High sensitivity M-protein detection in a case of light-chain cardiac amyloidosis without evidence of plasma cell dyscrasia
1 CASE PRESENTATION
Sixty-three-year-old female presented with progressive shortness of breath and pedal edema over 6 months. Her only comorbidity was hypertension, which was well controlled. Physical examination revealed signs of congestive heart failure. ECG displayed sinus rhythm with low voltage. Echocardiography showed severe concentric left ventricular thickening of 17 mm, bi-atrial enlargement and impaired diastolic dysfunction. The patient's well controlled hypertension and the absence of other contributing comorbidities did not account for the relatively rapid course of her symptoms. Interestingly, her echocardiographic global longitudinal strain (GLS) was severely reduced (Figure 1 A), with a strain pattern typical for cardiac amyloidosis.1 Furthermore, late gadolinium enhancement on cardiac magnetic resonance was pathognomonic for cardiac amyloidosis. A diagnosis of cardiac amyloidosis was made and workup was initiated to identify the sub-type.
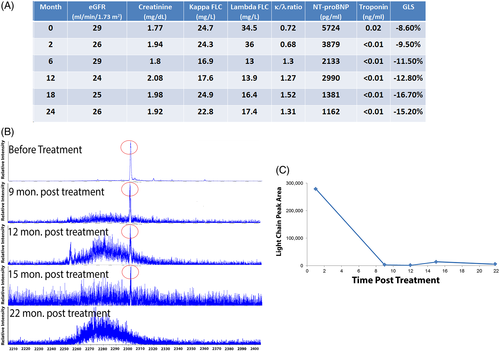
Additional laboratory testing (Figure 1 A) revealed impaired renal function and elevated cardiac biomarkers. Despite the clinical evidence implicating amyloidosis, serum and urine protein electrophoresis (PEL) and immunofixation electrophoresis (IFE) repeatedly failed to detect any monoclonal protein (M-protein) clones. Both kappa (κ) and lambda (λ) FLC serum levels were elevated with a normal κ/λ FLC ratio, consistent with impaired renal excretion. However, an unusual feature was a higher λ than κ FLC, as opposed to expected disproportionate elevation of κ FLC with renal impairment—potentially indicating an overproduction of λ FLC. A bone marrow biopsy showed normal plasma cell percentage without evidence of an expanded clonal population.
Technetium pyrophosphate scanning, which is nearly 100% sensitive and specific for transthyretin amyloidosis2 was performed, and was negative with no radiotracer uptake in the heart. Ultimately, an endomyocardial biopsy stained positive for amyloid and mass spectrometry typing demonstrated λ AL amyloidosis—despite the absence of standard markers of a plasma cell dyscrasia. These findings strongly supported light chain (AL) amyloidosis as the cause of the patient's symptoms.
(AL) amyloidosis is a systemic plasma cell dyscrasia involving many organs. Without treatment, the median survival time from the onset of congestive heart failure is as short as 6 months, but prompt and profound reduction of the free light chain (FLC) results in rapid improvement of cardiac function and extended survival, even in patients with advanced cardiac damage. Given the aggressiveness of cardiac (AL) amyloidosis, earlier diagnosis is of paramount importance to limit irreversible cardiac damage.3, 4 The absence of a clonal M-protein in serum or urine hampers assessment of treatment response and progression.
Recent data demonstrate that microflow liquid chromatography (micro LC) coupled with electrospray ionization (ESI) and Q-TOF MS (micro LC-ESI-Q-TOF MS) can be used to identify and monitor M-proteins in a patient's serum and urine (a method termed miRAMM).5-7 Relative to PEL, IFE and FLC, miRAMM was demonstrated to have higher analytical sensitivity and specificity, implying that such high accuracy mass measurements can be used to definitively track the clone. This method was applied to a cohort of myeloma patients in “strict complete remission” (sCR) and was able to detect residual M-proteins in 81% of sCR patients 100 days post stem-cell transplant allowing potential prognostic stratification of sCR patients.8
The patient's serum and urine were tested by miRAMM which detected a urinary monoclonal LC peak fragmentation that corresponded to lambda light chain (Figure 1 B). The same monoclonal LC peak was detected in the serum but at a lower relative concentration than urine. These findings provided a reference point to monitor disease progression and response to treatment.
