Acquired alpha thalassemia associated with erythroleukemia
Conflict of interest: Nothing to report.
Hemoglobin H (HbH) inclusions are typically seen in alpha thalassemia, inherited as an autosomal recessive hemoglobinopathy. Occasionally, HbH inclusions can occur as acquired defects in bone marrow disorders associated with ineffective erythropoiesis.
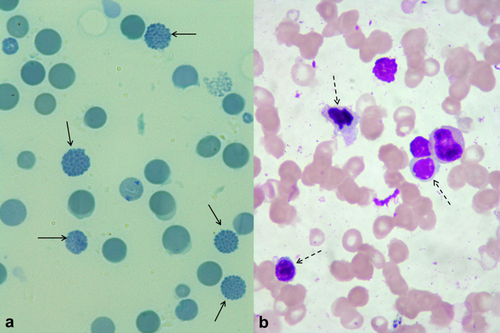
A 33-year-old male presented with history of anemia and leukopenia over a 6-month period, with no lymphadenopathy or hepatosplenomegaly. His hemogram showed Hb concentration of 8.5 g/dl, total white cell count 1,700/mm3, and platelet count of 242,000/mm3, with low mean corpuscular volume (74 fl) and mean corpuscular Hb (23 pg). Peripheral smear showed hypochromic microcytic erythrocytes, mild polychromasia, 5% nucleated red cells, trilineage dysplasia, and occasional myeloblasts. He was transfusion independent and had normal levels of serum ferritin, total iron binding capacity, and transferrin saturation. There was no family history of hemoglobinopathies. He had reticulocytosis (2.6%) and a normal level of HbA2 (1.54%) on electrophoresis. Serum levels of vitamin B12 (490 pg/ml) and folic acid (19.6 ng/ml) were within normal limits. An HbH supravital stain preparation revealed “golf ball” type inclusions in 15% of red cells (Image 1a). Bone marrow aspirate (Image 1b) showed trilineage dysplasia, 69% erythroblasts, and 11% myeloblasts (35% of non-erythroid nucleated cells), consistent with transformation of myelodysplastic syndrome (MDS) to M6 subtype of acute myeloid leukemia (AML). Erythroleukemia often results from transformation of MDS 1, 2.
Patients with clonal hematological disorders may occasionally undergo changes in alpha globin synthesis, leading to acquired alpha thalassemia (AAT). This has been reported in association with MDS, AML, acute lymphoblastic leukemia, myelofibrosis, and essential thrombocythemia 3. It is now known that the underlying molecular defect in such acquired cases is with the trans-acting regulator of globin gene expression, in contrast to cis-acting mutations in inherited alpha thalassemia 4. Over 85% of patients with AAT reported to date have been elderly males, with a median age at diagnosis of 68 years. A review by Steensma et al. identified that AAT has been reported in only 10 patients younger than 50 years of age 3. Anecdotal evidence indicates that the development of AAT is associated with a worsening of the underlying clonal disorder 3.
Acknowledgments
The technical and photographic support from BC Sangamesh and LB Manigantan is acknowledged with gratitude.




