Characteristics of translocation (16;16)(p13;q22) acute myeloid leukemia†
Conflict of interest: Nothing to report.
A subgroup of patients with core binding factor acute myeloid leukemias (AML) is characterized by the presence of the fusion gene CBFb-Myh11. At the cytogenetic level, most of these patients are identified by the presence of an inversion of chromosome 16 [inv(16)(p13q22)] and rarely by a translocation t(16;16)(p13;q22). The aim of this study is to describe the natural history of patients with t(16;16) [N = 6] treated at MD Anderson Cancer Center and compared them with a cohort of patients with inv(16)(p13q22) [n = 61]. In patients with t(16;16) the complete remission rate (CR) was 100% when treated with a combination of fludarabine and high-dose cytarabine. Median overall survival (OS) had not been achieved. There was no difference in response or OS or progression free survival between both groups. Presence of additional chromosomal abnormalities and molecular aberrations had no effect on prognosis. In conclusion, and consistent with previous reports, the natural history of patients with t(16:16)(p13;q22) is similar to that of classic patients with inv16 AML and therefore should be treated similarly.
Acute myeloid leukemia (AML) is the result of genetically disturbed hematopoietic precursor cells leading to an abnormal accumulation of increased number of immature myeloid cells in the peripheral blood and bone marrow. Distinct chromosomal translocations lead to the activation of proto-oncogenes and generation of leukemia specific fusion proteins. Inv(16)(p13q22) and t(16;16)(p13;q22), that we refer as inv (16) and t(16;16) respectively from now, are recurring chromosomal rearrangements commonly associated with the M4Eo subtype of AML but also reported in the AML M2, M5 subtypes, and in patients with morphological features of higher risk MDS [ 1, 2]. In both inv(16) and t(16;16), the final genetic product is the fusion of the CBFB gene at 16q22 to the smooth muscle myosin heavy chain (MYH11) at 16p13 [3]. The translocation involves the same breakpoints on the long and short arms of the chromosome 16 homologes as does the inversion. The resulting CBFbeta-MYH11 fusion protein, including its AML1 interaction domain, is fused inframe to a variable amount of the C-terminal domain of MYH11 [4]. Presence of inv16 is associated with good prognosis if treated with high doses of cytarabine arabinoside. A10-year survival of 55% has been reported in patients with inv16 [5-7]. In general, presence of additional cytogenetic abnormalities do not have any effect prognosis either [8]. Other abnormalities of chromosome 16 like del(16)(q22) usually do not manifest as typical AML M4Eo and tend to have a worse prognosis and shorter remission after induction therapy [9]. Since the fusion gene product in t(16;16) is the same as inv(16) AML, there few are prior reports focusing on the characteristics of t(16;16) AML patients. In a study done by Larson et al. there was no statistical difference in terms of first remission duration and overall survival between 27 patients with inv(16) and 6 with t(16;16) [5]. In October 2010, two consecutive patients with AML and t(16;16) were admitted to MD Anderson Cancer Center Hospital (MDACC), a fact that resulted in the analysis of the experience at that center with this rare type of AML and this report.
Of 1,886 patients with AML evaluated at MDACC between Jan 2001 to September 2010: 6 (0.32%) had t(16;16) and 61 (3.2%) inv(16). All patients were treated with a high-dose ara-C program containing fludarabine (FA). They were analyzed retrospectively in regards to their hematologic, cytogenetic, molecular features, and response to therapy along with their survival.
The distribution of time-to-event end points (overall survival and progression free-survival) were estimated using the Kaplan-Meier method. Survival was measured from the time of induction therapy to the date of death from any cause or date of last follow-up. Progression free survival was estimated from the time of CR achievement to the date of relapse, death in CR or last follow-up. Survival curves were compared using a two-sided log-rank test. Results with P value of less than 0.05 were considered significant. Frequency tables were used to summarize baseline patient characteristics. All analyses were performed using the Statistica version 6 software package.
