Ocular adnexal lymphoma and Helicobacter pylori gastric infection†
Conflict of interest: Nothing to report.
Abstract
There is a causal association between Helicobacter pylori (Hp) gastric infection and the development of gastric MALT lymphoma. In contrast, the link between Hp gastric infection and the development of extragastric lymphoma has not been thoroughly investigated. We, therefore, studied the prevalence of gastric Hp infection at initial diagnosis of ophthalmologic and nonophthalmologic extragastric lymphoma patients. Three cohorts of patients were studied: a first one of 83 patients with OAL, a second one of 101 patients with extraophthalmologic extragastric lymphoma, and a third one of 156 control individuals (control) without malignant lymphoma. Gastric Hp infection was investigated by histopathological analysis and Hp-specific PCR assay on gastric biopsy tissue samples. We found gastric Hp infection in 37 OAL patients (45%), in 25 extraophthalmologic extragastric lymphoma cases (25%), and in 18 controls individuals (12%) (P < 0.0001 OAL/C and P < 0.01 OAL/extra-OAL cases). Gastritis was found in 51% and 9% of Hp-positive and Hp-negative lymphoma patients, respectively (P < 10−4). Gastric Hp infection only correlated with MALT/LPL lymphoma (P = 0.03). There is a significant association between gastric Hp infection and MALT/LPL OAL. This suggests a novel mechanism of indirect infection-associated lymphomagenesis whereby chronic local antigen stimulation would lead to the emergence of ectopic B-cell lymphoma. © 2010 Wiley-Liss, Inc. Am. J. Hematol.
Introduction
Growing evidence indicates that a number of lymphomas are associated with chronic antigenic stimulation triggered by microbial pathogens [1]. Helicobacter pylori (Hp) is a widely distributed bacterium that infects the human stomach mucosa and causes chronic active gastritis, leading to peptic ulcer disease, gastric adenocarcinoma, and mucosa-associated lymphoid tissue (MALT) lymphoma [2]. An association between Hp infection and gastric MALT lymphomas was first reported in 1991 [3]. Molecular progression from Helicobacter pylori-associated chronic gastritis to MALT gastric lymphoma has since been demonstrated [4], as well as the causal role of Hp in this process. According to a widely accepted pathophysiological scenario, persistence of gastric Hp infection leads to chronic and sustained antigen-driven lymphoproliferation, which, coupled with chronic inflammation, may lead to the emergence of allo- and auto-reactive lymphoid clones which proliferation may become antigen independent in the context of additional oncogenic events by passing B cell receptor stimulation such as translocation [1-5]. Hp now stands as a model microorganism associated with antigen-driven lymphomagenesis. Likewise, a number of other microbial pathogens such as B. burgdorferi, C. jejuni or Hepatitis C virus have since been implicated in microbial antigen-driven lymphomagenesis, also referred as “indirect” lymphomagenesis, as opposed to other microorganisms known to have a direct transforming activity such as the lymphotropic viruses HTLV1, EBV, and HHV8 [1].
A new comer in the field of infection-associated lymphoma is the ocular adnexal lymphoma (OAL), which has been reported to be associated with Chlamydia psittaci (Cp) ocular infection in around 20% of the more than 400 OAL biopsy samples analyzed until now [6, 7]. It has been proposed that Cp may play in OAL development the causal role that Hp plays in gastric MALT lymphoma, as a source of local chronic and sustained antigen-driven lymphoproliferation. In line with this hypothesis, antimicrobial therapy known to be active against Cp has been shown to be associated with OAL remission. However, an “antitumoral” efficacy of antimicrobial therapy has also been observed in Cp-negative OAL patients [8-10], thereby suggesting a possible association of other microbial agents and/or extraocular infection with OAL. We, therefore, investigated the possible association between gastric Hp infection and OAL.
Patients and Methods
Patient selection and characteristics.
