Fractionated gemtuzumab ozogamicin and standard dose cytarabine produced prolonged second remissions in patients over the age of 55 years with acute myeloid leukemia in late first relapse
Conflict of interest: SC received a research grant from Pfizer. Other authors have nothing to report.
Abstract
Gemtuzumab ozogamicin (fGO), a humanized anti-CD33 monoclonal antibody linked to calicheamicin in combination with intensive chemotherapy gives high response rates in adult acute myeloid leukemia (AML) patients in relapse. However, reduced intensity chemotherapy in combination with fractionated GO has not been tested in aged relapsing patients. Patients from our institution with CD33+ AML aged 55 years or more in first late relapse (≥6 months) were proposed participation in a GO compassionate use program. Induction therapy consisted in fractionated GO (fGO; 3 mg/m2, days 1, 4, 7) with standard-dose cytarabine (200 mg/m2/day, 7 days). Patients were consolidated with two courses of GO and intermediate dose cytarabine. Twenty-four patients (median age 68 years) received fGO with cytarabine. Median follow-up was 42 months. The response rate was 75%, including complete remission (CR) in 16 patients and CR with incomplete platelet recovery (CRp) in two patients. Two-year overall survival (OS) was 51% (95% CI: 28–69) and 2 years relapse-free survival (RFS) was 51% (95%CI: 25–72). Duration of second CR (CR2) was longer than first CR (CR1) in 9 out of 18 patients. Minimal residual disease (MRD) was negative in evaluable patients in CR2, particularly in NPM1 mutated cases. Toxicity was in line with that of the same fractionated single agent GO schedule. Fractionated GO with low intensity chemotherapy produced high response rates and prolonged CR2 in aged AML patients in first late relapse. Am. J. Hematol. 89:399–403, 2014. © 2013 Wiley Periodicals, Inc.
Introduction
Prognosis of adult patients with acute myeloid leukemia (AML) after first relapse remains poor. Complete response rates range from 25 to 40% 1, 2. Few patients survive after intensive chemotherapy with or without allogeneic hematopoietic stem cells transplant (HSCT) 3. Salvage regimens with anthracyclines and high dose cytarabine are generally unsafe for patients aged 55 years or older due to toxicity. Even for patients eligible for intensive salvage chemotherapy, age remain an independent adverse prognostic factor 4. In order to categorize patients eligible to salvage therapy, Breems et al. developed a clinical index based on four clinically relevant parameters (length of relapse-free interval after first complete remission, cytogenetics at diagnosis, age at relapse and whether previous stem-cell transplantation was performed). Using this stratification system, three risk groups were defined: a favorable-risk group A (overall survival [OS] of 70% at 1 year and 46% at 5 years), an intermediate-risk group B (OS of 49% at 1 year and 18% at 5 years), and a poor-risk group C (OS of 16% at 1 year and 4% at 5 years) 5.
Gemtuzumab ozogamicin (GO, Mylotarg®, Pfizer, New York, NY, and Ben Venue Laboratories, Bedford) is a humanized anti-CD33 monoclonal antibody linked to calicheamicin. GO targets CD33-expressing leukemic cells and induces cell death by internalization of the associated cytotoxic drug calicheamicin 6, 7. In phase 2 studies, GO was used as a single agent at a dose of 9 mg/m2 on day 1 and 14 which gave a 26% response rate in relapsed AML patients. Main toxicities were prolonged myelosuppression and hepatotoxicity 8. Subsequent studies evaluated GO administered as one or two doses ranging from 4.5 to 9.0 mg/m2 in combination with various agents in relapsed AML patients 9-20. Complete remission rates ranged from 12 to 63% and median survival from 2.0 to 9.5 months.
To reduce GO toxicity and enhance efficacy, we developed a new regimen of fractionated low doses of GO (the 3-3-3 regimen, GO 3 mg/m2 on days 1, 4, and 7). Results from two phase 2 studies in AML patients in first relapse showed this 3-3-3 regimen to be effective and safe. CR rates were 55 and 33% when fractionated GO was administered in the presence or absence of conventional dose daunorubicin and cytarabine, respectively, and median survival was 15 and 8.4 months respectively 21, 22. These studies in relapsed patients were followed by a phase 3 study in previously untreated AML patients aged 50–70 years which demonstrated the superiority of the addition of fractionated GO to the standard “3+7” regimen versus standard 3+7 alone 23.
