The impact of sex on disease phenotype and prognostic thresholds of anemia in myelodysplastic syndromes
Current information on sex-specific genetic (including mutations) and clinical details in myelodysplastic syndromes (MDS) is limited. Similarly, the impact of sex on the use of the conventional 10 g/dl hemoglobin threshold, for prognostication in MDS, has yet to be clarified. The original International Prognostic Scoring System (IPSS) for primary MDS included a hemoglobin level of <10 g/dl as one of its risk variables, alongside of karyotype, bone marrow blast percentage, platelet count of <100 × 109/l and absolute neutrophil count of <1.8 × 109/l).1 The revised IPSS (IPSS-R), published in 2012, retained anemia as an independent prognostic marker and accounted for its severity by distinguishing hemoglobin level of <8 g/dl from that of 8 to <10 g/dl.2 However, neither IPSS nor IPSS-R accounted for the significant differences in hemoglobin levels between men and women. We recently addressed the latter issue in the setting of primary myelofibrosis and were able to show that using a hemoglobin threshold of 10 g/dl undermined the prognostic contribution of lesser degrees of anemia in men, but not in women.3 In the current study, we examined the impact of sex on the distribution of mutations, cytogenetic risk and other clinical features in MDS. In addition, we queried the individual prognostic contribution of red cell transfusion need and hemoglobin level of <10 g/dl, in transfusion-independent cases, in women vs. men with MDS.
The current study was approved by the institutional review board of the Mayo Clinic, Rochester, MN, USA. The diagnosis of primary MDS was according to the 2008 World Health Organization criteria.4 All patients were evaluated at time of their first referral to the Mayo Clinic, which coincided, in most instances, with the time of initial diagnosis. Statistical analyses considered clinical and laboratory parameters obtained at time of initial diagnosis or referral. During estimation of overall survival, patients receiving allogeneic stem cell transplantation were censored at the time of their transplant. Overall survival curves were prepared by the Kaplan–Meier method and compared by the log-rank test. Cox proportional hazard regression model was used for multivariable analysis. P values of less than .05 were considered significant. The JMP® Pro 13.0.0 software from SAS Institute, Cary, NC, USA, was used for all calculations.
A total of 357 consecutive patients (median age 74 years; 70% males) with primary MDS were considered; the study population included patients from recently published documents.5, 6 IPSS-R risk distribution was 11% very high, 15% high, 17% intermediate, 40% low, and 16% very low. Cytogenetic risk distribution, according to the recently described Mayo model,5 was 11% high, 30% intermediate, and 59% low. Next-generation sequencing-derived mutation information was available in all patients and the most frequent were SF3B1 (32%), ASXL1 (27%), TET2 (24%), U2AF1 (15%), DNMT3A (14%), SRSF2 (13%), TP53 (12%), and RUNX1 (10%). Additional data on clinical and laboratory parameters at presentation are outlined in Table 1.
