Sorafenib, a multikinase inhibitor, is effective in vitro against non-hodgkin lymphoma and synergizes with the mTOR inhibitor rapamycin†
Conflict of interest: SK: research support from Celgene, Millennium, Novartis, Merck, Cephalon, Genzyme and Bayer; SK: advisory board, Merck
Abstract
Non-Hodgkin lymphoma (NHL) represents a heterogenous group of neoplasias originating from lymphoid cells. Increased angiogenesis and expression of Vascular Endothelial Growth Factor (VEGF) and its receptors (VEGFR) have been found to be associated with NHL disease progression. Increase in VEGF and other cytokines stimulate signaling cascades, including the Ras/Raf/Mek/Erk pathway, resulting in increased proliferation and decreased apoptosis. Here, we report the in vitro antilymphoma activity of sorafenib, an inhibitor of VEGFR and Raf kinase. Sorafenib induced potent cytotoxicity in NHL cell lines and patient samples. This induction of cytotoxicity was associated with a corresponding increase in apoptotic cell death. Mechanism of action of sorafenib was investigated in follicular (DoHH2) and Burkitt lymphoma (Raji) cell lines. pStat3, pAkt, Mcl1, and Xiap were downregulated in both cell lines, whereas pErk decreased in Raji but not in DoHH2 cells following sorafenib treatment. IL6 was unable to prevent sorafenib induced repression of pStat3, pAkt, Mcl1, and Bcl-Xl. Sorafenib in combination with an mTORC1 inhibitor rapamycin demonstrated synergy in inducing cytotoxicity in NHL cells. Sorafenib/rapamycin combination resulted in downregulation of pAkt, pmTOR, p-p70S6K, p4EBP1, pGSK3β, Mcl1, and Bcl-Xl. On the basis of our results, a clinical trial is underway using sorafenib with everolimus in NHL patients., 2012. © 2011 Wiley Periodicals, Inc.
Introduction
Angiogenesis and its relevance to cancer progression and metastasis has been extensively studied [1]. Several antiangiogenic agents have been approved by the FDA as anticancer drugs and more are in preclinical and clinical evaluations as treatment options in various cancers [2]. In hematological malignancies, including non-Hodgkin lymphoma (NHL), it has been found that the increase in tumor microvessel density correlates with disease progression [3-6]. Although several proangiogenic cytokines have been identified, VEGF has emerged as one of the most important [7, 8]. Increased angiogenesis associated with increased levels of VEGF and VEGF receptors (VEGF-R) have been observed in B and T-cell NHL with higher levels in advanced stages of the disease [9-13]. Coexpression of VEGF-A and VEGF-R1 was observed in bone marrow of NHL patients with extranodal disease involvement indicating a role for VEGF in cell migration and tumor spreading [11]. Indeed, expression of these molecules on NHL cells has been associated with an adverse prognosis [10]. Despite the expression of VEGF and VEGFR's on NHL cells, a large trial of single-agent bevacizumab in 52 patients with relapsed NHL demonstrated only a 2% overall response rate (ORR) [14].
This suggests that agents that target other pathways in the angiogenesis signal cascade may be needed. For example, aberrant signaling through the Raf-MEK-Erk pathway plays a critical role in tumor progression in a variety of cancers, including lymphoma [15]. This can result from mutations involving the signal transduction protein leading to constitutive activation as well as increased levels of receptor ligands, such as cytokines. Elevated Raf levels have been reported in mantle cell lymphoma [15]. In addition, Raf has been shown to act synergistically with the transcription factor myc to induce B-cell tumors in BALBC mice [16].
