Novel type of red blood cell pyruvate kinase hyperactivity predicts a remote regulatory locus involved in PKLR gene expression
Conflict of interest: Nothing to report
Abstract
Red blood cell pyruvate kinase (PK-R) is a key regulatory enzyme of red cell metabolism. Hereditary deficiency of PK-R is caused by mutations in the PKLR gene, leading to chronic nonspherocytic hemolytic anemia. In contrast to PK deficiency, inherited PK hyperactivity has also been described. This very rare abnormality of RBC metabolism has been documented in only two families and appears to be without clinical consequences. Thus far, it has been attributed to either a gain of function mutation in PKLR or to persistent expression of the fetal PK isozyme PK-M2 in mature red blood cells. We here report on a novel type of inherited PK hyperactivity that is characterized by solely increased expression of a kinetically normal PK-R. In line with the latter, no mutations were detected in PKLR. Mutations in regulatory regions as well as variations in PKLR copy number were also absent. In addition, linkage analysis suggested that PK hyperactivity segregated independently from the PKLR locus. We therefore postulate that the causative mutation resides in a novel yet-unidentified locus, and upregulates PKLR gene expression. Other mutations of the same locus may be involved in those cases of PK deficiency that fail to reveal mutations in PKLR. Am. J. Hematol. 89:380–384, 2014. © 2013 Wiley Periodicals, Inc.
Introduction
Pyruvate kinase (PK) deficiency (OMIM 266200) is an autosomal recessive disorder and represents the most common defect of red blood cell glycolysis 1. As the enucleated red blood cell relies entirely on glycolysis for its energy production, a deficiency of the key regulatory enzyme PK results in an energy-deprived red blood cell that is eventually prematurely cleared from the circulation. The associated type of anemia is designated hereditary nonspherocytic hemolytic anemia. PK deficiency is caused by mutations in the PKLR gene which is located on chromosome 1q22. To date, more than 200 mutations have been identified in association with PK deficiency (www.lovd.nl/pklr, this website replaces pklrmutationdatabase.com). The majority of PK-deficient patients are either homozygous or compound heterozygous for a mutation in PKLR. However, in a number of cases the anticipated causative mutation(s) remains to be indentified 2-6 (van Wijk, unpublished results, Bianchi, personal communication, with permission).
In contrast to deficient enzyme activity, inherited hyperactivity of red blood cell PK has also been described (OMIM 102900). This very rare abnormality of red blood cell metabolism has been confirmed in only two families 7, 8. In both of these families PK hyperactivity presented as a dominantly inherited disorder without apparant clinical consequences. The etiology in both cases, however, was found to be quite different. In the first described family 7, increased PK activity was found to be because of altered enzymatic properties of PK-R 9. In line with these findings a heterozygous missense mutation in PKLR was later identified 10. In the second family 8 increased PK activity was attributed to persistent expression of the fetal isozyme PK-M2 11, 12.
Recently, we encountered a family with inherited red blood cell (RBC) PK hyperactivity. We set out to investigate the nature of increased PK enzymatic activity in this family by extensive genetic analysis of PKLR, RBC PK protein expression, and kinetic characterization of RBC PK.
Materials
Patients
The index patient concerns a healthy 2-year old female, referred to our Institution for measurement of red blood cell enzyme activities. The finding of unexplained strongly elevated activity of PK prompted further study of additional family members to investigate the cause of PK hyperactivity. Informed consent was obtained from all family members in accordance with the Helsinki Declaration of 1975.
Genetic analysis of PKLR
DNA sequence analysis of PKLR exons 1–12 (including the 5′- and 3′-UTR), flanking intronic sequences, and the erythroid-specific promoter was performed as previously described 13. In addition, approximately 1 kbp of the upstream region, intron 1, and a predicted enhancer region in the 5′-end of intron 3 14 were analyzed for the presence of mutations (for primer sequences see Supporting information Table I). In order to detect any variation in PKLR copy number Multiplex Ligation-dependent Probe Amplification (MLPA) was performed using the P203 PKLR Probemix (MRC Holland), according to the manufacturer's instructions. Fragments were amplified and analyzed on an ABI 3130 automated sequencer (PE Applied Biosystems). Peaks were analyzed with GeneMarker (SoftGenetics, LLC, State College).