| Variables | All patients (n = 357) | Males (n = 250; 70%) | Females (n = 107; 30%) | P value |
|---|---|---|---|---|
| Age; median years (range) | 74 (28–96) | 74 (35–94) | 73 (28–96) | .09 |
| Age >70 years; n (%) | 225 (63) | 162 (65) | 63 (59) | .29 |
| Hemoglobin, g/dl; median (range) | 9.6 (6.8–14.8) | 9.4 (6.8–14.8) | 9.9 (6.8–14.1) | .5 |
| Hemoglobin categories; n (%) | .59 | |||
| Transfusion dependent | 134 (38) | 98 (39) | 36 (34) | |
| Transfusion independent but <10 g/dl | 101 (28) | 68 (27) | 33 (31) | |
| ≥10 g/dl | 122 (34) | 84 (34) | 38 (36) | |
| Presence of anemia using sex-adjusted reference range; n (%) | 342 (96) | 243 (97) | 99 (93) | .04 |
| Leukocytes, × 109/l; median (range) | 3.8 (0.6–37.1) | 3.6 (0.6–37.1) | 4.4 (1.1–31.9) | .03 |
| ANC, × 109/l; median (range) | 1.8 (0.02–20.4) | 1.8 (0.02–13.3) | 1.9 (0.08–20.4) | .02 |
| Platelets, × 109/l; median (range) | 116 (4–667) | 108 (4–667) | 143 (7–599) | |
| Platelet categories: n (%) | .38 | |||
| Platelets <50 × 109/l | 74 (21) | 55 (22) | 19 (18) | |
| Platelets 50-<100 × 109/l | 86 (24) | 63 (25) | 23 (22) | |
| Platelets ≥100 × 109/l | 197 (55) | 132 (53) | 65 (61) | |
| Circulating blasts %; median (range) | 0 (0–18) | 0 (0–18) | 0 (0–9) | .09 |
| Circulating blasts ≥1%; n (%) | 49 (14) | 40 (16) | 9 (8) | .047 |
| BM blasts %; median (range) | 2 (0–19) | 2 (0–19) | 1 (0–18) | .02 |
| BM blasts ≥10%; n (%) | 43 (12) | 36 (14) | 7 (7) | .03 |
| IPSS-R prognostic risk categories; n (%) | .02 | |||
| Very low | 58 (16) | 31 (12) | 27 (25) | |
| Low | 143 (40) | 99 (40) | 44 (41) | |
| Intermediate | 62 (17) | 50 (20) | 12 (11) | |
| High | 55 (15) | 42 (17) | 13 (12) | |
| Very high | 39 (11) | 28 (11) | 11 (10) | |
| Mayo cytogenetic risk categorya | .03 | |||
| High (monosomal karyotype) | 38 (11) | 26 (10) | 12 (11) | |
| Intermediate (not high or low) | 108 (30) | 86 (34) | 22 (21) | |
| Low (normal or 5q-) | 211 (59) | 138 (55) | 73 (68) | |
| Mutations | ||||
| SF3B1 mutated; n (%) | 113 (32) | 74 (30) | 39 (37) | .2 |
| ASXL1 mutated; n (%) | 98 (27) | 79 (31) | 19 (18) | .006 |
| TET2 mutated; n (%) | 86 (24) | 58 (23) | 28 (26) | .55 |
| U2AF1 mutated; n (%) | 52 (15) | 46 (18) | 6 (6) | <.001 |
| DNMT3A mutated; n (%) | 50 (14) | 35 (14) | 15 (14) | .99 |
| SRSF2 mutated; n (%) | 47 (13) | 38 (15) | 9 (8) | .07 |
| TP53 mutated; n (%) | 43 (12) | 28 (11) | 15 (14) | .46 |
| RUNX1 mutated; n (%) | 36 (10) | 30 (12) | 6 (6) | .053 |
| IDH2 mutated; n (%) | 14 (4) | 12 (5) | 2 (2) | .16 |
| EZH2 mutated; n (%) | 16 (4) | 13 (5) | 3 (3) | .3 |
| IDH1 mutated; n (%) | 12 (3) | 10 (4) | 2 (2) | .28 |
| CBL mutated; n (%) | 12 (3) | 7 (3) | 5 (5) | .38 |
| SETBP1 mutated; n (%) | 10 (3) | 8 (3) | 2 (2) | .47 |
| CEBPA mutated; n (%) | 10 (3) | 7 (3) | 3 (3) | .99 |
| CSF3R mutated; n (%) | 9 (3) | 6 (2) | 3 (3) | .83 |
- Only mutations with frequency of at least 2% were included.
- Abbreviations: ANC, absolute neutrophil count; IPSS-R, revised international prognostic scoring system; BM, bone marrow.
- a Mayo high risk cytogenetic category included monosomal karyotype only; Mayo intermediate-risk karyotype included all abnormalities other than single/double del(5q); and Mayo low risk karyotype included normal karyotype or single/double del(5q).