In this study, we investigated the efficacy and tolerability of fractionated GO in combination with standard dose cytarabine without anthracycline (7+fGO) in AML patients in first late relapse aged 55 years or over.
Methods
Patients
From October 2007 to November 2011, patients from our institution with CD33+ AML aged 55 years or more in first relapse were proposed participation in the GO compassionate use program if they were considered suitable to receive intensive chemotherapy (ICT) and if CR duration before relapse was 6 months or more. Patients with refractory AML, liver dysfunction, who had already had received GO as part of their first-line therapy, or with prior HSCT were not considered eligible. The GO compassionate use program is a named-patient program approved by the French Health Authorities and referred to “authorization for temporary use (ATU)” in France. All patients provided informed consent to enter the program that covers the combination of GO and cytarabine.
Treatment and evaluations
Patients received an induction course with intravenous cytarabine, 200 mg/m2/day as a continuous infusion on days 1–7 and GO at a dose of 3 mg/m2 infused over 2 hr on days 1, 4, and 7 after premedication with methylprednisolone (7+fGO). Daily granulocyte colony stimulating factor (lenograstim 263 μg, intravenous) was given from day 9 until neutrophil recovery. Response assessment was performed after 7+fGO on days 35–45. Responses were classified as either complete remission (CR), defined as fewer than 5% blasts within a normocellular marrow, an absolute neutrophil count (ANC) of >1 × 109/L and a platelet count of ≥100 × 109/L in peripheral blood, or as CR with incomplete platelet recovery (CRp), defined as CR with platelet recovery <100 × 109/L at day 45 following the induction course. Patients in CR or CRp on day 45 were given two consolidation courses of intermediate dose cytarabine (ID-AraC 1000 mg/m2 every 12 hr, infused over 2 hr on days 1–4), with one injection of GO (3 mg/m2) on day 1. A maintenance phase with low dose cytarabine (10 mg/m2 every 12 hr, subcutaneously, on days 1 to 7–10, every 28 days) was given at the physician's discretion.
Immunophenotyping and cytogenetic analyses
Immunophenotyping and cytogenetic analysis were performed locally. CD33 expression level was determined by dividing the mean fluorescence intensity of CD33 on the leukemic blasts by the mean fluorescence intensity of its isotypic control. The threshold for positivity was defined by a ratio of ≥ 2 as previously described 23. Cytogenetics was classified according to standard ISCN criteria 24. Central screening was performed for the nucleophosmin gene (NPM1) mutation, the FMS-like tyrosine kinase 3 gene (FLT3) internal tandem duplication (ITD), and the CCAAT/enhancer-binding protein alpha gene (CEBPA) mutation. Patients were categorized according to the genetic risk classification of the European LeukemiaNet recommendations 25. Minimal residual disease (MRD) based on the chimeric fusion transcript NPM1 mutations or WT1 overexpression was assessed in bone marrow or peripheral blood samples from all informative and evaluable patients. Total RNA was extracted and reverse transcribed using the standardized protocol developed in the Europe Against Cancer (EAC) program. Real-time quantitative PCR (RQ-PCR) assays were performed using an ABI7900 machine (Applied Biosystems, Courtaboeuf, France) for the control gene (ABL or PBGD) and various target genes (PML-RARA, AML1-ETO, CBFB-MYH11 fusion transcript, NPM1 mutant and WT1 transcripts), as previously described 26-29. Standard curves were established using plasmids from Ipsogen (Ipsogen, Marseille, France) and MRD results were expressed as normalized copy numbers (per 100 ABL copies).