PKLR linkage analysis was performed using intragenic markers rs2071053 (intron 5), rs57484820 (intron 10), rs201306934 (intron 11), rs1052176 and rs1052177 (exon 11), and extragenic microsatellite markers at chromosome positions 155,133,067, 155,136,017 (5′ to PKLR), 155,302,252, and 155,326,870 (3′ to PKLR) (primer sequences available on request).
PK enzymatic properties, immunological specific activity, and thermostability
Enzymatic activities of red blood cell PK, HK, and G6PD, as well as PK kinetic properties and thermal stability were determined according to standard methods 15, 16. Immunological specific activity of PK was determined by immunoinactivation with anti-PK-L antiserum 17 on crude hemolysates according to previously described methods 18. Results were expressed as the amount of milli-units of PK activity inhibited per μl of antiserum. Thermostability of PK was investigated by performing the heat stability test according to standard methods 16 with the exception that the used temperature was 56°C (instead of 53°C).
Western blot analysis of PK-R and PK-M2
Western blot analysis of PK was performed as previously described 19, using polyclonal antibodies directed against PK-L 17 and a monoclonal antibody raised against PK-M2 20. Actin was used as a loading control (mouse monoclonal anti-α-actin, GE Healthcare-Amersham). Blots were visualized using Alexa Fluor® 680-conjugated goat anti-mouse and goat anti-rabbit antibodies (Invitrogen) and quantified using the Odyssey Infrared Imaging System and ImageJ shareware (http://imagej.nih.gov/ij/). Results were expressed as the ratio PK-M2/actin and PK-R/actin.
Quantification of PK antigen levels by enzyme-linked immunosorbent assay
Quantitative measurements of red blood cell PK was performed by enzyme-linked immunosorbent assay (ELISA) using rabbit antibodies against the A and C domains of PK-R as previously described 13. PK antigen levels were calculated from a standard curve, obtained from eight dilutions (range: 1:50–1:1000) of recombinant human PK-R protein, expressed in HEK 293T cells. The amount of PK antigen in the undiluted solution of recombinant PK was arbitrarily set to 1000 antigen units (au). Final results were corrected for the amount of hemoglobin in each sample and expressed per gram of hemoglobin, analogous to PK enzymatic activity.
Results and Discussion
The index patient (individual III-1 in Fig. 1) concerns a healthy 2-year old female. She was referred to our Institution for routine measurements of red blood cell enzyme activities because glucose-6-phosphate dehydrogenase (G6PD) deficiency was known to segregate in the maternal family line. G6PD enzymatic measurements were inconclusive but subsequent DNA analysis revealed the propositas to be a carrier for the p.Leu128Pro substitution in G6PD (G6PD Vanua Lava) 21. The same G6PD variant had previously been identified in the child's mother. Notably, enzymatic activities of hexokinase (HK) and PK revealed an approximately three-fold increase in activity of the latter enzyme in the index patient's RBCs (Fig. 1). Because both the reticulocyte count and HK activity were normal, this increase could not be attributed to a red blood cell population of relatively young red cell age 22. Enzymatic measurements of additional family members revealed high PK activity also in the index patients' father, paternal aunt, and grandfather. PK activity in the child's mother and grandmother was normal (Fig. 1). A cord blood sample from the newborn sister of the index patient (individual III-2) also displayed high PK activity. Even though it is well established that cord blood displays higher activities of many red blood cell enzymes 23, the disproportionately higher activity of PK compared to the slightly elevated activities of HK and G6PD, makes it reasonable to assume that this individual is also affected by PK hyperactivity. Therefore, similar to the two previous reported families 7, 12, PK hyperactivity in this family segregated in a dominant manner. All family members were clinically and hematologically normal (Supporting information Table II).
