Targeting survival and cell trafficking in multiple myeloma and Waldenstrom macroglobulinemia using pan-class I PI3K inhibitor, buparlisib
Abstract
The phosphatidylinositol-3 kinase (PI3K) pathway is activated in multiple myeloma (MM) and Waldenstrom Macroglobulenima (WM), and plays a crucial role in tumor progression and drug resistance. In this study, we characterized the role of pan-class I PI3K inhibition on cell trafficking and survival of MM and WM cells. We tested the effect of pan-class I PI3K inhibition by siRNA silencing or pharmacologic inhibition with buparlisib on MM cell survival, apoptosis and cell cycle in vitro and tumor growth and mobilization of MM cells in vivo. We then evaluated buparlisib-dependent mechanisms of induced MM cell mobilization. Moreover, the effect of buparlisib on cell survival, apoptosis, and adhesion of WM cells to bone marrow stromal cells (BMSCs) has been evaluated. We showed that buparlisib induced toxicity in MM cells, supported by induction of apoptosis and cell cycle arrest. Buparlisib was also found to reduce tumor progression in vivo. Importantly, buparlisib enhanced MM cell mobilization in vivo which was driven by decreased adhesion of MM cells to BMSCs and increased chemotaxis via up-regulation of CXCR4 expression. Similar to its effects on MM cells, buparlisib also induced cell survival and apoptosis, and decreased adhesion in WM cells. These data highlight the critical contribution of class I PI3K signaling to the regulation of survival and cell dissemination in B-cell malignancies. Am. J. Hematol. 89:1030–1036, 2014. © 2014 Wiley Periodicals, Inc.
Introduction
The phosphatidylinositol-3 kinase (PI3K) pathway has been shown in many malignancies to be a critical regulator of tumor progression, protein translation and cytoskeletal dynamics, collectively required for cell division, growth, survival and motility 1-8. Activation of this pathway is frequently observed in numerous human cancers 9, and may occur due to, either external signaling from the bone marrow milieu including signaling through insulin growth factor (IGF-1), receptor tyrosine kinases (RTKs), or due to cell autonomous mechanisms through mutations in regulatory pathways including mutations of signaling genes such as RAS, PTEN, FGF, c-Myc, and CDKN pathways 1-8. Although no mutations have been described in the PI3K/Akt genes in MM, it was shown that this pathway is constitutively activated in MM cells and has pleiotropic effects influencing proliferation, drug resistance, angiogenesis, and cell adhesion, which are mediated by multiple broad downstream effectors such as mammalian target of rapamycin (mTOR), NF-kB and BAD 8, 10. Given the important downstream targets that affect key components of this pathway, such as Akt or mTOR, there has been a focus on the development of PI3K pathway-specific agents 9, 11. Rapamycin analogues such as everolimus and temsirolimus have been tested in MM in preclinical and clinical trials and display incomplete inhibition of the signaling cascades downstream of the TORC1 complex, eventually leading to increased phosphorylation of Akt due to loss of the feedback inhibitory circuit mediated by S6K 12-19. It was also demonstrated that DEPTOR, an mTOR-interacting protein downstream of PI3K is highly overexpressed in patients with MM with t(11;14) and t(6;14) (Cyclin D1/D3) or in patients with t(14;16) (with c-MAF/MAFB), which represent 30% of patients with MM 7. In these cells, high DEPTOR expression is necessary to maintain PI3K and Akt activation by relieving feedback inhibition from TORC1, and reducing DEPTOR levels leading to apoptosis 7.
Drug development of pan-PI3K inhibitors (Buparlisib, GDC-0941 and XL147); dual pan-PI3K–mTOR inhibitors (SF1126, BEZ235, XL765, and GSK1059615) and isoform-specific inhibitors (such as the p110δ-selective inhibitor, idelalisib or GS-1101) has increased during the past decade 20. Interestingly, p110δ inhibitors such as idelalisib have led to intriguing changes in the localization and cell trafficking of malignant cells in Chronic Lymphocytic Leukemia (CLL), highlighting the critical role of the PI3K pathway in cell motility and localization in specific niches 21. Moreover, PI3K was shown to play an important role in cell adhesion and migration in many different cell types 22, through the assembly of membrane signaling complexes and intracellular trafficking of proteins 23. PI3K-dependent migration in leukocytes is thought to be controlled by PI3K through its association to G- protein coupled receptor (GPCRs), especially downstream of chemokine receptors 24-26. Specifically, PI3K is a major downstream transducer in CXCR4-mediated chemotaxis, which in turn regulates adhesion and migration 27.