Statistical analysis
Relapse-free survival (RFS), leukemia free survival (LFS), and OS were estimated according to the Kaplan–Meier method and compared between groups using the Gehan-Wilcoxon test 30. RFS was calculated from the first reported date of response to the earliest date of relapse or death, LFS was calculated from the first day of therapy to the earliest date of relapse or death. Duration of response was censored at the last examination date for patients with an ongoing response. Overall survival (OS) was calculated from the time of the start of treatment to the date of death and was censored at the last recorded contact or evaluation for patients alive at the time of analysis. The two-tailed Fisher's exact test was used for categorical variables. Adverse events were evaluated throughout the study in all patients who received at least one dose of GO.
Results
Patient characteristics
Twenty-four consecutive AML patients in first relapse aged 55 years or over diagnosed in our institution between October 2007 and November 2011 received combined fractionated GO plus cytarabine (7+fGO). Median age was 68 years (range, 55–77). All patients received a 3+7 regimen as CR1 induction. Consolidation therapy was based on conventional dose Ara-C combined with anthracycline in three patients, intermediate-dose Ara-C in 20 patients and ATRA-ATO in the remaining APL patient. No patient received allogenic HSCT in first CR (no sibling or 10/10 match donor available for the four patients eligible for allogenic HSCT in first CR). Median duration of first CR was 16 months (range, 6–33). Distribution of cytogenetics, genetic risk classification and Breems score are described in Table 1. According to Breems score, 4 patients (17%) belonged to the low relapse risk, 7 (29%) to the intermediate risk, and 13 (54%) to the poor risk categories.5 Following salvage therapy, 13 patients in CR received the 2 planned consolidation cycles with GO single dose and ID-AraC and 6 of them received LD-AraC as a maintenance therapy. Five patients in CR2 did not receive GO (three were not considered fit and received LD-AraC, two received ID-ARAC alone before allogenic HSCT). Patients were followed up for a median of 42 months (range, 19–70).
| Patients | Age (y) | Sex | FAB subtype | Cytogenetic risk groupa | Molecular markers | First line therapy | CR1 (months) | Breems score | |
|---|---|---|---|---|---|---|---|---|---|
| Induction | Consolidation | ||||||||
| 1 | 61 | M | AML2 | Intermediate | EVI1 overexpressed | DNR80 x3 + AraC 7d | DNR80x1 + ID AraCx4, 2 cycles | 20 | 7 |
| 2 | 73 | F | Unclassified | Unfavorable | MLL | DNR45 x4 + AraC 7d | DNR45x1 + AraCx5, 3 cycles | 30 | 7 |
| 3 | 67 | M | AML5b | Intermediate | NPM1+ / FLT3-ITD+ | DNR80 x3 + AraC 7d | DNR80x1 + ID AraCx4, 2 cycles | 15 | 10 |
| 4 | 57 | F | AML 2 | Intermediate | NPM1+ | DNR 80x3 + AraC 7d | DNR80x1 + ID AraCx4, 2 cycles | 19 | 7 |
| 5 | 72 | F | AML 1 | Intermediate | NPM1 + | DNR60 x3 + AraC 7d | DNR60x1 + AraCx5, 6 cycles | 13 | 10 |
| 6 | 74 | M | AML 1 | Intermediate | NPM1+ / FLT3-ITD+ | DNR45 x4 + AraC 7d | DNR45x1 + AraCx5, 6 cycles | 6 | 12 |