Sorafenib (BAY 43-9006) is a small molecule inhibitor of Raf kinase that has demonstrated anticancer activity against pancreatic, renal, colon, ovarian, nonsmall cell lung cancers, and multiple myeloma [17-20]. In addition, sorafenib also inhibits VEGFR namely VEGFR2, VEGFR3, and platelet derived growth factor receptor beta (PDGFR-β) [21]. The broad antiangiogenic activity of sorafenib led us to hypothesize that it may have activity in NHL. In this report, we demonstrate the ability of sorafenib to induce apoptosis in NHL cell lines and patient samples and inhibit angiogenesis in vitro.
Material and Methods
Lymphoma cell lines.
The lymphoma cell lines used were representative of different types of NHL and included Raji and Ramos (high grade lymphoma), Dohh2 (indolent follicular lymphoma) and Karpas 1106, Granta 519 and RL (diffuse large B-cell lymphoma). All the cell lines were cultured in RPMI 1640 media (Sigma Chemical, St. Louis, MO) that contained 10% fetal bovine serum, 2 mM L-glutamine (GIBCO, Grand Island, NY), 100 U/mL penicillin, and 100 μg/mL streptomycin.
Primary lymphoma samples.
Fresh lymphoma samples were obtained through the Lymphoma SPORE tissue biobank from patients who provided written informed consent for research use of surgical waste tissue. This protocol was approved by the Mayo Clinic Institutional Review Board. Lymph nodes or spleen were forced through wire screens to suspend cells, then ficolled and resuspended in RPMI with 10% FCS.
Sorafenib (Nexavar™).
Sorafenib was provided by Bayer HealthCare Pharmaceuticals. Stock solutions were made in DMSO at a concentration of 20 mM, aliquoted and stored at −20°C to avoid repeated freeze thaw. For individual experiments, the drug aliquot was thawed and diluted taking care to avoid final DMSO concentrations of over 0.01%.
Rapamycin.
Rapamycin (Sirolimus) was purchased from EMD Chemicals, (Gibbstown, NJ). Stock solutions were made in DMSO at a concentration of 5 mM, aliquoted and stored at −20°C to avoid repeated freeze thaw. For individual experiments, the drug aliquot was thawed and diluted taking care to avoid final DMSO concentrations of over 0.01%.
Cytotoxicity and proliferation assays.
Lymphoma cells were incubated in 96-well culture plates (Costar, Cambridge, MA) in media (RPMI 1640 media containing 10% fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin) alone, or with indicated concentrations of sorafenib for 48 hr at 37°C. For experiments with rapamycin, indicated concentrations of rapamycin and sorafenib were added alone or in combination and incubated for 48 hr at 37°C. To confirm cytotoxicity of sorafenib, colorimetric assays were performed using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrasodium bromide (MTT; Chemicon International, Temecula, CA) as previously described [22, 23]. All experiments were performed in triplicate. Cell proliferation assays measured tritiated thymidine uptake as described previously [22, 23]. Briefly, lymphoma cells were cultured in 96-well plates alone or with indicated concentrations of drug for 48 hr. Cells were pulsed with 3H-TdR during the last 16 hr, harvested and incorporated radioactivity determined using a scintillation counter (PerkinElmer, Waltham, MA).
Detection of apoptosis in lymphoma cell lines and patient samples.
Dohh2 cells were cultured with 5 μM of sorafenib for 8, 12, or 24 hr, while Raji cells were cultured with 7 μM of sorafenib for 12, 24, or 48 hr. All the experimental and control cultures were set up at the same time and incubated with drug for indicated periods of time. All cultures were harvested for further analysis at the same time. Cells were washed once with PBS and once with Annexin binding buffer (ABB) and then incubated with Annexin V-FITC (Invitrogen, Camarillo, CA) for 15 min in the dark at room temperature. Cells were washed with 2 mL ABB. The pellet was resuspended in 0.5 mL annexin binding buffer with 10 μg/mL propidium iodide (PI). The apoptotic fraction was identified as Annexin V-positive and PI-negative cells when analyzed using a FACS Canto (BD Biosciences, San Jose, CA).