In the current study, we investigated the role of pan-class I PI3K inhibition on cell survival and trafficking of MM and WM. Buparlisib (BKM120), a 2,6-dimorpholino pyrimidine derivative, is a potent pan-class I PI3K inhibitor in biochemical assays with at least 50-fold selectivity against other protein kinases 28, and currently being tested in clinical trials in solid tumors 29. Herein, we demonstrated inhibition of PI3K regulates cell survival and trafficking in MM and WM cells providing rationale for targeting PI3K in B-cell malignancies.
Methods
Cells
The MM cell lines, OPM1, OPM2, H929 and RPMI8226 were kindly provided by Prof Jesús F. San Miguel, University of Salamanca, Salamanca, Spain. MM.1S was purchased from ATCC. BCWM.1 and MWCL1 are B-cell lines developed in patients with WM; BCWM.1 was a gift from Dr S.P. Treon from the Dana-Farber Cancer Institute (Boston, MA) 30, and MWCL1 was a gift from Dr S. Ansell (Mayo Clinic, Rochester, MN) 31. All cell lines were cultured at 37°C in RPMI-1640 (IMDM for MWCL1) containing 10% FBS (Sigma Chemical), 2 mmol/l glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin (Gibco). Bone marrow stromal cells (BMSCs) cultures were established as described previously 32. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki for primary MM patient samples.
Animals
Female SCID-Bg mice (7–9 weeks of age) were obtained from Taconic and injected with MM.1S cells. Approval for the studies was obtained from the Dana-Farber Cancer Institute and Massachusetts General Hospital Institutional Animal Care and Use Committees.
Reagents
Buparlisib was provided by Novartis Inc. (Basel, Switzerland), diluted in dimethylsulfoxide (DMSO), and stored at −20°C until use; it was then diluted in culture medium immediately before use. The maximum final concentration of DMSO (<0.1%) did not affect cell proliferation and did not induce cytotoxicity on the cell lines and primary cells tested (data not shown). Recombinant SDF-1α was purchased from R&D Systems (Minneapolis, MN). Calcein-AM cell-labeling dye and Lipofectamin-2000 were obtained from Invitrogen (Carlsbad, CA). Scramble-siRNA and SmartPool siRNA's against all PI3K isoforms from Dharmacon (Lafayette, CO).
Immunoblotting
For the effect of buparlisib on cytoskeletal dynamics, adhesion, apoptosis and cell cycle signaling, MM or WM cells were cultured, harvested and lysed. Proteins were detected by the use of immunoblotting as previously described 32. α-tubulin was used as a loading control. The following mAbs were used for immunoblotting: Akt, P-Akt, caspase-3, caspase-9, PARP, Bcl-XL, cyclin D1, pRb, P27, P21, pSRC, pFAK, pMLC, pS6R, pP70S6K and pmTOR. All mAbs were purchased from Cell Signaling (Technologies Danvers, MA).
Quantitative reverse transcriptase PCR
RNA extraction was performed using the RNeasy kit (QIAGEN). RNA was analyzed using an Agilent 2100 bioanalyzer. All PCR reactions were run in triplicate, and mRNA expression, relative to 18S rRNA or glyceraldehyde-3-phosphate dehydrogenase, was calculated using the 2−ΔΔCt method 33.
Cell viability test
Cell growth was assessed by measuring MTT (Chemicon) dye absorbance, as described previously 34.
Cell-cycle and apoptosis assays
Cell-cycle analysis was profiled by flow cytometry using 5 μg/ml propidium iodide (PI) (Sigma-Aldrich) after 24 hr of culture with buparlisib. Apoptosis was quantified using Annexin V-FITC Apoptosis Detection kit (BD Biosciences).
Effect of buparlisib on MM tumor progression in vivo
To assess the effect of pan-PI3K inhibition on tumor progression, 14 SCID-Bg mice were injected with 5 × 106 MM.1S-GFP+/luc+ cells intravenously. At day 3, mice were divided into two groups of seven animals each: one group received treatment 50 mg/kg of buparlisib once a day by oral gavage, whereas the second group received vehicle treatment once a day. Tumor progression was monitored once a week by bioluminescence imaging (BLI) for 5 weeks at which point mice were sacrificed.