Three cohorts of patients were investigated for Hp infection, a first one of 83 consecutive primary OAL cases, a second one of 101 consecutive non randomly chosen extraophthalmologic extragastric lymphoma patients, all treated at a single center, the Institut Curie, during the same time frame (1970 to 2007) and whom initial staging included gastroscopy with systematic biopsy performed at the Institut Curie, and a third one of 156 individuals who underwent gastroscopy performed at the Institut Mutualiste Montsouris during the same period for digestive symptoms or anemia without evidence of malignant lymphoma and be used as a control population (control). Staging of the disease included laboratory work-up, CT scan or chest radiography plus abdominal ultrasound scan, and, in most cases, bone marrow biopsy, and the disease at diagnosis was defined according to the Ann Arbor staging system [11]. Apart from the existence of an initial ophthalmologic or extraophthalmologic extragastric lymphoma, no selection was performed. Patients with classical pathological features associated with gastric Hp infection, such as gastric malignant lymphoma or ulcer or cancer, were excluded from each of these groups. In concordance with national practices, no ethics committee was required for this retrospectice study; similarly, no written consent given by the patients were required.
Initial characteristics of the overall lymphoma patients' populations are presented in Table I. Pathological review was centralized and performed by a single experimented hematopathologist (AVS) according to the WHO classification [15].s As plasmocytic differentiation is very common in MALT lymphomas, tumor samples came from occular sites, and lymphoplasmocytic lymphoma (LPL) are very rare in ophthalmologic localizations, we pooled MALT and LPLs. As a consequence, our pathological review showed a majority of MALT/LPL lymphomas among OAL patients (74%), and as expected for a Western country, a majority of follicular and diffuse large B-cell lymphoma among extraophthalmologic extragastric NHL patients (25% and 53%, respectively). For the 83 OAL patients, the site of the ophthalmologic lymphomatous disease was the conjunctiva in 36 patients (43%), intraorbital in 32 patients (33%), the lachrymal gland in 10 patients (12%), and palpebral in four cases (5%). Bilateral ophthalmologic involvement was observed in 10 patients (12%). The 5-year disease-free survival (DFS) was 66% and 62% for the OAL and the extraophthalmologic lymphoma patients, respectively. The 5-year overall survival was 84% and 77% for the OAL and the extraophthalmologic lymphoma patients, respectively.
| Parameters | Overall N (%) | OAL | Extra-OAL N (%) | |
|---|---|---|---|---|
| Overall N (%) | MALT/LPL N (%) | |||
| Total number | 184 | 83 | 61 | 101 |
| Gastric abnormalities: | ||||
| Lympho-epithelial lesions | 4 (2) | 4 (5) | 2 (4) | 0 (0) |
| Gastritis | 39 (21) | 19 (23) | 15 (32) | 20 (20) |
| Ulcerous | 2 (1) | 1 (1) | 0 (0) | 1 (1) |
| Pathologic review (WHO classif.): | ||||
| Lymphocytic NHL | 11 (6) | 5 (6) | / | 6 (6) |
| MALT/LPL NHL | 63 (35) | 61 (74) | 61 (100) | 2 (2) |
| Nodal MZL | 2 (1) | 0 | / | 2 (2) |
| Follicular NHL | 31 (17) | 6 (7) | / | 25 (25) |
| B-DLCL NHL | 63 (34) | 6 (7) | / | 53 (53) |
| Others | 14 (7) | 5 (6) | / | 13 (13) |
| M/F sex ratio | 0.8 | 0.9 | 0.8 | 0.7 |
| Age <60 years | 78 (42) | 24 (29) | 20 (33) | 54 (53) |
| B symptoms | 27 (15) | 1 (1) | 0 | 26 (26) |
| PSa ≥2 | 10 (6) | 2 (3) | 0 | 8 (8) |
| Nodal site | 99 (54) | 20 (24) | 13 (21) | 79 (79) |
| Extranodal sites ≥2 | 53 (29) | 28 (34) | 21 (35) | 25 (25) |
| Stage III.IVb | 101 (55) | 34 (41) | 25 (41) | 67 (67) |
| Gastric involvement | 8 (5) | 8 (10) | 4 (6) | 0/94 (0) |
| Bone marrow involvement | 36/178 (20) | 7/78 (9) | 6/59 (10) | 29/100 (29) |
| ESR ≥30 | 35/163 (22) | 12/67 (18) | 7/52 (13) | 23/96 (24) |
| Elevated LDH level | 29/176 (16) | 7/79 (9) | 4/59 (7) | 22/99 (22) |
| Elevated β2 microglobulin level | 29/139 (21) | 8/54 (15) | 6/45 (13) | 21/85 (25) |
| Albumin <40 g/l | 69/163 (42) | 22/69 (32) | 17/53 (32) | 47/94 (50) |
| IPI scorec: | 172 (93) | 80 (96) | 45 (96) | 92 (91) |
| 0–1 | 99 (58) | 48 (60) | 48(81) | 51 (55) |
| 2 | 40 (23) | 17 (21) | 10 (17) | 23 (25) |
| 3 | 24 (14) | 14 (18) | 1 (2) | 10 (11) |
| 4–5 | 9 (5) | 1 (1) | 0 | 8 (9) |
- Other histopathological subtype of lymphomas included mantle cell lymphoma, Burkitt's lymphomas, T-lymphoblastic lymphoma, anaplastic T-cell lymphoma, Hodgkin's lymphoma, and unspecified low-grade lymphomas.