As comprehensively illustrated in Table 1, men with MDS were more likely to display ASXL1 and U2AF1 mutations and higher bone marrow blast percentage while their women counterparts were more likely to display a more favorable karyotype and lower risk disease by IPSS-R. Borderline significance was also noted for an association between male sex and RUNX1 mutations. Of note, we found no difference between the sexes in terms of age distribution, red cell transfusion need or hemoglobin or platelet levels (Table 1). However, although not apparent from the crude comparisons of hemoglobin levels, the incidence of anemia, defined as a hemoglobin level below the sex-adjusted lower limit of the institutional reference range, was somewhat higher in males (Table 1).
After a median follow-up of 14 months, 217 (61%) deaths, 35 (10%) leukemic transformations, and 19 (5%) stem cell transplants were documented. In order to clarify the individual prognostic contribution of red cell transfusion need and the conventional hemoglobin threshold of 10 g/dl, patients were stratified into 3 groups: (i) red cell transfusion-dependent, (ii) transfusion-independent but with hemoglobin level of <10 g/dl, and (iii) hemoglobin level ≥10 g/dl. Figure 1A (female patients) and Figure 1B (male patients) illustrate the impact of sex on the prognostic value of using a hemoglobin threshold of 10 g/dl, in otherwise transfusion-independent cases. In women, the only thing that mattered for survival, in regards to hemoglobin level, was whether or not the patient was transfusion-dependent. In other words, the hemoglobin threshold of 10 g/dl did not predict survival in transfusion-independent women with MDS and the overall scenario was not affected by lowering the hemoglobin threshold to 9 g/dl (data not shown). In univariate analysis of survival in men with MDS, the presence of transfusion need, as well as hemoglobin level of <10 g/dl, in transfusion-independent cases, were associated with inferior survival (Figure 1B). However, in multivariable analysis that included the recently described age-adjusted genetic risk model for MDS,5 the significance of using the hemoglobin threshold of <10 g/dl, in transfusion-independent cases, was no longer apparent, while transfusion need retained its significance, in both men and women with MDS (data not shown).
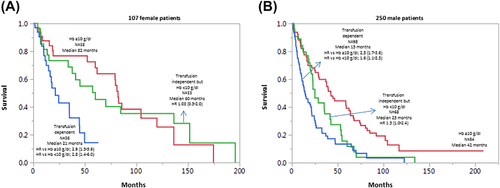
Overall survival data on 357 Mayo Clinic patients with primary myelodysplastic syndromes stratified by sex and severity of anemia. Key: Hb, hemoglobin; HR, hazard ratio (95% confidence interval); A, female patients; B, male patients [Color figure can be viewed at wileyonlinelibrary.com]
The current study highlights a number of novel observations. First, women with MDS, compared to their male counterparts, were less likely to harbor adverse genetic features, including ASXL1, U2AF1, and RUNX1 mutations. Second, in both men and women, the prognostic contribution of anemia might be limited to the presence or absence of red cell transfusion need. In other words, in transfusion-independent patients with MDS, the value of conventional hemoglobin thresholds (eg, 10 g/dl) appears to be questionable, especially in women (Figure 1A) and in the context of genetic risk stratification. We fully recognize the fact that our observations require validation by additional studies but such studies should (i) distinguish between the 2 sexes when assigning hemoglobin thresholds and (ii) determine genetics-independent prognostic contribution.
ACKNOWLEDGMENTS
None.
AUTHORSHIP AND CONFLICT-OF-INTEREST STATEMENTS
All authors declare no conflict of interest regarding matters pertinent to the current manuscript.
All authors have reviewed and approved the manuscript.
CAH reviewed pathology data.
RPK reviewed cytogenetic data.
NG and AP contributed patients.
TLL was in charge of molecular studies and analysis.
DP, MN, RRV and NS were in charge of data collection and organization.
AT developed the study concept and design, contributed patients, assisted in data extraction, performed statistical analysis and wrote the paper.