| 7 | 76 | M | AML 4 | Favorable | AML1-ETO | DNR60 x4 + AraC 7d | AMSA90x1 + ID AraCx4, 2 cycles | 33 | 5 |
| 8 | 69 | F | AML 2 | Intermediate | EVI1 overexpressed | DNR80 x3 + AraC 7d | DNR80x1 + ID AraCx4, 2 cycles | 28 | 7 |
| 9 | 68 | M | AML 1 | Intermediate | NPM1+ | DNR80 x3 + AraC 7d | DNR80x1 + ID AraCx4, 2 cycles | 21 | 7 |
| 10 | 60 | F | AML 1 | Intermediate | NPM1+ | DNR80 x3 + AraC 7d | DNR80x1 + ID AraCx4, 2 cycles | 18 | 10 |
| 11 | 61 | F | AML 1 | Intermediate | NPM1+ | DNR60 x3 + AraC 7d | DNR60x1 + ID AraCx4, 2 cycles | 15 | 10 |
| 12 | 55 | M | AML 1 | Unfavorable | None | DNR80 x3 + AraC 7d | DNR80x1 + ID AraCx4, 2 cycles | 9 | 10 |
| 13 | 67 | M | AML 0 | Intermediate | None | DNR80 x3 + AraC 7d | DNR80x1 + ID AraCx4, 2 cycles | 12 | 10 |
| 14 | 55 | M | AML 2 | Intermediate | CEBPA+ | DNR80 x3 + AraC 7d | DNR80x1 + ID AraCx4, 2 cycles | 13 | 10 |
| 15 | 58 | M | AML 4 | Intermediate | NPM1+ /MLLdup / FLT3-ITD+ | DNR80 x3 + AraC 7d | DNR80x1 + ID AraCx4, 2 cycles | 12 | 10 |
| 16 | 75 | F | AML 4 | Intermediate | NPM1+, FLT3-ITD+ | DNR45 x4 + AraC 7d | DNR80x1 + ID AraCx4, 2 cycles | 16 | 10 |
| 17 | 58 | F | AML 1 | Intermediate | FLT3-ITD+ | DNR80 x3 + AraC 7d | DNR80x1 + ID AraCx4, 2 cycles | 7 | 10 |
| 18 | 77 | F | AML 3 | Intermediate | PML-RAR | DNR80 x3 + ATRA+ATO | ATRA + ATO | 27 | 5 |
| 19 | 71 | F | sAML | Intermediate | None | IDA9 x4 + AraC 7d | IDA9x1 + ID AraCx4, 2 cycles | 19 | 7 |
| 20 | 67 | F | AML 2 | Intermediate | None | DNR80 x3 + AraC 7d | DNR80x1 + ID AraCx4, 2 cycles | 12 | 10 |
| 21 | 72 | F | AML 4 | Intermediate | NPM1+ | DNR45 x4 + AraC 7d | DNR80x1 + ID AraCx4, 2 cycles | 12 | 10 |
| 22 | 59 | M | AML 4 Eo | Favorable | CEBPA-MYH11 | DNR60 x3 + AraC 7d | DNR80x1 + ID AraCx4, 2 cycles | 11 | 5 |
| 23 | 71 | F | AML 2 | Intermediate | None | DNR80 x3 + AraC 7d | DNR80x1 + ID AraCx4, 2 cycles | 34 | 7 |
| 24 | 78 | M | AML 4 Eo | Favorable | CEBPA-MYH11 | DNR80 x3 + AraC 7d | DNR80x1 + ID AraCx4, 2 cycles | 14 | 5 |
- fGO, fractionated gemtuzumab ozogamicin; CR1, first complete remission; DNR, daunorubicin; DNR or IDA aa x b, DNR or IDA aa mg/d for b days; IDA, Idarubicin; AraC, cytarabine, conventional dose; LD AraC, low dose AraC; ID AraC, intermediate dose AraC.
- a According to the European LeukemiaNet recommendations.25
Efficacy
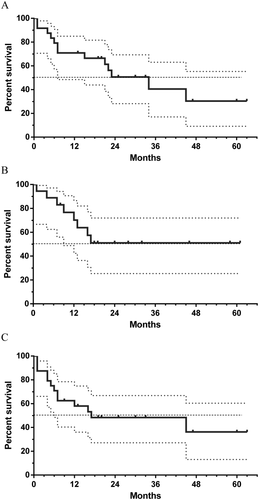
Overall response rate in patients treated with 7+f-GO was 75% (18 out of 24 patients), including CR in 16 patients and CRp in 2 patients. At 24 months, OS was 51% (95% CI: 28–69), LFS was 48% (95%CI: 27–67), and RFS was 51% (95%CI: 25–72). Median OS was 34 months, median LFS was 17 months, and median RFS was not reached (Fig. 1). Duration of second CR (CR2) was longer than first CR (CR1) in 9 out of 18 patients (50%), ranging from 3 to 46 months longer. Four patients underwent allogenic HSCT with reduced intensity conditioning 2, 4, 5, and 7 months after achieving CR2. One patient died 1 month post-transplant and the three other patients were alive 7, 30, and 33 months post-transplant. No difference in OS and DFS was evidenced in our patient population according to the Breems score.