- N, number of cases; pts, patients; OAL, ocular adnexal lymphoma; MZL, marginal zone lymphoma; LPL, lymphoplasmocytic lymphoma; M, male; F; female; PS, performance status; LDH; lactate dehydrogenase; IPI, international prognostic index.
- a Oken et al [12];
- b Carbone et al [13];
- c Shipp et al [14].
Detection of gastric and ophthalmologic Hp infection.
Histopathological diagnosis of Hp infection was determined using H&E and methyl blue coloration. Hp-specific PCR analysis was performed with total DNA obtained from gastric biopsy samples. Total DNA extraction was performed as previously described [16]. Briefly, total DNA was extracted from four 15 μm-thick tissue sections from AFA (acetic acid, formalin, ethylic alcohol)-fixed tissue samples obtained at the time of diagnosis, using the QIA amp DNA mini kit (Qiagen, Courtaboeuf, France) and quantified. DNA was stored at −20°C until use. All samples were tested for DNA integrity by PCR using primers amplifying the human GAPDH gene. All samples gave a positive GAPDH signal indicating good DNA preservation in all samples. The quantity and purity of extracted DNA were assessed by measuring the absorbance at 230, 260, and 280 nm using a NanoDrop ND 1000 spectrophotometer (Wilmington, USA). TaqMan PCR was performed to amplify fragments of the 16S rRNA gene of Hp, as previously described [17].
Statistical Analysis of HP infection detection.
Correlations between Hp infection, histopathological, clinical, and biological characteristics of ophthalmologic and extraophthalmologic lymphoma patients, as well as correlation between histopathological and PCR analyses of Hp infection were determined using the Chi-square test. DFS was defined from the date of diagnosis to the date of first relapse or death (all causes of death). Overall survival (OS) was defined from the date of diagnosis to the date of death or the date of last follow-up. Survival curves were drawn using the Kaplan-Meier method [18], and the level of significance between various outcomes evaluated using a log-rank test.
Results
Detection of gastric Hp infection
The detection of gastric Hp infection was first performed by histopathological analysis for all 156 controls and 119 lymphoma patients, including 51 with OAL, among whom 41 were MALT/LPL OAL, and 68 with extraophthalmologic lymphoma. The detection of gastric Hp infection was also performed by PCR analysis in all 156 controls and ophthalmologic and nonophthalmologic lymphoma patients (184 cases). Sixty-five cases had exclusive PCR analysis because of the lack of availability of paraffin-embedded gastric tissue at the time of the histopathological analysis. As shown in Table II, among the 119 lymphoma patients for whom both histopathological and PCR analyses were performed, gastric Hp infection was detected in 29/51 cases of OAL patients (57%; 13 cases with both positive histopathological and PCR analyses, eight with positive histopathological analysis and negative PCR analysis, and eight with negative histopathological analysis and positive PCR analysis) and in 22/68 cases of extraophthalmologic extragastric lymphoma patients (32%; 10 cases with both positive histopathological and PCR analyses, six with positive histopathological analysis and negative PCR analysis, and six with negative histopathological analysis and positive PCR analysis) (see Fig. 1). Among OAL cases, 22/41 MALT/LPL OAL were considered as Hp+ (54%). The two extraophthalmic MALT/LPL lymphoma cases were both negative for gastric Hp infection. Among the seven cases of non-MALT/LPL OAL in whom gastric Hp infection was diagnosed, all but two cases (both mantle-cell lymphomas) were of low grade (3 follicular lymphomas and 2 lymphocytic lymphomas), excluding the diagnosis of a transformation of MALT/LPL lymphomas in high-grade NHL. Hp detection was negative both histopathologically and by PCR assay in 68/119 lymphoma patients (57%), among whom 22/51 OAL patients (43%) and 46/68 extraophthalmologic lymphoma patients (68%). Among the 156 controls who underwent a fiberoptic gastroscopy, 15 cases were positive for Hp-specific PCR detection (histopathological and PCR determinations) (10%).