Lymphoma cells were harvested from tissue samples of lymphoma patients. Lymph nodes or spleen were forced through wire screens to suspend cells, then ficolled, resuspended in RPMI with 10% FCS and incubated with indicated concentrations of sorafenib for 48 hr. The cells were harvested, washed twice with PBS, and apoptosis was measured by annexin/PI staining as described above.
Western blotting.
Dohh2 and Raji cells were cultured with or without 5 μM or 7 μM of sorafenib, respectively, for the indicated time points and lysed in buffer (50 mM HEPES (pH 7.4), 150 mM NaCl, 1% Triton X-100, 30 mM sodium pyrophosphate, 5 mM EDTA, 2 mM Na3VO4, 5 mM NaF, 1 mM phenylmethyl-sulfonyl-fluoride (PMSF), 5 μg/mL leupeptin, and 5 μg/mL aprotinin. For experiments with sorafenib and rapamycin, Dohh2 cells were cultured with 6 μM of sorafenib or 6 nM of rapamycin or 6 μM of sorafenib in combination with 6 nM of rapamycin for the indicated time points. Cell lysates were subjected to SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted with the relevant antibody. The antibodies used, namely pErk, Erk, pStat3, Stat3, pAkt, Akt, Mcl1, Bcl2, Bcl-xl, Xiap, pmTOR, mTOR, p-p70S6K, p70S6K, pGSK3β, GSK3β, p4EBP1, 4EBP1, caspase 8, caspase 9, PARP, and beta-actin were purchased from (Cell Signaling Technology). Antigen–antibody complexes were detected using enhanced chemiluminescence (Amersham, Arlington Heights, IL). Blots were stripped and reprobed with antiactin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) to ensure equivalent protein loading. All experiments were repeated on three separate occasions. In the experiments done to evaluate the effect of growth factors, Dohh2 cells were incubated with sorafenib (5 μM) for 6 hr. In the last 30 min of incubation with the drug, cells were induced with recombinant IL6 (25 ng/mL) for 0, 10, 20, or 30 min. Immunoblots for pStat3, Stat3, pAkt, Akt, and Mcl1 (Cell Signaling Technology) were performed. Beta-actin (Cell Signaling Technology) was used as a loading control.
Angiogenesis assay.
The in vitro human angiogenesis assay using Angiokit (TCS Cellworks, Buckinghamshire, UK) was used for evaluating the antiangiogenic activity of sorafenib as described previously [4]. Briefly, after coculturing human fibroblasts with endothelial cells in a 24-well plate, either VEGF, suramin, sorafenib, or a no treatment control was added to the wells. Two weeks post-treatment, we quantified tubule formation as a measure of the process of angiogenesis.
Isobologram analysis
The interaction between sorafenib and rapamycin was analyzed using the CalcuSyn™ software program (Biosoft, Ferguson, MO). This program is based upon the Chou–Talalay method, which calculates a combination index (CI), and analysis is performed based on the following equation: CI = (D)1/(Dx)1 + (D)2/(Dx)2 + (D)1(D)2/(Dx)1(Dx)2, where (D)1 and (D)2 are the doses of drug 1 and drug 2 that have x effect when used in combination, and (Dx)1 and (Dx)2 are the doses of drug 1 and drug 2 that have the same x effect when used alone [24]. Data from the MTT viability assay was expressed as the fraction of cells killed by the individual drug or the combination in drug-treated cells compared with untreated cells. A CI of 1.0 indicates an additive effect, whereas CI values below 1.0 indicate synergism.
