whitney tests (non-normal data). Normality was assessed with the Shapiro-Wilk test. Categorical variables, presented as number (n) (% of population), were compared with Fisher's exact test. For the illustration of pulmonary, circulatory and psychologic data, two-factor ANOVAs [groups (non-training and training) × time (before and after the experiment)] were performed. When a main effect or a significant interaction was found, a post-hoc analysis was made using Fisher's test. The critical level for statistical significance was set at an α-level of 0.05. Analyses were computed with Statistica 8 (Statsoft, Tulsa, OK). For muscle structural and energetic characteristics, some patients were excluded from the analyses because of the poor quality of the biopsy or extracted samples.
3 RESULTS
Power output and V˙O2 at LT1, [lactate]b at 40 or 60 W (for women and men, respectively), CS and type I fiber COx activities have previously been reported24 but are repeated here for discussion and the convenience of the reader.
3.1 Baseline data and comparisons
At baseline, no significant between-group differences were found for anthropometric, hemoglobinic or hematologic data (Table 1). Neither muscle structural and energetic characteristics or integrative data measured at baseline were different between groups (Table S1). Fifteen [eight in the non-training group and seven in the training group (no difference between groups, Table S1)] of 32 SCD patients (47%) displayed some muscle fibers with COx-/SDH+ (blue fibers), suggesting mitochondrial dysfunction at baseline.
3.2 Training impact on muscle fiber characteristics
No alterations in muscle fiber type distribution were observed in either group over the 8-week period (Table S2). The mean cross-sectional area of muscle fibers was unchanged in both groups and the per fiber-type analysis showed that type I fiber cross-sectional area increased in the training group relative to the non-training group (P = .038; Figure 1B). Muscle cross-sectional surface area of type IIa and IIx fibers were unchanged and did not differ between groups (Table S2).
3.3 Adaptations in muscle enzyme activities
No changes or differences between groups were observed for MK, CK, ENO, LDH or CII activities (Figure 1C). However, β-HAD, CS and CIV activities increased in the training group relative to the non-training group (P = .009, P < .001 and P = .017, respectively; Figure 1C). The change in CIV/CII activity ratio increased in the training group relative to the non-training group (P = .026; Table S2). COx activity increased in the training group relative to the non-training group in type I (P = .042; Figure 1D) but not in other fiber types. After the 8-week period, the number of SCD patients that displayed some muscle fibers with COx-/SDH+ was unchanged.
3.4 Changes in muscle content of respiratory chain complexes
The muscle content of CI, CIII, CIV and CV subunits of the respiratory chain increased in the training group relative to the non-training group (P = .049, P = .005, P = .003 and P = .002, respectively, Figure 1E-F; Table S2). No changes were reported for the content of the CII subunit or CIV/CII subunit ratio (Figure 1E-F; Table S2).
3.5 Changes in muscle content of proteins involved in glucose and lactate transport and pH regulation
The muscle content of GLUT4, MCT1, MCT4, CAII and CAIII did not change in the training group relative to the non-training group (Figure 2; Table S2).
3.6 Changes in integrative physiologic and metabolic parameters
At rest, fR, V˙E, V˙O2, RER, HR and [lactate]b were unchanged in the training group relative to the non-training group. At the second stage of SIT (30 W for women and 45 W for men), [lactate]b (P = .022) and RPE (P = .010) decreased in the training group relative to the non-training group. All other parameters were unchanged at the second stage. At stages 3 and 4 (40 W and 50 W for women and 60 W and 75 W for men, respectively), fR (P = .019, P = .002, respectively), RER (P = .005, P = .029, respectively), [lactate]b (P = .009, P = .011, respectively) and RPE (P = .003, P = .018, respectively) decreased in the training group relative to the non-training group. No changes were reported for V˙E, V˙O2 or HR for stages 3 or 4 (Figure 3, Table S2). In addition, at the first lactate threshold, an increase in power output and V˙O2 were reported in training patients compared to non-training counterparts (P < .001 and P < .001, respectively; Table S2).
