Improved outcomes in acute myeloid leukemia patients treated with washed transfusions
Conflict of interest: NB has received a lecture fee in the past (>2 years ago) from Terumo, a manufacturer of blood cell processing equipment and a consulting fee from Biomet, the manufacturer of rejuvenation solutions for red cells. The other authors have no conflicts relevant to the manuscript.
Epidemiologic data suggested that patients with solid tumors receiving whole blood replete with stored plasma had substantially worse survival than patients receiving plasma depleted red cell transfusions 1. We hypothesized that washed transfusions might mitigate the immunomodulatory effects of blood transfusions in AML. In a pilot randomized trial, improved survival with washed transfusion was statistically significant for the subgroup of patients < 50 years of age. Compared with typical long-term survivals of 30% for younger adult patients with acute myeloid at that time, patients in the washed arm had a 75% survival rate 2.
In January 2006, based upon these data, our standard of care became washed, ABO identical, leukocyte-reduced, irradiated red cell and platelet transfusions for all patients <50 years of age with AML (n = 69). This study is registered at clinical trials.gov (NCT01976442) and conducted with institutional human subjects review board approval.
Previously published protocols were used to saline wash ABO identical, leukocyte-reduced, irradiated red cell 3 and platelet transfusions 4 immediately prior to administration. Approximately 95% of the original supernatant is removed, with recovery of 80–95% of the cells. All patients received induction chemotherapy with curative intent between January 2000 and April 2014. Patients receiving washed transfusions, beginning in 2006, remained on this protocol throughout all phases of their care. To examine any role of changes in treatment and supportive care regimens, we also compared a subset of patients treated from 2003 to 2005 with the patients treated in 2006–2008, a more restricted period of time. We also compared contemporaneous NY State cancer registry data with our center's data.
The only outcome measured was survival. Short-term survival was recorded starting from the date of diagnosis until death or survival at 30, 60, or 100 days. Comparisons were by Fisher's exact test. Long-term survival was assessed using Kaplan–Meier analysis with log rank and Wilcoxon tests (JMP 10, SAS Institute).
Pretreatment demographics of the two cohorts were similar (Supporting Information Table 1). The survival of patients receiving washed transfusions was superior (58%) to that in the unwashed cohort (38%), approaching conventional statistical significance (Supporting Information Fig. 1) (P = 0.08). Mortality at 30, 60, and 100 days in the washed transfusions recipients was one-half to one-third that in the recipients of unwashed transfusions, differences that were not statistically significant (Supporting Information Table 2).
Because the cohorts were heterogeneous in cytogenetics and age, we performed subset analyses to examine survival in more homogeneous patient groups (Supporting Information Table 2). Survival did not differ between the washed and unwashed cohort for patients with unfavorable cytogenetics. Subset analysis of AML patients with favorable or intermediate cytogenetics, regardless of age (Supporting Information Fig. 2), demonstrated statistically significant and substantially superior survival in the washed recipients (P = 0.029).
There was an age disparity between the cohorts. There were 20 AML patients ages 46–50 in the washed cohort, but only 5 patients in the historical cohort. These older patients had substantially worse outcomes in both groups. The short-term and long-term mortality in age cohorts for patients with favorable or intermediate cytogenetics is shown in Supporting Information Table 2. Short-term mortality (100 days) was significantly lower in the subset of AML patients <46 years old receiving washed transfusions at 0% (n = 49) compared with 12% (n = 26) in patients receiving unwashed transfusions (p = 0.039). AML patients <46 years of age with favorable or intermediate cytogenetics had strikingly and significantly better long-term survival if receiving washed transfusions (Supporting Information Fig. 3; Supporting Information Tables 3 and 4). Mortality was reduced by half in the recipients of washed transfusions. Cox regression analysis of known prognostic factors (age; cytogenetics) and type of transfusion demonstrated that the receipt of washed transfusions was the only significant predictor of survival in AML patients <46 years of age with favorable or intermediate cytogenetics (Supporting Information Table 3).
Limiting the analysis to AML patients with favorable or intermediate cytogenetics receiving washed transfusions during 2006–2008 demonstrated statistically significant improved survival compared with patients receiving unwashed transfusions in the preceding three years, 2003–2005. Five year survival was 29% in the unwashed recipients (n = 14), in contrast to 61% in the comparable washed recipients (n = 18) (P = 0.036).