The patient subsequently received chemotherapy (cyclophosphamide-bortezomib-dexamethasone) for 6 monthly cycles. Following chemotherapy, she improved symptomatically, and λ FLC concentration decreased disproportionately causing a reversal of the κ/λ FLC ratio. Cardiac biomarkers and GLS improved (Figure 1A). Urine and serum miRAMM testing was repeated 9 months after starting chemotherapy. The λ LC peak area remarkably decreased in urine by 99% in response to treatment, and was no longer detected in serum. Repeat measurements at 12, 15, and 22 months after initiation of chemotherapy showed maintenance of post chemotherapy low levels of λ LC (Figure 1B,C).
2 DISCUSSION
Serum and urine detection of a M-protein is the standard approach for diagnosing AL amyloidosis. Recommended testing includes serum PEL, immunofixation electrophoresis (IFE), serum FLC measurements and a 24-hour urine IFE.9 A definitive diagnosis requires amyloid detection in a biopsy with protein identification usually by mass spectrometry. Once a diagnosis is made and treatment begins, the response to therapy is evaluated using the FLC concentration and disappearance of M-protein.10 The goal of treatment is eradication of the plasma cell clone producing the amyloidogenic light chain. Complete response (CR) is defined as the disappearance of the involved amyloidogenic FLC with restoration of normal FLC ratio and negative serum and urine immunofixation. Recent data suggest that minimal residual disease, as assessed by next generation flow cytometry, may hinder organ recovery indicating that trace amounts of the amyloid light chain can sustain organ dysfunction.11
The miRAMM method successfully detected a monoclonal LC peak in the patient's urine, and allowed closer interrogation of the patient's serum for the same monoclonal LC peak. The relatively high biologic and analytic variability of FLC measurements and ratios presents a challenge for physicians. In clinically stable patients, the coefficient of variance (CV) of serum monoclonal FLC measurements >100 mg/L is around 29%. About 20% to 80% of this variance is attributed to analytic and biologic factors, respectively.12 At lower baseline monoclonal FLC levels, as in our patient or in non-stable disease, this variability would be even higher. High-resolution mass spectrometry allows direct quantitation of the monoclonal LC apart from the polyclonal background and even therapeutic monoclonal antibodies, and hence decreasing reliance on the less accurate LC ratios. In this case, miRAMM also demonstrated that the LC had related +162 masses which are consistent with LC glycosylation post-translational modifications. Perhaps this glycosylation pattern may have reduced the ability of electrophoretic and immunonephelometric assay to detect the LC.
This technique may be also utilized to risk-stratify patients based on detection of clones or monoclonal LCs that are not detected on standard assays, as we have shown in this report and a previous report by our group in which we were able to detect residual M proteins in 81% of myeloma patients in sCR.8 This is even more relevant for AL amyloidosis as the role of maintenance therapy is not well defined, with different approaches adopted in specialized centers. miRAMM has the potential to guide this decision and identify patients with a low-level clone who may be at-risk for progression or relapse and require maintenance therapy.
In summary, this report illustrates the clinical utility of miRAMM in both diagnosis and monitoring response to therapy in AL amyloidosis, with the potential to risk-stratify patients with a presumed complete hematological response. This report also demonstrates the applicability and higher performance of mass spectrometry methods in the detection of M-proteins. Currently, we are working to bring the miRAMM as an orderable test available for clinicians. Larger studies are needed to validate these findings and assess the impact on clinical outcomes.
AUTHOR CONTRIBUTIONS
D.M., R.H.F., G.M., and D.B. designed and oversaw experiments. A.M.S., A.S., and M.K. performed experiments and wrote manuscript.
CONFLICT OF INTEREST
David Murray and David Barnidge receive royalties from the Binding Site Company.