3.7 Effect of sex in training-induced adaptations
The changes in type I fiber type distribution and in type IIx fiber COx activity in response to endurance training were significantly higher in training men compared to training women (P = .009, P = .031, respectively; Table S3). No changes were reported between training women and men for any other muscle characteristics or integrative data (Table S3).
4 DISCUSSION
The present study is the first clinical randomized controlled trial to evaluate changes in muscle structural and energetic characteristics induced by an 8-week individualized moderate-intensity endurance training program and their effects on physiological adaptations with graded physical exercise in adult SCD patients. The main findings are that endurance exercise training (a) increased the surface area of type I muscle fibers, (b) improved the muscle oxidative enzyme activities and content of several subunits of the respiratory chain, (c) did not alter the muscle content of proteins involved in glucose and lactate transport or pH regulation and (d) improved indexes of physical ability. Together, these results support our hypothesis that moderate-intensity endurance exercise training is beneficial for muscle morphology, energetics and function. In addition, the lack of change in muscle content of glucose and lactate transporters suggests that the training program did not challenge muscle energetics or pH regulation.
4.1 Muscle structural characteristics of adult SCD patients at baseline and after endurance exercise training
Fiber type distribution and mean cross-sectional areas in the French SCD patients of the present study at baseline were similar to those obtained in stable conditions (at distance from any VOC) in Cameroonian SCD patients.6 After 8 weeks of moderate-intensity endurance exercise training, patients displayed a slight increase in type I fiber cross-sectional area compared to controls. These findings are in agreement with several studies demonstrating the ability of endurance training to increase muscle type I fiber surface area in healthy and pathological populations.16, 18 However, compared to these previous studies,16, 18 the increase in type I fiber cross-sectional area was relatively modest in the present study. This lower response was expected and can mainly be attributed to the relatively low training load performed. Indeed, to maintain patient safety, the present endurance exercise training program was only comprised of short-duration moderate-intensity training sessions. The low training load also explains the lack of adaptation in other fiber types. Indeed, only a very small portion of type II fibers are recruited during moderate-intensity exercise.36 Nevertheless, the small increase in type I fiber surface area is of importance because it suggests that muscle hypotrophy in SCD patients6 can be counteracted and minimized even by moderate-intensity training or activity. This is of utmost importance for patients' functional aptitude for daily life, well-being24 and quality of life.15
4.2 Muscle oxidative capacity at baseline and after endurance exercise training
SCD is associated with altered muscle oxidative capacity compared to healthy persons.6 This has been evidenced by lower activities of enzymes β-HAD, CS and COx, which influence fatty acid beta-oxidation, the Krebs cycle and the respiratory chain, respectively.6 In the present study, the coupled COx/SDH histochemical analyses revealed that 47% of SCD patients displayed some COx-deficient but SDH-normal muscle fibers at baseline. This abnormality was observed in the oldest SCD patients (39 vs 30 years old; P < .01, data not shown). The presence of COx-deficient SDH-normal muscle fibers is usually observed with human aging37 and in a variety of clinical conditions38-40 and indicates mitochondrial dysfunction.41 Therefore, this result constitutes an additional argument for altered muscle oxidative capacity in SCD patients that can play a key role in their exercise intolerance.6, 15
After 8 weeks of moderate-intensity endurance exercise training, SCD patients displayed improved muscle oxidative capacity. Patients in the training group had increased (a) activity of key enzymes of oxidative metabolism (CS, β-HAD, CIV), (b) COx activity of type I fibers, that is, the muscle fibers specifically recruited during this type of endurance training and (c) muscle content of CI, CIII, CIV and CV respiratory chain subunits. Because muscle oxidative capacity is associated with muscle mitochondrial volume density,42 we can infer that our training program stimulated mitochondrial biogenesis in the training SCD patients, a common training-induced adaptation in health and disease.43 In addition, the large increase in the CIV/CII enzyme activity ratio may indicate better efficiency of the respiratory chain in training patients. Further investigations are needed to confirm the underlying mechanisms of the mitochondrial adaptations. As a whole, higher muscle oxidative capacity (enzyme activity) after endurance training clearly indicates a greater ability to utilize oxygen at the muscle level. After the training program, non-oxidative glycolytic enzyme activities (ENO and LDH) were unchanged. As a result, the enzyme activity ratios β-HAD/ENO (+28%, P < .001), CS/ENO (+35%, P = .006) and CIV/ENO (+37%, P = .008) strongly increased in training SCD patients compared to non-training controls (data not shown). Taken together, the results of enzyme activities indicate that muscle energetic metabolism in SCD training patients relies more on oxygen-derived pathways and that the imbalance between glycolytic and oxidative pathways observed in previous reports,44 including in SCD mice,45 is reduced after training. The lower blood lactate accumulation for a given power output reported in the present study results, at least in part, from these enzymatic and metabolic adaptations. The greater post-training muscle oxidative capacity (greater capacity for muscle to utilize oxygen) may also result from improved oxygen supply to skeletal muscle via the densified capillary network we observed in the present study after training.24
4.3 Endurance exercise training did not modify the muscle content of proteins involved in glucose and lactate transport or pH regulation
In contrast to our hypothesis, the present endurance exercise training program did not improve the content of glucose (GLUT4) or lactate (MCT1, MCT4) transporters in human skeletal muscle. The low exercise intensity used in the present study may explain the lack of change in substrate transporter content.20 Indeed, exercise intensity is a key factor for transport system adaptations.46, 47 The lack of adaptations also indicates that exercise intolerance at baseline was not driven by limitations in energy substrate supply necessary for muscle contraction.
Lactate transport via MCT1 and MCT4 is coupled with H+, meaning that MCT1 and MCT4 are also involved in muscle pH regulation. In the present study, we completed our analysis by quantifying muscle content of CAII and CAIII, two intramyocellular carbonic anhydrases buffering protons with bicarbonate. Collectively, MCT1, MCT4, CAII and CAIII act as a first line of defense against muscle acid/base disturbances, especially during exercise.20, 46 Several studies have demonstrated that high-intensity exercise challenges myocellular pH and that high-intensity training sessions improve mechanisms of myocellular pH regulation via increases in the content of MCTs and CAs in muscle.48, 49 The lack of change in MCT and CA content in the present study suggests that our training protocol did not lead to specific adaptations in muscle pH regulatory mechanisms, validating the goal of our training program, which was to not disturb acid/base balance during exercise for the safety of the patients.13
4.4 Endurance exercise training improves integrative physiologic and metabolic parameters and overall physical ability in adult SCD patients
The improved muscle oxidative capacity we observed in endurance-trained SCD patients may explain, at least partly, the lower [lactate]b and RER after training. It is also noticeable that fR decreased for a given power output after training. This demonstrates that ventilatory function was improved by endurance training in adult SCD patients. Importantly, RPE was also lower at a given power output after endurance training in SCD patients. In other words, a given (submaximal) physical task was perceived to be less difficult after training. This clearly indicates better exercise tolerance after endurance training. As a whole, these adaptive responses to endurance exercise training (lower [lactate]b, RER, fR and RPE) suggest that exercise-related physiological stress is reduced for a given task after endurance training.
Indexes of physical ability improved in the training patients. More specifically, endurance training increased power output and oxygen uptake at the first lactate threshold. Taken together, the present results support the idea that the observed muscle structural and metabolic adaptations could contribute to the improvement of physical ability in the training patients.
4.5 Sex did not modulate training-induced adaptations
Some studies have shown that women with SCD display higher foetal Hb (HbF) and lower HbS levels, more severe anemia, better peripheral oxygen saturation and both greater nitric oxide (NO) bioavailability and NO responsiveness than men with SCD.50-53 Since these differences may modulate the exercise-related physiological responses between men and women,11, 51, 54 an effect of sex in exercise-induced adaptations can be suspected. In the present study, among all muscle characteristics or integrative data analyzed (ie, 62), only two were differently changed by training between men and women, suggesting that sex did not drastically influence training-induced adaptations. However, the small number of patients investigated in the present study requires us to be cautious. Further studies are warranted.
4.6 Clinical implications and perspectives
This study demonstrates meaningful benefits of a moderate-intensity endurance exercise training program in SCD patients. Indeed, the hypotrophy and altered oxidative capacity previously reported in SCD patients6 are at least partially counteracted by the proposed training regimen. Based on both previous24, 55 and present findings, this intervention appears to improve muscle microvascular, structural and energetic characteristics, muscle function, physical ability, exercise tolerance and some parameters of quality of life of SCD patients.