RESULTS
Sorafenib induces cytotoxicity and inhibits proliferation in lymphoma cell lines
We first tested the ability of sorafenib to inhibit proliferation of NHL cells in vitro. We observed dose dependent inhibition of proliferation of all NHL cell lines at 48 hr post incubation with the drug with a median inhibitory dose of 3–6 μM (Fig. 1A). Incubating NHL cell lines with sorafenib for 48 hr induced dose dependent cytotoxicity as observed by a decrease in cell viability (Fig. 1B). The IC50 values ranged from 4–8 μM, a concentration clinically achievable. We then cultured Dohh2 cells alone or in coculture with either of the two important proangiogenic cytokines of the NHL microenvironment, namely VEGF, IGF or IL6 followed by incubation with various doses of sorafenib for 48 hr. Sorafenib was able to induce cytotoxicity with similar IC50 values in the presence or absence of cytokines (Fig. 1C).
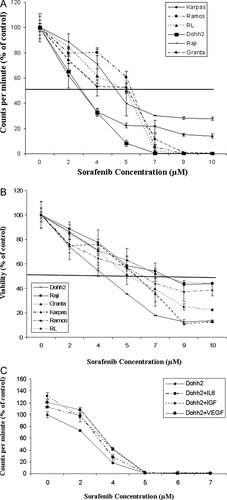
Cytotoxicity of sorafenib in lymphoma cells. Panel A: Sorafenib inhibits proliferation of NHL cells. Raji, Dohh2, Karpas, Granta, Ramos, and RL cells were incubated with sorafenib for 48 hr and proliferation inhibition was measured by thymidine uptake. Sorafenib concentrations (μM) are indicated on the x-axis and % cpm is indicated on the y-axis. Experiments were done in triplicates. Error bars represent one standard deviation. Panel B: Sorafenib induces cytotoxicity in various lymphoma cell lines. Raji, Dohh2, Granta, Ramos, RL or Karpas cells were incubated with sorafenib for 48 hr and cytotoxicity measured using MTT assays. The IC50 value was between 4–8 μM. Sorafenib concentrations (μM) are indicated on the x-axis and viability (as a percentage of the control) is indicated on the y-axis. All experiments were done in triplicates. Error bars represent one standard deviation. Panel C: Sorafenib overcomes the protective effects and inhibits proliferation of NHL cells in the presence of cytokines. Dohh2 cells were cultured alone or cultured in the presence of IL6 (25 ng/ml), VEGF (50 ng/ml) or IGF (50 ng/ml) and incubated with indicated concentrations of sorafenib for 48 hr and proliferation inhibition was measured by thymidine uptake. Sorafenib concentrations (μM) are indicated on the x-axis and % cpm is indicated on the y-axis. Experiments were done in triplicates. Error bars represent one standard deviation.
Sorafenib induces apoptosis in lymphoma cells and patient samples
To determine whether the cytotoxicity induced by sorafenib in lymphoma cells was predominantly due to induction of apoptosis, we incubated Dohh2 and Raji cells with 5 μM and 7 μM of sorafenib, respectively, for indicated time points. Following the incubation, we measured cells undergoing apoptosis by performing annexin/PI staining and flow cytometry. As shown in Fig. 2A and 2B, sorafenib treatment increased the number of apoptotic cells in a time dependent manner in Dohh2 and Raji cells. Activated caspase 8, caspase 9, and PARP levels were found to be slightly increased following sorafenib treatment in both Dohh2 and Raji cells (Fig. 2C,D) indicating that sorafenib induces both the intrinsic and extrinsic apoptotic pathways. However, pretreating cells with the pan caspase inhibitor z-vad fmk was not able to inhibit the apoptotic effect induced by sorafenib indicating that sorafenib could also induce apoptosis through caspase independent mechanisms (data not shown). More importantly, when NHL patient derived primary cells were incubated with indicated concentrations of sorafenib, we observed a dose dependent decrease in cell viability (Fig. 2E). In all the three patient samples used for this analysis sorafenib was able to induce apoptosis, albeit at higher concentrations than observed in NHL cell lines. Next, we incubated PBMC's derived from two healthy donors with indicated concentrations of sorafenib. Cells from both donors were fairly resistant to sorafenib treatment (Fig. 2F). There was a slight reduction in cell viability when 20 μM sorafenib was used.