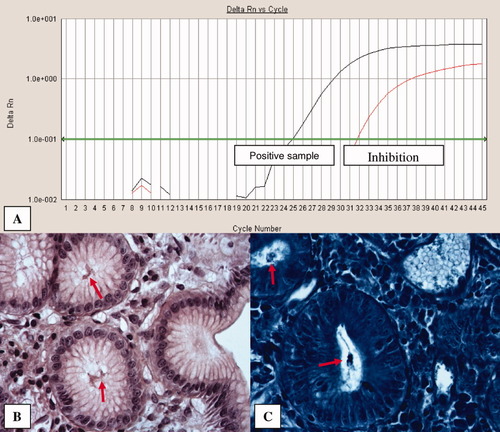
A: Real time PCR of Hp. Result obtained from a positive sample. B: Chronic gastritis with numerous intraglandular Hp (HES, ×60). C: Gastric glands with intraluminal Hp (methyl blue, ×60). In both B and C figures, Hp are indicated with red arrows.
| Hp − | Hp + | |||||
|---|---|---|---|---|---|---|
| PCR − Histo NA | PCR − Histo − | PCR + Histo + | PCR − Histo + | PCR + Histo − | PCR + Histo NA | |
| Controls (N = 156) | 0 | 138 | 10 | 3 | 5 | 0 |
| 138 (88%) | 18 (12%) | |||||
| Extra-OAL (N = 101) | 30 | 46 | 6 | 10 | 6 | 3 |
| 76 (76%) | 25 (25%) | |||||
| OAL (N = 83) | 24 | 22 | 13 | 8 | 8 | 8 |
| 46 (55%) | 37 (45%) | |||||
| MALT/LPL OAL (N = 61) | 14 | 19 | 10 | 7 | 5 | 6 |
| 33 (54%) | 28 (46%) | |||||
- Histo, histopathological analysis; NA, gastric tissue not available.
We first compared the rate of gastric infection for the three studied patient groups with concordant histopathological and PCR analyses, i.e., 35 OAL patients (13 double positive and 22 double negative), 56 extraophthalmologic extragastric lymphoma patients (10 double positive and 46 double negative), and 148 controls (10 double positive and 138 double negative), and found a significant difference when comparing OAL patients with histologically and PCR proven gastric Hp infection versus control cases (37% vs. 7%; P < 10−3), extraophthalmologic extragastric lymphoma patients versus controls (18% vs. 7%; P < 0.02), and OAL patients versus extraophthalmologic extragastric lymphoma cases (37% vs. 18%; P = 0.04).
Because the strength of the correlation between histopathological and Hp-specific PCR analyses was very high (P < 10−4), we extended our analyses and included cases with gastric Hp infection defined by a positive result for at least one technique. Using this diagnostic criterion, among the 184 patients with lymphoma enrolled in our study, 62 were considered as infected (34%), in whom 37/83 OAL cases (45%), 28/61 MALT/LPL OAL patients (46%), and 25/101 extraophthalmologic extragastric lymphoma cases (26%)(see Fig. 2). Together, these results show a significant higher proportion of gastric Hp infection in OAL patients compared to healthy cases (P < 10−4), in extraophthalmologic extragastric lymphoma patients versus controls (26% vs. 13%; P < 10−3), and in OAL patients versus extraophthalmologic extragastric lymphoma cases (P < 10−2). Similar results were also obtained when comparing patients with MALT/LPL OAL to control cases (P < 10−3) and extraophthalmologic extragastric lymphoma patients (P< 10−2).
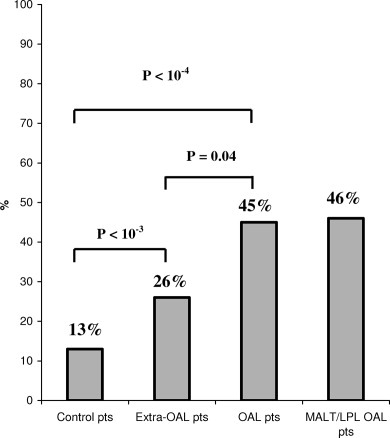
Proportion of patients with positive detection of gastric Hp infection.