The six patients with t(16;16) in the study underwent induction and consolidation therapy with aforementioned regimen and all achieved complete remission (CR). Median duration of follow-up is 85 weeks (range, 27–419). The median age was 44 years (range, 34–63) and all are alive and in remission after induction and consolidation therapy except one patient who expired due to an unrelated cause after six months in remission. Four patients had isolated t(16;16). One patient had additional +22 chromosomal alteration and another one had trisomy 8, trisomy 22, and trisomy Y. Bone marrow analysis of patients showed increased presence of abnormal eosinophils consistent with previous reports [ 2, 10]. Of interest, five of the six patients had a RAS and one a FLT3-ITD mutation respectively. Presence of c-Kit mutation was evaluated in four patients and was negative in three of them. The patient with c-Kit positive mutation achieved molecular negativity for c-Kit and has been in remission for +36 months. Only one patient had prior malignancy (sigmoid colon cancer) and died in complete remission due to severe pneumonia. All patients had detectable CBFb-MYH11fusion transcript by real time PCR (RT-PCR) at diagnosis and one patient continued to be positive despite achieving complete remission 31 weeks into response. Table I summarizes the characteristics of patients with t(16;16) and those with inv(16). Figures 1 and 2 show the overall survival and progression-free survival curves of the two cytogenetic cohorts. There are no statistically significant differences, including overall survival between t(16;16) and inv(16). None of the characteristics were different between both cytogenetic groups. Additional cytogenetic alterations and molecular aberrations had no impact on either subgroup.
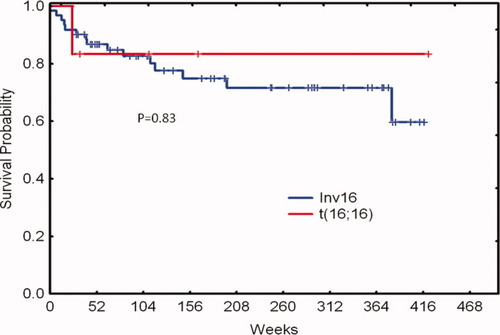
Overall survival by cytogenetic group. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
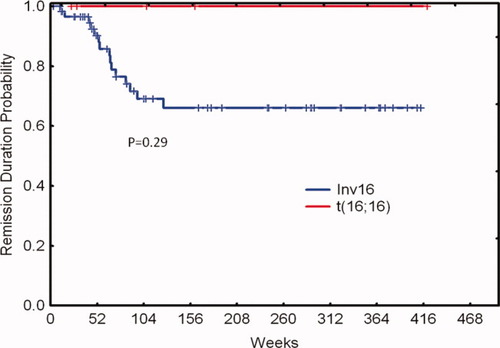
Remission duration probability by cytogenetic group. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
| Characteristic | t (16;16) | Inv (16) | P |
|---|---|---|---|
| Number | 6 | 61 | |
| Median Age years (range) | 44 (34–62) | 51 (18–88) | 0.43 |
| Median WBC (range) | 36 (2–97) | 16 (1–109) | 0.22 |
| Median BM Eos | 12 (2–45) | 7 (0–50) | 0.16 |
| Response (%) | |||
| CR | 6 (100) | 57 (93) | 0.52 |
| CRp | 0 | 3 (5) | |
| Failure | 0 | 1 (2) | |
| Prior Malignancy (%) | 1 (16) | 10 (16) | 0.98 |
| PS (%) | |||
| 0/1 | 6 (100) | 53 (89) | 0.35 |
| 2 | 0 | 8 (13) | |
| FAB (%) | |||
| M4 | 5 (84) | 38 (62) | 0.31 |
| M1 | 0 | 3 (5) | |
| M2 | 0 | 4 (7) | |
| M5 | 0 | 1 (2) | |
| RAEB–T | 0 | 3 (6) | |
| Unknown | 1 (16) | 11 (18) |
- WBC, white blood cell count in 103 u/L; BM, bone marrow; CR, complete remission; CRp, complete remission with incomplete platelet recovery; PS, performance status; FAB, French American Bristish Classification; RAEB–T, refractory anemia with excess blasts in transformation.
In conclusion, t(16:16) is a rare chromosomal abnormality in AML and constitutes 10% of patients with core binding factor leukemias and less than 1% of all cases of AML evaluated at our center. Presence of t(16;16) is associated with an excellent prognosis despite presence of additional cytogenetic or molecular alterations if treated with a high-dose ara-C containing program. Patients with t(16;16) should be treated and monitored with the same approaches used in inv(16).
References
Alireza Eghtedar*, Gautam Borthakur*, Farhad Ravandi*, Elias Jabbour*, Jorge Cortes*, Sherry Pierce*, Hagop Kantarjian*, Guillermo Garcia-Manero*, * Department of Leukemia, University of Texas MD Anderson Cancer Center, Houston, Texas.