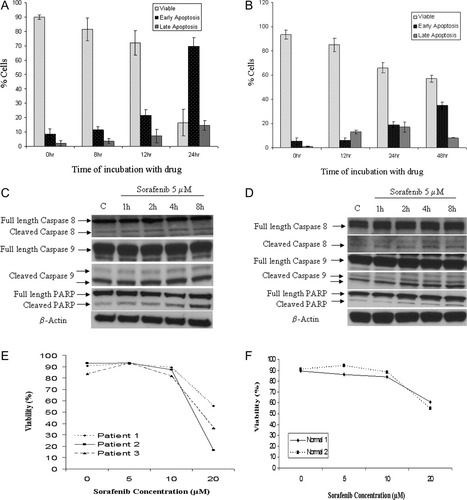
Sorafenib induces apoptosis in lymphoma cells and patient samples. Time dependent increase in apoptosis in (A) Dohh2 and (B) Raji cells following incubation with 5 μM or 7 μM of sorafenib, respectively, for indicated time points as measured by annexin/PI staining and flow cytometry. X-axis represents time of incubation with sorafenib and y-axis denotes percentage of cells. Viable cells were the ones that were both annexin and PI negative. Early apoptotic cells included annexin positive and PI negative cells and late apoptotic cells included annexin positive/PI positive cells and annexin negative and PI positive cells. Error bars represent one standard deviation. Panel C: Dohh2 cells were incubated with sorafenib (5 μM), and Panel D: Raji cells were incubated with sorafenib (7 μM) for the indicated time points. Time dependent cleavage of caspase 8, caspase 9 and PARP in both cell lines was observed post treatment with sorafenib. Panel E: Sorafenib induces apoptosis of NHL patient cells. Primary patient samples were incubated with indicated concentrations of sorafenib for 48 hr and induction of apoptosis was measured using annexin/PI staining and flow cytometry. Sorafenib concentrations (μM) are indicated in x-axis and % viable cells are indicated in y-axis. Panel F: PBMC's from two healthy normal donors were incubated with indicated concentrations of sorafenib for 48 hr and induction of apoptosis was measured using annexin/PI staining and flow cytometry. Sorafenib concentrations are indicated in x-axis and % viable cells are indicated in y-axis.
Mechanism of antilymphoma action of sorafenib
To better understand the mechanism of action of sorafenib, we performed immunoblots on Dohh2 and Raji cells after incubating them with 5 μM or 7 μM of sorafenib for indicated time points (Fig. 3A,B). In Dohh2 cells, sorafenib treatment resulted in a time dependent reduction in the levels of pStat3, pAkt, Xiap and Mcl1 expression (Fig. 3A). In Raji cells, sorafenib treatment led to down regulation of pStat3, pErk, pAkt, Xiap and a slight reduction of Mcl1 (Fig. 3B). Sorafenib treatment was not able to downregulate Bcl2 or Bcl-Xl in both cell lines tested. We then tested the ability of sorafenib to antagonize signaling pathways when cells were cocultured with IL6. Sorafenib was able to effectively inhibit pStat3, pAkt, and Mcl1 in the presence of IL6 (Fig. 3C).
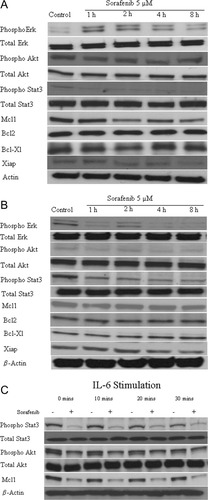
Mechanism of action of sorafenib in lymphoma cells. Immunoblots of extracts from Panel A: Dohh2 and Panel B: Raji cells treated with sorafenib (5 μM or 7 μM, respectively) for indicated time points indicate a downregulation of pStat3, pAkt, Mcl1, and Xiap protein levels in Dohh2 cells. In Raji cells, sorafenib treatment resulted in down regulation of pAkt, pErk, pStat3, and Xiap levels. Mcl1 level was only slightly down regulated in Raji cells. β-actin was used as a loading control. Panel C: Dohh2 cells were incubated with IL-6 (25 ng/ml) for the indicated times with (+) or without (-) a 6 hr preincubation with sorafenib (5 μM ). Sorafenib effectively inhibits the cytokine induced upregulation of pStat3, pAkt, and Mcl1 protein levels.