Fluorescence microscopy for BM specimens
Femurs were isolated from buparlisib and vehicle treated mice at 5 weeks, washed with cold PBS, fixed with 4% formaldehyde in PBS, dehydrated with ethanol, embedded in paraffin blocks, and sectioned. Sections were stained with DAPI, mounted, and analyzed by a fluorescent microscope (Nikon Eclipse 80i).
Mobilization of MM cells to the circulation and expression of CXCR4 in vivo
MM.1S-GFP+/luc+ cells (3 × 106 cells /mouse) were injected intravenously into six SCID-bg mice and tumor development was observed using BLI at 4 weeks. Mice were then randomized and administered with vehicle (three mice) or 50 mg/kg buparlisib (three mice) by oral gavage. Blood (100 ml) was obtained from each mouse before treatment (baseline) and 5 hr after treatment. RBCs were then lyzed, and mononuclear cells were resuspended in PBS and stained with an APC-anti-CXCR4 antibody or isotype control. Flow cytometric analysis of CXCR4 and GFP expressing cells were performed.
Adhesion of MM cells to fibronectin and bone marrow stromal cells (BMSCs)
For adhesion assay we used fibronectin coated plates or plates coated with a confluent monolayer BMSCs which was generated by plating 1 × 104 cells/well in 96-well plates overnight. The next day MM cell lines (MM.1S,OPM1, H929) or WM cell lines (BCWM.1 and MWCL1) (1 × 106 cells/mL) were serum starved for 4 hr, prelabeled with calcein-AM, treated with increasing concentrations of buparlisib, washed and then added to the fibronectin coated plates or BMSCs. In some cases, cells with PI3K knockdown were starved and then added to the plates. Cells were co-cultured for 2 hr at 37°C and non-adherent cells were washed off. Adherent cells were detected by measuring the fluorescence intensity in the wells using a fluorometer (Ex/Em = 485/520 nm).
Chemotaxis of MM cells
MM cell lines (MM.1S, OPM1, H929) were serum-starved and treated with increasing concentrations of buparlisib (0–1 µM) for 4 hr and then left to migrate to 0 or 30 nM of SDF-1α or primary bone marrow stromal media in the lower chamber for 5 hr. Cells in the lower chambers were counted by flow cytometry. In some cases, MM.1S cells were treated with buparlisib (0.5 µM). In some cases, MM cells with PI3K knockdown were used in the migration assay. Moreover, in some experiments SDF-1α 30 nM was added to the upper chamber to examine the migration of cells in response to the SDF-1α gradient.
Expression of CXCR4 in vitro by flow cytometry
MM cells were pretreated with 0 or 0.5 µM buparlisib for 5 hr and then treated with increased concentration of SDF-1α (0–10 nM) for 15 min which were then stained on ice with anti-CXCR4 or an isotype control, washed and analyzed by flow cytometry for expression of CXCR4. The expression was quantified as the ratio of the mean-fluorescence intensity (MFI) of CXCR4 to the MFI of isotype control.
Statistical analysis
Statistical differences between experimental groups were analyzed using Microsoft Office Excel 2007 software using t tests (two-tailed; α 0.05) or ANOVA. P values less than 0.05 were considered significant. Exact P values are provided in the ures.
Results
PI3K inhibition regulates survival of MM cells
We tested the effect of increasing concentrations of buparlisib on the survival of five MM cell lines (RPMI-8226, OPM1, MM.1S, OPM2 and H929). Buparlisib induced cell toxicity after 48 hr treatment in all MM cell lines tested; with an IC50 between 0.5 and 1 µM (Fig. 1A). In addition, buparlisib decreased the activation of signaling proteins downstream of PI3K including pAkt, pS6R, pP70S6K, and p-mTOR in MM.1S cells in a dose dependent manner (Fig. 1B).