Among the eight OAL patients for whom PCR analysis could be performed on the ophthalmologic lymphoma tissue sample, no Hp infection was detected.
Correlations between gastric Hp infection and patients' characteristics
The proportion of lymphoma patients (overall cases, OAL patients and extraophthalmologic lymphoma cases) with gastric Hp infection was analyzed according to their initial characteristics. On the overall studied population of lymphoma patients (184 cases), and as one would expect, a higher and significant correlation was observed between the detection of gastric Hp infection and the presence of gastritis lesions as determined by histopathological analysis, namely gastritis in 51% of Hp-positive infection cases, whatever the initial lymphoma localization, when compared with gastritis in only 9% of Hp-negative infection patients (P< 10−4). A significant correlation of gastric Hp infection was also found between MALT/LPL vs. non-MALT/LPL lymphomas (44% and 29%, respectively)(P = 0.03). In contrast, neither gender, age, presence of B symptoms, PS (performance status) greater than 1, nodal involvement, gastric involvement, extranodal involvement greater than 1, advanced stage, bone marrow involvement, elevated ESR level, elevated LDH level, and albumin level lower than 40 g/L were more frequently associated with Hp gastric infection in both studied populations. In particular, the median age of the three studied populations according to Hp status and methodologies were not different. For OAL patients, a bilateral ophthalmologic involvement and a specific ophthalmologic site involvement (intraorbital, conjunctiva, palpebral, and lachrymal gland) were not associated with a higher rate of gastric Hp infection. Moreover, on the overall lymphoma patients' population, the four subgroups of patients defined by their International Prognostic Index (IPI) score were not significantly different in their proportion of gastric Hp infection. However, when the IPI score was reclassified in two groups (0-1-2 vs. 3-4-5), a higher proportion of gastric Hp infection was detected in the high IPI score group, namely 31% of cases with an IPI score lower than 3 and 51% of patients with an IPI score of 3 or more (P = 0.02). Nevertheless, this was not observed in the three subpopulations of OAL patients, MALT/LPL OAL cases, and extraophthalmologic lymphoma patients. Finally, gastric Hp infection detected at the initial diagnosis of lymphoma had no impact on DFS and overall survival of the overall studied population, as well as for OAL and extraophthalmologic lymphoma patients (data not shown).
Discussion
In this study, we investigated the prevalence of gastric Hp infection in patients with either OAL, extraophthalmologic lymphoma, or who underwent gastric biopsy for digestive symptoms or anemia and in whom no evidence of malignant lymphoma or gastric ulcerous and cancer was found. We demonstrate a significant association between gastric Hp infection and OAL, this association being the highest for MALT/LPL OAL. Our conclusions are based on the use of two control populations: a first one constituted by cases without malignant lymphoma, for whom a 13% rate of gastric Hp positivity was found [19, 20]; and a second one of extraophthalmologic lymphoma patients who presented classical distribution in the different subtype of lymphomas. As we have not used an extragastric and extraophthalmologic MALT/LPL control population, our data demonstrating that gastric Hp infection may play a role in extragastric OAL may also applied to extra-OAL lymphomagenesis.
It is known that infections may contribute to lymphomagenesis, according to at least two types of mechanisms referred to as direct and indirect lymphoid transformation [1]. Lymphotropic viruses such as Epstein-Barr virus, Human Herpes virus 8, and Human T-Lymphotrophic virus 1 directly infect a subset of lymphoid cells in which they express viral oncogenes that favor cell transformation. Other microbial species such as Helicobacter pylori [21], Campylobacter jejuni [22], and Borrelia burgdorferi [23], may induce chronic inflammation together with protracted antigenic stimulation inducing chronic lymphoid proliferation and leading to lymphoid transformation indirectly, in the absence of lymphoid infection. In all these situations, inflammation and lymphoid infiltration displaying MALT architecture develop at the primary site of the bacterial infection and, therefore, lead to lymphoid transformation at this local infection site. In this view, Chlamydia psittaci infection, which has been associated with OAL [6], could be one event that induces a local ocular adnexal chronic inflammation, antigenic stimulation, and lymphoid infiltration.