Sorafenib inhibits angiogenesis
Because sorafenib is known to inhibit Raf kinases and VEGFR, we wanted to examine if the drug is able to inhibit angiogenesis in vitro. For this, we performed an in vitro angiogenesis assay. We observed a significant reduction in angiogenesis post sorafenib treatment, as measured by the ability to form tubules (Fig. 4). This inhibitory effect of sorafenib was observed at nanomolar ranges (1–50 nM), concentrations that are much lower than cytotoxic concentrations of sorafenib. VEGF treatment along with sorafenib was not able to overcome the inhibitory effect of the drug on angiogenesis.
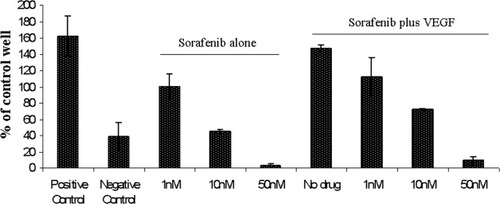
Antiangiogenic ability of sorafenib. In this experiment, we performed in vitro angiogenesis assay to observe possible antiangiogenic capability of sorafenib. Suramin was used as a negative control and VEGF as a positive control. Sorafenib caused a dose dependent decrease in tubule formation. VEGF treatment along with sorafenib was not able to overcome this decrease. Experiments were performed in triplicates. Error bars represent one standard deviation.
Sorafenib synergizes with the mTOR inhibitor rapamycin
mTOR inhibitors, such as rapamycin, and its analogs have shown activity in numerous solid tumors and hematological malignancies including lymphoma [25]. However, cells develop acquired resistance to mTOR inhibition by reactivating Akt through mTORC2 [26]. Since sorafenib treatment led to reduced pAkt levels in NHL cells, we tested the combination of sorafenib with rapamycin to learn if the mTORC1 induced pAkt could be blocked by sorafenib. We observed clear cytotoxic synergy when sorafenib and rapamycin were used in combination in Dohh2, Ramos and Raji cells (Fig. 5A). We next examined the effects of sorafenib/rapamycin combination on the PI3K/Akt pathway downstream of pAkt. We examined levels of pAkt, pmTOR, p-p70S6K, p4EBP1, and pGSK3β when Dohh2 cells were either untreated or treated with indicated concentrations of sorafenib or rapamycin as single agents or treated with the drugs in combination (Fig. 5B). pAkt level was down regulated at 8 hr post treatment with both drugs together. Similarly pmTOR was slightly downregulated at 8 hr following rapamycin treatment. When rapamycin was used in combination with sorafenib, pmTOR was down regulated at 4 hr with a more pronounced down regulation after 8 hr of incubation with the drug combination. p-p70S6K was downregulated when Dohh2 cells were treated with rapamycin alone or when rapamycin was used in combination with sorafenib. In contrast, p4EBP1 and pGSK3β were down regulated when cells were incubated with sorafenib alone or when sorafenib was used in combination with rapamycin. In addition, we also examined levels of important down stream antiapoptotic proteins, namely Mcl1, Bcl2, and Bcl-Xl post treatment (Fig. 5C). Although sorafenib treatment reduced levels of Mcl1, there was no further decrease in Mcl1 levels when sorafenib was used in combination with rapamycin. However, using the drugs in combination appears to bring down Bcl2 and Bcl-xL expression levels at 8 hr post incubation, though not significant. Thus, from the above experiment it is clear that sorafenib inhibits pAkt and down stream members pGSK3β, pmTOR, and p4EBP1, whereas rapamycin inhibits pmTOR and p-p70S6K. Together, these effects lead to synergistic impact on viability of lymphoma lines when the drugs are used in combination.