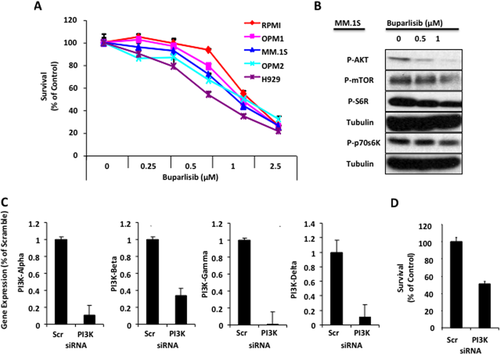
Pan-PI3K inhibition regulates survival of MM cells. (A) The effect of the pan-PI3K inhibition, mediated by increased concentrations of buparlisib on MM cell survival using MTT assay. (B) The effect of buparlisib treatment on PI3K signaling pathway was assessed by immunoblotting, which showed decreased expression of pAKT, pS6R, pP70S6K, and pmTOR. (C) Pan-PI3K inhibition by down-regulation of all the isoforms α, β, γ, and δ in MM.1S cells using a pooled siRNA compared with a scrambled siRNA control, detected by RT-PCT, showing down-regulation of all the different isoforms. (D) The effect of pan-PI3K inhibition by siRNA on survival of MM cells using MTT assay, showing decreased cell survival due to inhibition of the PI3Ks.
To mimic the pan-inhibition of buparlisib in MM cells, we used siRNA to knockdown the different isoforms of PI3K, and tested the effect of these siRNAs compared with scramble siRNA on the expression of the different PI3K isoforms in MM.1S cells using RT-PCR. As shown in Fig. 1C, all the PI3K isoforms were down-regulated in MM.1S using the PI3K siRNA compared with the scramble siRNA. Down-regulation of all the PI3K isoforms resulted in inhibition of cell survival to 50% of control compared with the scramble siRNA (Fig. 1D).
PI3K inhibition by buparlisib induces apoptosis and G1 arrest in MM cells
Increasing concentrations of buparlisib induced apoptosis in MM cells, using annexin V-PI staining as assessed by FACS. Buparlisib induced apoptosis of MM cells after 48 hr treatment in a dose–response manner in both MM.1S and H929 cell lines (Supporting Information Fig. 1A). These results were confirmed using apoptosis signaling in MM.1S cells; buparlisib- induced caspase-3, caspase-9 and PARP cleavage and decreased the expression Bcl-XL in a dose dependent manner (0, 0.1, 0.25, 0.5, and 1 µM) (Supporting Information Fig. 1B).
We further tested the effect of increasing concentrations of buparlisib on cell-cycle regulation in MM cells; apoptotic cells (sub-G1/G0) were excluded. Buparlisib induced G1 cell cycle arrest in MM cells after 24 hr treatment in a dose–response manner in MM.1S and H929 cell lines, 90% and 80% of MM.1S and the H929 cells were arrested in G1 using buparlisib 1 µM, respectively (Supporting Information Fig. 1C). These results were confirmed by testing cell cycle signaling in MM.1S cells; buparlisib decreased the expression of the cell cycle inducers cyclin-D1 and pRb, and increased the expression of the cell cycle inhibitors P21 and P27 in a dose dependent manner (Supporting Information Fig. 1D).
Buparlisib reduces MM tumor progression in vivo
Treatment of mice with buparlisib significantly decreased the rate of tumor progression (P < 0.01) compared with the vehicle treated group, as shown in representative images of the BLI (Fig. 2A) and quantification of the BLI (Fig. 2B). These results were further confirmed by fluorescence microscopy, showing that the number of MM.1S-GFP+/luc+ cells present in the BM of mice treated with buparlisib decreased significantly compared with those present in the BM of mice treated with vehicle, as shown in representative images of immunofluorescence (Fig. 2C).

Buparlisib reduces MM tumor progression in vivo. The effect of buparlisib on MM tumor proliferation in SCID-Bg mice was tested. While at day 10 no difference was observed in the tumor progression level, buparlisib decreased the rate of tumor progression overtime compared with the vehicle treated group, as shown in representative images (A) and quantification of BLI (B). Confirmation of the effect of buparlisib in inhibition of MM tumor progression in the BM by detection of MM.1S-GFP+ cells at day 30 by fluorescent microscopy (C), showing representative from vehicle and buparlisib treated group (blue: DAPI and green GFP). Buparlisib treatment decreased the number of MM cells in the BM compared with the vehicle treated group.