Many studies testify to the beneficial effects of regular physical exercise on cardiovascular and metabolic profiles of patients.56, 57 By increasing energy expenditure, namely utilization of glucose and fat as energy substrates, physical training contributes (a) to reduce insulin resistance, hyperglycemia and hyperlipidemia, (b) to ameliorate insulin sensitivity, glucose uptake/transport (via GLUT4) and blood lipid profile and (c) to prevent metabolic syndrome, type 2 diabetes, obesity, hypertension and cardiovascular disease.56, 57 From that point of view, regular physical exercise may be of paramount importance for sedentary patients potentially at risk for cardiovascular and metabolic disease as in the case of SCD.58-60 However, this point will need to be specifically investigated.
The low bioavailability of NO is implicated in the pathophysiology of SCD.61, 62 Nitrate supplementation, which improves production of NO by the NOS-independent pathway, may be helpful in the context of exercise where vasomotion is of paramount importance. Besides, nitrate supplementation reduces the oxygen consumption during submaximal exercise,63 possibly via a reduced ATP cost of muscle force production64 and an improved mitochondrial efficiency.65 This point is very important because SCD patients already display lower O2 consumption for a given work rate than controls.23, 25 At the present time, we do not know the real meaning of this lower O2 uptake vs power output slope in SCD patients, but it is tempting to interpret it as a lower ability to supply and consume O26 rather than a lower O2 cost/better efficiency in SCD. Therefore, if nitrate supplementation decreases muscle O2 needs, this intervention might help the patients to match oxygen supply with needs and therefore to cope with the exercise requirement. However, nitrate supplementation in the context of exercise in SCD patients has yet to be investigated. These hypotheses might constitute interesting perspectives.
It is not known if a training program of longer duration may have led to greater improvements in the training group. Therefore, further research is needed to assess the long-term effects of moderate-intensity endurance training in SCD patients. There is also the need to study a larger cohort of SCD patients with more diverse (including more severe) clinical profiles.
5 CONCLUSION
In conclusion, the present study demonstrated for the first time the beneficial effects of an 8-week moderate-intensity endurance exercise training program on skeletal muscle morphology, energetics and function in SCD patients without severe chronic complications. These benefits include (a) increases in type I muscle fiber surface area and muscle oxidative capacity (oxidative enzyme activities and content of respiratory chain subunits in muscle) and (b) decreases in blood lactate concentration, RER, fR and RPE at a given power output. These findings demonstrate improved working capacity and better exercise tolerance in the training patients. Besides, the lack of changes observed in glucose and lactate transport and some mechanisms of pH regulation suggest that substrate supply is not the limiting factor at baseline and that acid/base balance was not challenged during the training program. The lack of change in acid/base balance indicates the safety of this mode of training for SCD patients. The safety and benefits of endurance training demonstrated in the present study in SCD patients suggest that endurance training is a potential therapy strategy in SCD.
6 ACKNOWLEDGMENTS
We thank John Temesi for editorial assistance (Faculty of Health and Life Sciences, Northumbria University, Newcastle, UK). We thank Marie-Pierre Blanc, Dominique Gouttefangeas and Marion Ravelojaona (Myology Unit, Referent Center of Rare Neuromuscular Diseases, Euro-NmD, University Hospital of Saint-Etienne, France) for technical assistance.
7 CONFLICT OF INTEREST
P.B. and L.A.M. declare no conflict of interests for this study but report conflict of interests related to the general field of sickle cell disease. Others authors declare that there are no competing interests associated with the manuscript.
8 AUTHOR CONTRIBUTION
L.A.M., L.F., B.G., F.G. and P.B. designed and conducted this study. C.H., L.D., M.F. and A.N.M. designed muscle analyses. C.S. provided determinant technical assistance. A.N.M., performed the analyses. A.N.M. and L.A.M. wrote the first draft of the article. All authors critically reviewed the draft and approved the final version for publication.