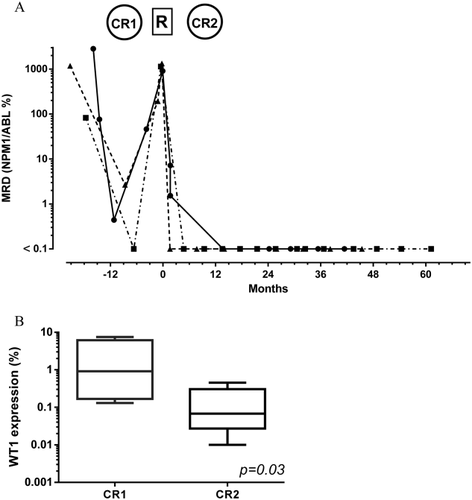
MRD in CR2 was evaluable in 13 patients. All five evaluable patients with NPM1 mutations were below the threshold of detection in blood or bone marrow after 7+fGO salvage. As an example, a lower MRD level was achieved in CR2 as compared to CR1 in 3 NPM1 mutated patients who were evaluable for MRD in CR1 and in CR2 (Fig. 2A). Eight evaluable patients with WT1 over-expression were within the normal range in blood after CR2. This included six patients who were evaluated in CR1 and in CR2. Again, the MRD levels after CR2 were significantly inferior to MRD levels after CR1 (Fig. 2B). Two patients with CBFβ-MYH11 and PML-RARA transcripts were negative.
Safety
All 24 treated patients developed grade 3–4 neutropenia and/or thrombocytopenia. Median duration of neutropenia (<0.5 × 109/L) was 25days (range 13–34) and the median duration of thrombocytopenia (<50 ×109/L) was 28 days (range 20–39). Two patients had persistent thrombocytopenia post-induction and did not receive GO during the consolidation courses. Two patients (8.3%) died during the induction period, one from a VOD and one from septic shock arising during a VOD. None of the four patients who went on to receive a transplant developed VOD post-transplant. Ten patients (41.7%) developed documented infections, including eight with bacterial septicaemia, and two with fungal infections. No treatment-related mortality was observed during the consolidation courses.
Discussion
Despite the omission of anthracyclines, the 7+fGO, combining fractionated doses of GO with conventional doses of cytarabine, produced a 75% response rate associated with prolonged RFS in aged AML patients in first late relapse. We decided not to include aged AML patients that were refractory to chemotherapy or in early relapse (<6 months) due to their very poor prognosis. Recommendations suggest that these patients may be eligible for supportive care or experimental therapies only 31. As a matter of fact, only 54% of our patients fall in the high risk category according to Breems et al 5. However, no significant differences were observed in our study between high risk and low/intermediate risk patients in late relapse.
Other studies combining GO and chemotherapy in relapsing adult AML patients have been reported 9-20. The largest study tested the MIDAM regimen with intermediate dose cytarabine (1 g/m2, every 12 hr, days 1–5) and mitoxantrone (12 mg/m2/day, days 1–3) combined with a single dose of GO (9 mg/m2, day 4). Patients with refractory CD33+ AML (18 patients) or CD33+ AML in relapse (44 patients) were included, and median patient age was 55.5 years. Response rate (CR and CRp) was 63%. The CR rate in patients aged 60 years or more was 68% (15 of 22 patients). Median time to neutrophil recovery was 25 days. Of note, 47 out of 62 patients (76%) experienced bacterial sepsis or fungal infections 9. The median age of patients included in our study was older than that in the MIDAM study. However, we did not observe more toxicity and time to neutrophil recovery was comparable with fewer episodes of documented infections. Our results thus suggest that it may not be necessary to add anthracycline when fractionated GO is used.