Buparlisib induces mobilization of MM cells in vivo and decreases adhesion of MM cells to BMSCs and fibronectin in vitro
Given that idelalisib, a selective inhibitor of PI3Kδ, leads to mobilization of tumor cells to the peripheral blood 21, we sought to examine the effect of pan-PI3K inhibition on cell mobilization in vivo. SCID mice with advanced tumor burden were randomized into two groups (vehicle vs. buparlisib) and 100 µl peripheral blood was drawn from each mouse, before and after 5 hr of treatment. The number of circulating MM cells in peripheral blood samples of mice was measured by flow cytometry. We found that buparlisib (50 mg/kg) increased the mobilization of MM cells into the circulation in all treated mice compared with baseline samples from the same mice, while there was minimal circulation of tumor cells in the vehicle treated mice (Fig. 3A).
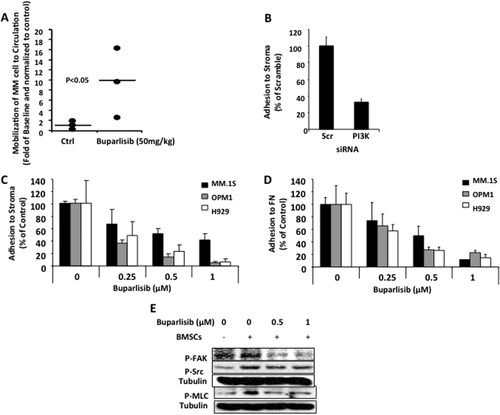
Pan-PI3K inhibition increases MM cells mobilization in vivo and decreases adhesion of MM cells to BMSCs. The effect of pan-PI3K inhibition with buparlisib on the mobilization of MM cells from the BM to the circulation was tested in SCID-Bg mice with advanced disease. Changes in the number of circulating MM cells in the peripheral blood compared with baseline of the same mice after 5 hr of treatment was measured by flow cytometry. (A) Increased number of MM cells to the circulation in buparlisib treated group was found, while no change was observed in the vehicle treated group. (B) The effect of pan inhibition of PI3K by siRNA on adhesion of MM.1S cell to BMSCs, showing decreased cell adhesion due to inhibition of the PI3K's by siRNA compared with scrambled siRNA. (C) Similarly, buparlisib induced dose-dependent inhibition of adhesion of MM.1S, OPM1 and H929 cells to BMSCs. (D) The effect of increased concentrations of buparlisib on adhesion of MM cells revealed that buparlisib also induced dose-dependent inhibition of adhesion of MM.1S OPM1 and H929 cells to fibronectin. (E) The mechanism of inhibition of adhesion by buparlisib in MM cells by testing its effect on adhesion signaling in MM.1S cells using immunoblotting, showing decreased adhesion signaling in MM.1S cells.
To better understand the role of PI3K on mobilization of MM cells, we studied adhesion of MM cells to BMSCs derived from MM patients and to fibronectin representing the extracellular matrix. We found that MM.1S cells treated with pooled siRNAs against all PI3K isoforms compared with a scramble siRNA decreased the adhesion of MM.1S cells to BMSCs (Fig. 3B). Similarly, we found that buparlisib decreased the adhesion of MM cells (MM.1S, OPM1, and H929) to BMSCs (Fig. 3C) and fibronectin (Fig. 3D) in a dose-dependent manner. These results were further confirmed by immunoblotting, in which buparlisib decreased adhesion signaling induced by co-culture with BMSCs in MM.1S cells; including decrease of expression of pFAK, pSrc and pMLC (Fig. 3E). These studies were performed within 5 hr of exposure to buparlisib while the viability of the cells was still intact.
Buparlisib regulates chemotaxis of MM cells
To examine the role of PI3K on chemotaxis of MM cell in vitro, we used MM.1S cells that were treated with pooled siRNA against all PI3K isoforms compared with a scramble siRNA, and found that pan-PI3K inhibition increased chemotaxis of MM.1S cells toward SDF-1α 30 nM (Fig. 4A). To further confirm the effect of PI3K inhibition, we treated three MM cell lines (MM.1S, OPM1, H929) with increasing doses of buparlisib (0, 0.5, and 1 µM), and found that PI3K inhibition by buparlisib increased the chemotaxis of MM cells towards SDF-1α (30 nM) in a dose dependent manner (Fig. 4B). Similar results were observed with MM.1S cells which were exposed to conditioned media from BMSCs isolated from three different MM patients and then treated with buparlisib 0.5 µM (Fig. 4C). The increase of chemotaxis due to pan-inhibition of the PI3K was abolished when the SDF-1α gradient was reversed by the addition of SDF-1α to the upper chamber of the chemotaxis assay (Fig. 4D), indicating that this is directed cell motility in response to activation of cell receptors by SDF-1α.