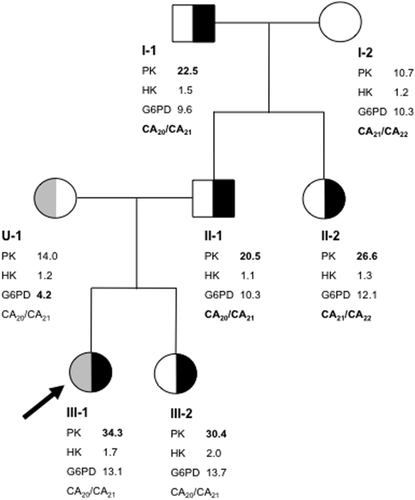
To elucidate the nature of PK hyperactivity we first considered the presence of a gain of function mutation in PKLR. However, DNA sequence analysis of individual exons and flanking intronic sequences of PKLR displayed no mutation (results not shown). At the same time, PK kinetic properties were determined using RBCs from subject III-1. Table 1 shows that substrate binding, cooperativity, inhibition by ATP, and immunological properties of PK from the subject displaying PK hyperactivity were all normal. The obtained values were also in line with values previously reported in the literature 9. Hence, the lack of any mutation in PKLR combined with normal kinetic properties of RBC PK led us to conclude that a quantitative defect rather than a qualitative defect was the cause of increased PK activity in this family.
| III-1 | Control | |
|---|---|---|
| Enzymatic activity (U/gHb) | 34.3 | 9.4 |
| n-Hill | 2.2 | 2.6 |
| K0.5 PEP (mM) | 0.72 | 0.80 |
| K0.5 PEP in presence of FBP (mM) | 0.14 | 0.18 |
| Km ADP (mM) | 0.26 | 0.29 |
| ATP inhibition (% residual activity) | 46 | 25 |
| Immunoinactivation of PK (mU/μl) | 69.5 | 64.5 |
| Thermostability (% residual activity) | 21 | 25a |
- n-Hill, Hill coefficient; PEP, phosphoenolpyruvate; FBP, fructose-1,6-biphosphate; ADP, adenosine diphosphate.
- a N = 6, median: 25%, range 19–34%.
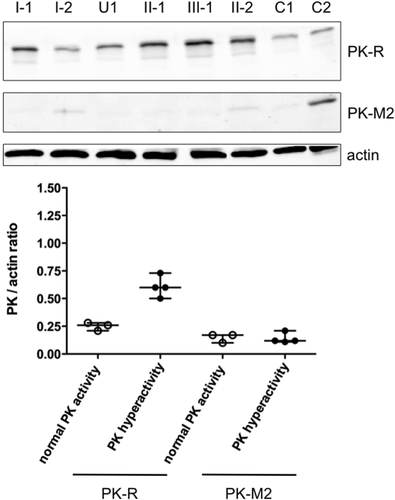
We therefore next investigated the PK isozyme composition in RBCs from all family members, except for individual III-2 who was excluded for further biochemical analysis because of the alternate sample source (cord blood). Western Blot analysis using antibodies directed against PK-R and PK-M2 showed that PK-M2 levels were low but detectable in all family members (Fig. 2, top). They did not differ, however, between affected (median PK-M2/actin ratio: 0.12, range: 0.11–0.21, N = 4) and nonaffected individuals (median ratio: 0.17, range: 0.10–0.17, N = 3, Fig. 2, bottom). In contrast, PK-R protein levels were clearly increased in individuals displaying hyperactivity: when corrected for actin content, the median PK-R/actin ratio in RBCs from nonaffected individuals was 0.26 (range: 0.21–0.28, N = 3) whereas this was approximately 2.5-fold higher in individuals with PK hyperactivity (median: 0.60, range: 0.50–0.73, N = 4, Fig. 2, bottom).