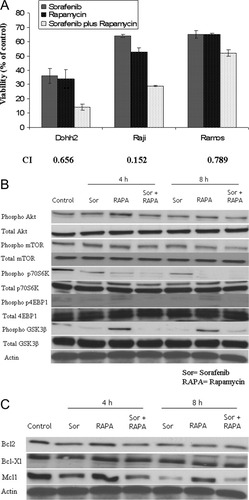
Synergy of sorafenib with other anticancer agents in lymphoma cells. Panel A: When sorafenib was combined with the mTOR inhibitor rapamycin, synergistic cytotoxicity was observed in Dohh2, Raji and Ramos cells as measured using the MTT assay. The graph represents the concentrations of sorafenib, rapamycin or the combination at which maximum synergy was observed. The concentrations are: (1) Dohh2-6 μM sorafenib, 6 nM rapamycin, (2) Raji-7 μM sorafenib, 20 nM rapamycin and (3) Ramos-5 μM sorafenib, 10 nM rapamycin. Cell lines used are indicated on the x-axis and Viability (% of control) indicated on the y-axis. All experiments were performed in triplicates. Error bars represent one standard deviation. The combination index (CI) values are indicated below the bar graph. CI values <1 indicate synergy. Panel B and C). Immunoblots of extracts from Dohh2 cells either left untreated or treated with 6 μM of sorafenib or 6 nM of rapamycin or 6 μM of sorafenib in combination with 6 nM of rapamycin for the indicated time points. As shown in Panel B, sorafenib and rapamycin combination led to downregulation of pAkt, pmTOR, p-p70S6K, p4EBP1, and pGSK3β. βactin was used as a loading control. Sorafenib as a single agent was able to inhibit pAkt, pGSK3β, and p4EBP1. Rapamycin as a single agent on the other hand led to a slight upregulation of pAkt and a strong upregulation of pGSK3β. Rapamycin was able to downregulate pmTOR and p-p70S6K Panel (C) Sorafenib as a single agent was able to inhibit Mcl1 but not Bcl-xL. In contrast, rapamycin, as a single, agent was not able to downregulate either Mcl1 or Bcl-xL. In contrast, sorafenib and rapamycin combination led to downregulation of Mcl1 and Bcl-xL. βactin was used as a loading control.
DISCUSSION
In this report, we demonstrate that sorafenib, a multi-kinase inhibitor with demonstrated inhibitory activity on Raf kinase and VEGF receptors, has significant antitumor activity in NHL cell lines and patient samples. Although originally identified to inhibit the Raf/MEK/Erk pathway [21, 27] it is now known that sorafenib could elicit its functions through different mechanisms in different tumor systems. We demonstrated that in NHL cells apoptosis was induced and the primary signaling effects were a downregulation of pSTAT3, Mcl1, and pAkt. In Dohh2 cells, sorafenib treatment caused downregulation of pStat3 and pAkt similar to results obtained in MM and a human pancreatic cell line [20, 28]. There was no reduction in the level of pErk with in fact a slight upregulation in levels of pErk. This was observed in a nonsmall cell lung cancer cell line A549. However, sorafenib was able to inhibit tumor growth in A549 though pErk levels were not affected [21]. Thus, it is clear that sorafenib could exert its antitumor effects irrespective of its ability to inhibit pErk. The inhibitory effect observed in Dohh2 cells might be due to the ability of sorafenib to inhibit VEGFR and other receptor tyrosine kinases. In Raji cell line, sorafenib treatment resulted in downregulation of pErk and pStat3. Mcl1 has been reported to be a common mediator involved in the mechanism of action of sorafenib [20, 28, 29]. Similar to previous reports, we observed a decrease in Mcl1 protein levels following sorafenib treatment. Mcl1 downregulation was more pronounced in Dohh2 cells than in Raji cells. We have shown that Dohh2 is more sensitive to sorafenib treatment than is Raji (Figs. 1B and 2A). However, sorafenib was unable to inhibit the phosphorylation of Erk in Dohh2 cells. There is evidence to suggest that this type of unexpected increase in pErk may be due to dimerization and transactivation of Raf by Raf inhibitors that is both time and dose-dependent. From this, it is clear that the extent of pErk inhibition does not dictate sensitivity to sorafenib. Our results also indicate that the extent of sorafenib induced Mcl1 downregulation might actually be the reason for increased apoptosis observed in Dohh2 cells when compared with Raji cells.