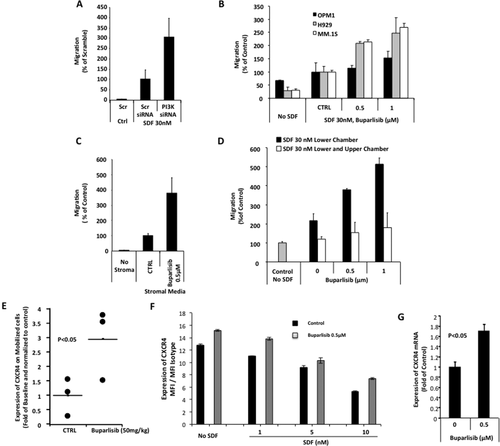
Buparlisib increases chemotaxis and CXCR4 expression in MM cells. The effect of pan-PI3K inhibition by siRNA on chemotaxis of MM.1S cells to SDF-1α gradient (30 nM SDF-1α in the lower chamber and no SDF-1α in the upper chamber), showing increased chemotaxis in cells treated with PI3K siRNA compared with scrambled siRNA. (B) The effect of increasing concentrations of buparlisib on chemotaxis of MM cells towards an SDF-1α gradient revealed that buparlisib induced a dose-dependent increase in chemotaxis of MM.1S, OPM1 and H929 cells. (C) Similarly, buparlisib induced increased chemotaxis of MM.1S to conditioned media from BMSCs. (D) The effect of buparlisib on chemotaxis of MM cells without SDF-1α gradient conditions (30 nM SDF-1α in both the upper and lower chamber), showing the lack of effect of buparlisib without the SDF-1α gradient. (E) Measurement of the expression of CXCR4 on mobilized MM cells due to treatment with buparlisib by flow cytometry, showing that pan-PI3K inhibition by buparlisib increased the expression of CXCR4 in MM cell in vivo. (F) The effect of buparlisib in the presence or absence of SDF-1α on expression of CXCR4 in MM cells in vitro, represented as the MFI of APC of CXCR4 compared with an isotype control, as detected by flow cytometry, showing that buparlisib increased the surface expression of CXCR4; (G) similarly the expression of CXCR4 mRNA was increased as detected by RT-PCR and normalized to the untreated control.
Inhibition of PI3K increased surface expression of CXCR4 in MM cells
To further elucidate the mechanism by which increased chemotaxis occurs in response to pan-PI3K inhibition, CXCR4 expression was examined on MM cells that were circulating in the peripheral blood in mice treated with buparlisib or vehicle for 5 hr. Increase in CXCR4 expression in circulating tumor cells was observed by flow cytometry in mice treated with buparlisib compared with control mice (P < 0.05). Similarly, an increased surface expression of CXCR4 in MM cells was observed when MM cells were treated with buparlisib in vitro (Fig. 4F). Additionally, buparlisib was able to partially compensate reduced CXCR4 expression, which was due to down-regulation/internalization induced by SDF-1α (Fig. 4F). We further examined the effect of buparlisib on the mRNA level of CXCR4 in MM cells and found that buparlisib increased the mRNA level of CXCR4 to 1.7-fold of the control level (Fig. 4G).
Buparlisib induces survival and decreases adhesion of WM to BMSCs
We finally tested the effect of increasing concentrations (0–4 µM) of buparlisib on the survival of WM cell lines (BCWM.1 and MWCL1). Buparlisib induced cell toxicity after 48 hr treatment in the cell lines tested by MTT assay (Supporting Information Fig. 2A). Buparlisib also induced apoptosis of MM cells after 48 hr treatment in a dose–response manner, which was shown by immunoblotting (Supporting Information Fig. 2B). We further tested the pan-PI3K inhibition on adhesion of WM cells to BMSCs from a WM patient and found that buparlisib decreased adhesion of WM cells to BMSCs in a dose dependent manner (Supporting Information Fig. 2C).