Table 2 recapitulates results obtained with the 7+fGO schedule and those obtained in our two previous phase 2 studies testing fractionated GO doses, either alone (Mylofrance 1 study 21) or in combination with a 3+7 regimen (Mylofrance 2 study 22) (Table 2). Although the patient populations were not strictly comparable, a higher CR + CRp rate was observed without an increase in duration of neutropenia in patients treated with the 7+fGO regimen as compared to fGO alone. Liver toxicity with or without VOD and persistent thrombocytopenia were the main GO-associated toxicities across the three clinical trials, independent of the addition of daunorubicin and cytarabine, suggesting that these adverse events are mainly driven by GO itself. This observation suggests that it may be possible to further optimize fractionated GO in future clinical trials.
| GO + cytarabine 7+fGO (this study) | Mylofrance 1 21 fGO alone | Mylofrance 2 22 3+7 and fGO | |
|---|---|---|---|
| Number of patients | 24 | 57 | 20 |
| Median age (years) | 68 | 64 | 60 |
| Median CR1 duration (months) | 16 | 10 | 10 |
| Prognostic classification,a n (%) | |||
| Favorable | 4 (16.7) | 0 | 2 (10) |
| Intermediate | 17 (70.8) | 44 (78) | 18 (80) |
| Unfavorable | 3 (12.5) | 12 (22) | 2 (10) |
| Response rate (CR + CRp) (%) | 75 | 33.3 | 55 |
| Neutrophils <0.5 × 109/L(median duration in days) | 25 | 23 | 30 |
| Platelets <50 × 109/L(median duration in days) | 28 | 20 | 32 |
| Median OS (months) | 34 | 8.4 | 15 |
| Median RFS (months) | Not reached | 11 | 12 |
- CR, complete remission; CR1, first complete remission; CRp, CR with incomplete platelet recovery; fGO, fractionated gemtuzumab ozogamicin; OS, overall survival; RFS, relapse-free survival.
- a According to the European LeukemiaNet recommendations.25
The safety and efficacy of GO in combination with conventional cytarabine was previously reported in elderly AML patients receiving frontline treatment 32. In a randomised phase 2 trial, GO was administered at 6 mg/m2 on day 1 and 4 mg/m2 on day 8, a higher and less fractionated dose of GO compared to our study. The GO + cytarabine arm was compared to the classic 3+7 regimen. Five patients out of 57 in the GO arm developed severe VOD and two patients died. However duration of neutropenia was comparable in both arms. Despite a higher rate of induction deaths in the GO arm, a trend was observed in favor of GO for a longer duration of remission. Recently, a retrospective study from the ALFA group suggested an increased efficacy of GO combined to intensive chemotherapy (ICT) as compared to ICT alone in relapsing AML patients aged 50 years or older if CR1 duration was superior to 12 months 33. These results are in line with those obtained in our study using fractionated GO and low intensity chemotherapy.
A remarkable effect of GO seen in this study was its capacity to lower MRD levels, especially in patients with NPM1 mutations as previously reported 34. MRD was shown as a major prognostic factor for remission duration in AML patients 35. The value of MRD after salvage therapy in first relapse has not been extensively studied 36. We were able to compare paired levels of molecular residual disease in CR1 and after salvage therapy with 7+fGO. We observed a gain in the extent of the response in evaluable samples. These results may partly explain the long RFS duration observed in our study.
In conclusion, fractionated doses of GO combined with standard dose cytarabine in aged AML patients in first relapse was associated with a high response rate, prolonged survival and a favorable toxicity profile. An improvement in MRD levels was seen in paired CR1 and CR2 evaluations suggesting a marked effect of GO on residual leukaemic cells.
Acknowledgment
We thank Sarah MacKenzie for medical editorial assistance with this manuscript.
Author Contributions
PR was the principal investigator and takes primary responsibility for the article. SC, SP, SR, CS, FR, ALT, HF, FM, MA, and SG recruited the patients. VR, CT, IG, AR, and CP performed the laboratory work. PR and SP participated in the statistical analysis. PR and SP wrote the article.