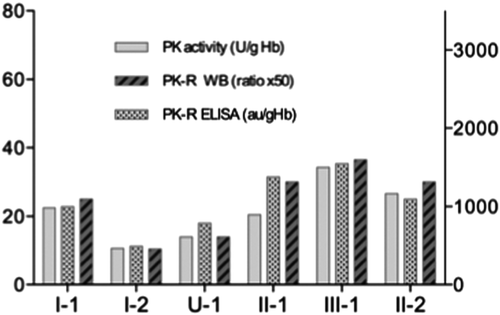
To further substantiate our findings on the cause of PK hyperactivity in this family we also quantified PK-R levels by means of ELISA 13, and compared the data with Western Blot results and PK activity measurements. Figure 3 demonstrates that PK-R levels as determined by ELISA parallel those determined by Western Blot. Furthermore, when compared to RBC PK enzymatic activity it is evident that the increase in PK activity in individuals displaying PK hyperactivity is accompanied by a concomitant increase in PK-R protein levels (Fig. 3.). Altogether, our results strongly suggest that PK hyperactivity in this family is caused by an increase in the levels of the PK-R isozym.
The here described family differs from both previous reported cases of PK hyperactivity. In the first described family 7, the enzyme showed a decreased K0.5 for the substrate phosphoenolpyruvate, a decreased Hill coefficient, a decreased response to ADP, and a lack of inhibition by ATP, whereas Km for ADP, nucleotide specificity, the thermostability, pH optimum, and immunological specific activity were normal 9. The authors concluded that PK hyperactivity likely resulted from a shift in the R(elaxed) versus T(ight) equilibrium towards the R(elaxed) form. In line with these altered kinetic properties a single nucleotide substitution in exon 2 of PKLR (c.110G>A) was later identified 10. This missense mutation predicts an amino acid substitution at residue 37 (p.Gly37Glu) that was postulated to interfere with phosphorylation of Ser42. As phosphorylation of this latter residue is known to inhibit PK enzymatic activity 24, abolishment of phosphorylation would account for increased enzymatic activity. In the second family 12 increased PK activity could be attributed to persistent expression of the fetal isozyme PK-M2 in mature RBCs from individuals with PK hyperactivity 8. Expression of this fetal isozyme normally declines during red cell differentiation and maturation 25, 26. However, in this particular family the authors demonstrated an absent decline of PK-M2 synthesis during RBC maturation 27, probably because of a disorder of the mechanism(s) responsible for the disappearance of PK-M2 during erythroid maturation 11. Extensive kinetic characterization of PK-R in this case revealed no abnormalities except for various levels of thermal instability 8.
PK hyperactivity in the here described family was not because of altered kinetic properties of PK-R, nor to persistent expression of PK-M2. Instead, it was caused by increased expression of a kinetically normal PK-R isozyme. This represents a novel cause underlying this disorder. Increased expression of PK-R could, for instance, be because of increased transcription of PKLR, or an increase in enzyme stability. We addressed the latter possibility by subjecting RBC PK of individual III-1 to a modified heat stability test (carried out at 56°C instead of 53°C). As shown in Table 1 there were no differences in the decrease of PK enzymatic activity between the individual with PK hyperactivity and six normal controls. This demonstrates that, at least under the conditions chosen, PK-R stability does not appear to be increased.