Discussion
The PI3K pathway plays an important role in tumor progression and resistance to antineoplastic drugs, including cytotoxic chemotherapies and targeted agents 20. Thus, a number of potential therapeutics targeting specific PI3K groups or isoforms were developed 9. In the present study, we examined the effect of pan-PI3K inhibition by siRNA silencing of PI3K or pharmacologic inhibition with an orally available pan-PI3K inhibitor, buparlisib on survival and cell trafficking of MM and WM.
It has been previously shown that buparlisib inhibits survival and induces apoptosis in different cancer types including lung, gastrointestinal cancers, diffuse large B-cell lymphoma (DLBCL) and chronic lymphocytic leukemia (CLL) 35-39. Similarly, we demonstrated that buparlisib leads to reduce cell survival, and induces apoptosis and G1 cell cycle arrest in MM cells. We also demonstrated that buparlisib inhibits PI3K downstream signaling in MM cells and reduces MM tumor progression in vivo.
Interestingly, we found that pan-PI3K inhibition enhances migration in addition to reduced adhesion. This combined effect led to enhanced mobilization in vivo, an effect that was also observed with idelalisib, a selective inhibitor of PI3Kδ, in CLL. Early lymphocytosis due to CLL cell redistribution from tissues into the blood is seen in CLL patients after starting idelalisib treatment 40. However, the mechanism by which idelalisib induces mobilization remains unknown. One hypothesis is that PI3K signaling has been linked to tandem pleckstrin homology domain protein (TAPP), which likely has multiple interaction partners in the cytoskeleton, and may lead to increased CLL cell adhesion to fibronectin and laminin 41. Here, we found that inhibition of PI3K decreased the adhesion of MM cells to fibronectin and BMSCs. We have previously shown that PI3K is a downstream target of CXCR4 and is involved in promoting its cytoskeletal signaling in MM 42, 43. Interestingly, inhibition of the PI3K increased the chemotaxis of MM cells by up-regulation of CXCR4. Similar to this finding, pan-PI3K inhibition (LY294002) in B-cell receptor-dependent DLBCLs was accompanied by transcriptional up-regulation of CXCR4 in a recent report 44. Mechanism(s) of enhanced CXCR4 expression is beyond the scope of our study but there are several possible mechanisms that could underlie up-regulation of CXCR4 upon buparlisib treatment; increased expression of CXCR4 through a feedback mechanism, inhibition of CXCR4 internalization, facilitation of re-expression of internalized CXCR4 and translocation of intracellular stores of CXCR4. It should be noted that the up-regulation of CXCR4 in our study was also observed in the absence of SDF-1α indicating that there might be some mechanism(s) other than inhibition of CXCR4 internalization induced by SDF-1α. This study begins to dissect the mechanism by which PI3K inhibition enhances tumor mobilization. However, further studies are needed to identify the role of CXCR4 and other chemokine receptors in the inside-out signaling effect of PI3K inhibitors.
In summary, these data suggest that pan-class I PI3K inhibition has direct action in killing of MM cells, and may enhance MM cell death by mobilization of MM cells to circulation and thus deprivation of MM cells from their supportive BM microenvironment. Future studies to determine the relative contributions of MM cell mobilization in treatment with PI3K inhibitors are also warranted.
We have previously shown that PI3K/Akt pathway is activated in WM and that BMSCs confer growth advantage to WM cells 34. We therefore investigated the role of pan-class I PI3K inhibition in WM cells. We found that buparlisib reduced cell survival and induced apoptosis. Moreover, buparlisib decreased adhesion of WM cells to BMSCs. Given that over 80% of patients with WM harbor mutations in MYD88 or CXCR4 45 that activates downstream PI3K signaling, we believe that PI3K inhibition might represent a rational therapeutic target that should be tested in clinical trials in WM.
Clinical trials using this agent (NCT01693614) as well as other PI3K inhibitors including PI3K delta isoform are ongoing in B cell malignancies. It has recently been shown that inhibition of PI3K delta isoform provides dramatic effect in certain B-cell cancers 46-48. The efficacy of PI3K inhibition arises not only from direct inhibition of survival signaling but also from disruption of malignant B cells in a protective niche 49. This study dissects potential mechanisms of PI3K inhibition on B cell egress from niche via the CXCR4 axis.
In conclusion, we demonstrated that buparlisib regulates survival of MM and WM cells and, importantly, regulates adhesion and migration of MM cells, indicating that these classes of agents are critical regulators of survival and cell trafficking in B-cell malignancies.