Burkitt lymphoma (BL) is often associated with Epstein-Barr virus (EBV) infection [30]. On this basis BL could be further classified as either EBV positive or negative. It has been demonstrated that EBV positive BL cell lines are positive for pMAPK, whereas EBV negative BL lines are negative for activated MAPK expression [31]. Raji is a EBV positive BL while Ramos is a EBV negative BL[31]. Sorafenib is able to induce cytotoxicity in both cell lines irrespective of their MAPK activation status. Hence, high basal levels of pERK expression do not seem to correlate with sensitivity to sorafenib in NHL cells unlike in hepatocellular carcinoma [32, 33].
mTOR inhibitors have demonstrated activity against lymphoma cell lines in vitro [26, 34]. Three analogs of rapamycin-temsirolimus, everolimus, and AP23573 are currently in clinical trials [35]. However, rapamycin and rapamycin analogs lead to an upregulation of pAkt by activating mTORC2 complex [36, 37]. It is known that cells develop resistance to rapamycin and its analogs due to this upregulation of pAkt. Also, significant cross-talk exists between the PI3K/Akt pathway and the Ras/Mek/Erk pathways [38]. Ras has been shown to directly activate PI3K [39]. pErk has been found to relieve the inhibition on mTORC1 by inhibiting TSC1/2 [40]. To further complicate things, mTOR inhibition often leads to activation of pERK [41]. In addition, it has been shown in hepatocellular carcinoma mouse models and in multiple myeloma that a combination of sorafenib and rapamycin inhibits tumor progression [20, 42]. Hence, we used sorafenib in combination with the mTOR inhibitor rapamycin and studied the effectiveness of this combination in the lymphoma setting. This combination demonstrated synergy in killing lymphoma cells. In an effort to better understand the intracellular events leading to the observed synergy, we performed western blots to analyze the levels of pAkt and other important members of this pathway post drug treatment. Sorafenib treatment resulted in down regulation of pAkt, pGSK3β, p4EBP1, and Mcl1. Single agent rapamycin at the concentration tested resulted in a slight down regulation of pmTOR and downregulation of p-p70S6K with no influence on p4EBP1. However, when the drugs were used in combination we observed more potent down regulation of pAkt, pmTOR and in addition we also observed down regulation of Bcl-xL. Thus, using rapamycin in combination with sorafenib leads to a more pronounced inhibition of pmTOR and its down stream targets p-p70S6K and p-4EBP1. More importantly, because if its ability to inhibit pAkt and its down stream target pGSK3β, sorafenib might be able to prevent rapamycin induced upregulation of pAkt, thereby preventing cells from developing acquired resistance to rapamycin. Currently, a phase 1/2 trial is underway to test the combination of sorafenib with RAD001 (an mTOR inhibitor) in patients with lymphoma and myeloma.
Acknowledgements
The authors like to acknowledge Roberta DeGoey and Christy Finke for their assistance with processing of tumor cells and all the patients who provided with the tumor samples. Sorafenib was provided by Bayer HealthCare Pharmaceuticals.