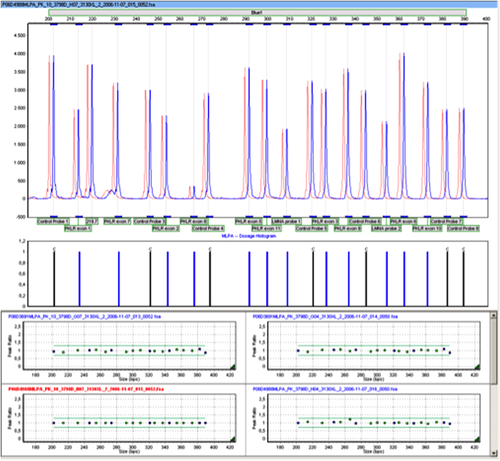
To investigate the possibility of a regulatory mutation influencing PKLR gene expression we performed extensive DNA sequence analysis of PKLR regulatory regions involving the erythroid-specific promoter 28, 29, approximately 1000 bp of the 5′ upstream region of PKLR, and predicted enhancer regions in intron 1 and the 5′-end of intron 3 14. No mutations were detected in any of these regions. In addition, MLPA analysis showed no abnormalities, which rules out variation in PKLR copy number that could account for increased PKLR expression (Fig. 4.). Ultimately, these results all argue against a mutation in PKLR as the cause of PK hyperactivity. To further substantiate this we performed PKLR linkage analysis. The intragenic markers used were not informative (Table 2). However, the segregation pattern of one particular microsatellite marker located just downstream of PKLR (AC repeat at 1:155,326,870) indicated that the paternal PKLR allele in subject II-1 was linked to 20 AC repeats, whereas the paternal PKLR allele in subject II-2 was linked to 21 AC repeats (Fig. 1 and Table 2, bold face). Thus, despite the fact that both these siblings displayed PK hyperactivity they inherited a different copy of PKLR from their father (I-1) who also showed PK hyperactivity.
| 5′ extragenic markers | PKLR (1:155,259,030–155,271,825) | 3′ extragenic markers | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1:155,133,067 (GT repeat) | 1:155,136,017 (AC repeat) | rs2071053 | rs57484820 | rs201306934 | rs1052176 | rs1052177 | 1:155,302,252 (AT repeat) | 1:155,326,870 (AC repeat) | |
| I-1 | GT16/GT17 | AC14/AC15 | C/T | T10/T10 | ATT14/ATT14 | C/C | T/T | AT10/AT12 | AC20/AC21 |
| I-2 | GT16/GT17 | AC14/AC15 | C/T | T10/T10 | ATT14/ATT15 | C/C | T/T | ND | AC21/AC22 |
| U-1 | GT17/GT18 | AC14/AC15 | C/T | T10/T10 | ATT14/ATT15 | C/C | T/T | AT10/AT12 | AC20/AC21 |
| II-1 | GT16/GT17 | AC14/AC15 | C/T | T10/T10 | ATT14/ATT15 | C/C | T/T | AT10/AT12 | AC21/AC22 |
| II-2 | GT16/GT17 | AC14/AC15 | C/T | T10/T10 | ATT14/ATT14 | C/C | T/T | ND | AC20/AC21 |
| III-1 | GT16/GT17 | AC14/AC15 | C/T | T10/T10 | ATT14/ATT15 | C/C | T/T | AT10/AT12 | AC20/AC21 |
| III-2 | GT16/GT17 | AC14/AC15 | C/T | T10/T10 | ATT14/ATT15 | C/C | T/T | AT10/AT12 | AC20/AC21 |
Therefore, our results ultimately lead us to conclude that PK hyperactivity in this family is caused by an inherited abnormality unlinked to PKLR. We postulate the existence of a novel, yet-unidentified, remote DNA region regulating PKLR gene expression. Gain of function by mutation in this locus results in this case in increased expression of PKLR, increased levels of PK-R, and, consequently, increased RBC PK activity. It is tempting to speculate that in a similar manner loss of function mutations of the same locus could result in PK deficiency. Such mutations may explain those cases of suspected PK deficiency in which extensive DNA sequence analysis of PKLR failed to identify a second anticipated mutation or any mutation at all 2-6 (van Wijk, unpublished results, Bianchi, personal communication, with permission). Similarly, the same locus may represent one of the yet-unknown (genetic) factors that contribute to the remarkable variable clinical picture associated with mutations in PKLR 1, 30. Future studies aimed at identifying the exact nature and location of the here predicted PKLR regulatory region may therefore shed new light on PKLR gene regulation and the phenotypic expression of PK deficiency.
Acknowledgements
The authors sincerely wish to thank the family members for their kind cooperation.