Lastly, we compared AML patients receiving washed transfusions with contemporaneous, similar patients (favorable or intermediate cytogenetics; age < 46) throughout New York State during the period 2006–2011. Patients treated with washed transfusions had substantially and statistically significant better five year survival (80%) compared with patients at other institutions in New York State (50%) (P < 0.01) (Fig. 1).
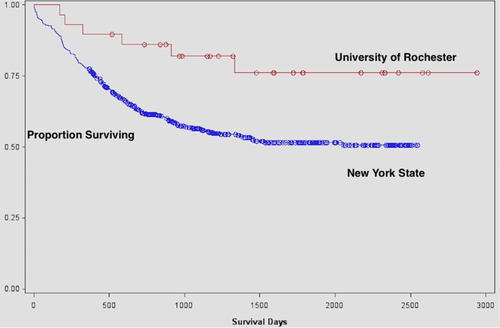
Survival in patients with favorable or intermediate cytogenetics <46 years of age with AML receiving washed (n = 29) or unwashed transfusions (n = 524) throughout New York State during 2006–2011 analyzed by the Kaplan–Meier method. Patients in the washed group experienced significantly better survival (80% vs. 50%) (P = 0.01 by log rank test). [Color figure can be viewed at wileyonlinelibrary.com]
These results in 69 younger patients with AML receiving washed transfusions mirror those in a small randomized trial 2. Washed, ABO identical, leukocyte-reduced, irradiated transfusions were associated with reduced short-term mortality compared with patients treated with conventional ABO identical, leukoreduced, irradiated transfusions. Long-term mortality in recipients of washed transfusions (20–40%) was half to two-thirds of that in the comparable historical comparison group and the current literature (60%) (Supporting Information Table 5). A limitation of these data, in addition to the lack of randomization, is that we did not collect detailed information on treatment regimens (e.g., choice and dose of anthracycline in AML). The striking differences we observed in long-term survival are unlikely solely due to progress in treatment regimens or supportive care. Identical differences were observed when we restricted the comparison to the years 2003–2005 and 2006–2008. For lower risk patients (favorable or intermediate cytogenetics; <46 years of age or younger) in New York State treated between 2006 and 2011 long-term mortality rate was 2.5-fold higher (50% versus 20%) in conventionally treated patients compared with recipients of washed transfusions.
This approach has the potential to substantially improve outcomes for many patients with AML. This may be limited, at present, to younger patients with favorable or intermediate cytogenetics. Larger randomized trials will be required to determine whether our promising results are generalizable and reproducible, and whether they might be applicable to older patients (who often receive less intensive therapy), and to patients with other hematologic malignancies or solid tumors.
Acknowledgment
Authors thank the nursing staff of the JP Wilmot Cancer Institute and the medical technologist and resident physician staff of the Transfusion Service/Blood Bank at the University of Rochester Medical Center for their tireless and devoted efforts to the care of the patients reported in this study. Authors thank the Cancer Registry and Margie Richardson for their assistance.
Authors Contribution
NB, KH, and DG had full access to the data. NB assumes full responsibility for the manuscript, and all authors contributed to the drafting, editing and finalizing of the manuscript.
-
Daniel Greener,1 Kelly F. Henrichs,1 Jane L. Liesveld,2 Joanna M. Heal,1 Christopher T. Aquina,3 Gordon L. Phillips II,2 Scott A. Kirkley,1 Laurie A. Milner,1,2 Majed A. Refaai,1 Jason H. Mendler,2 Jill Szydlowski,4 Debra Masel,1 Amy Schmidt,1 Francis P. Boscoe,5 Maria J. Schymura,5 and Neil Blumberg1*
-
1Transfusion Medicine Unit, Department of Pathology and Laboratory, University of Rochester Medical Center, Rochester, New York; 2Hematology-Oncology Unit, Department of Medicine, JP Wilmot Cancer Institute, University of Rochester Medical Center, Rochester, New York; 3Department of Surgery, University of Rochester Medical Center, Rochester, New York; 4Department of Public Health Sciences, University of Rochester Medical Center, Rochester, New York; 5New York State Department of Health, New York State Cancer Registry, Albany, New York