From our clinical observation, and for the subset of OAL lymphomas associated with gastric Hp infection, we propose the hypothesis of a new mechanism of indirect infection-associated lymphoid transformation: first, gastric Hp infection would constitute a persistent source of antigenic stimulation, leading to chronic gastric inflammation and lymphoid infiltration. Indeed, a highly significant proportion of gastric Hp infection is observed in OAL patients, when compared with extraophthalmologic lymphoma patients and controls, and gastritis is also significantly associated with OAL. The implication of a chronic gastric inflammation as a first indirect antigen-driven mechanism is also supported by the high rate of gastric Hp infection in MALT/LPL OAL than in all other subtypes of OAL. In contrast, we did not detect, in all eight studied cases, Hp DNA into ophthalmologic tumor samples, in agreement with what reported by Ferreri et al. [8]., but in contradiction with the recent report by Lee et al. [24]. This is also supported by the fact that at least a third of OAL patients exhibit extraophthalmologic lymphoma localization at initial diagnosis, with lymph node involvement in about 15 to 20% of cases [25, 26], bone marrow involvement in about 10% of patients [26], and multiorgan involvement ranging between 13 and 46% [25-27]. Third, circulating lymphomatous cells would be attracted to the ophthalmic mucosa, and may evolve to overt lymphoma, under the influence of additional mitogenic stimuli. In this view, it is of particular interest to note that OAL frequently occur in the course of ocular chronic inflammation or autoimmune diseases [8, 28]. These local ocular conditions may indeed lead to the local production of cytokines exerting a chemotactic effect on circulating lymphomatous cells according to classical B-cell homing mechanisms. Ophthalmic Cp infection could be one of the factors inducing this local chronic inflammation and attracting circulating lymphoid cells originating from the gastric mucosa. Importantly, the hypothesis of ocular attraction of Hp-induced gastric lymphoid cells to the inflamed ophthalmic mucosa is also supported by the observation that bilateral ophthalmologic involvement is frequently observed at the diagnosis of OAL (12% in our series) [29] and that a high rate of patients with bilateral and/or more than one MALT site involvement (i.e., 48% of cases) have been reported [9]. In line with this scenario, it is striking to note that blepharitis as also been strongly associated with gastric Hp infection (90% as detected by 13C-urea breath test in a large cohort of patients) [30]. Moreover, it has been reported the expression of the B-cell attracting chemokine 1 (CXCL13) in OAL biopsy specimens [30]. In the same way, dissociation of first Hp gastric infection and ophthalmologic implantation of activated or lymphomatous cells is in concordance with the lack of efficacy of anti-Hp antibiotic therapy in extragastric MALT localizations [9]. Finally, some Hp antigens display similarities with autoantigens such as the H(+)K(+)-ATPase expressed by the gastric epithelium. Whether ionic pumps of the lachrymal glands and the gastric mucosa share common epitopes also deserves further investigation [1, 31, 32]. A remaining question is the case of MALT lymphoma patients with neither gastric Hp nor ophthalmic Cp infection. These patients may have been previously treated with antimicrobials with anti-Hp or anti-Cp activity inducing false negative results. Alternatively, other microorganisms or autoantigens may also be implicated in OAL lymphomagenesis. It has to be mentioned that the search of Chlamydia psittaci DNA was performed in the 11 tumor samples available among the 83 OAL patients and was negative in all studied cases.
In conclusion, our results demonstrate a strong association between gastric Hp infection and MALT/LPL OAL and suggest a new mechanism of indirect infection-associated lymphomagenesis whereby chronic local antigen stimulation would lead to the emergence of ectopic B-cell lymphoma. This observation underlines the role of gastric Hp infection in lymphomagenesis and the usefulness of diagnosing and treating this infection in patients with lymphomas. A large prospective epidemiological study would evaluate the impact of gastric Hp infection on the occurrence of a MALT/LPL lymphoma, whatever its initial localization, and the role of antimicrobial therapy in its prevention and treatment. Furthermore, this new paradigm may apply for other pathogens infected other organs and that induced chronic inflammation and B cell stimulation.
Acknowledgements
The authors thank Gilles Quesnes (Department of Microbiology, Assistance Publique Hôpitaux de Paris, Necker-Enfants malades Hospital, Paris), Céline Campet and Martine Thioux (Laboratory of Molecular Pharmacology, Department of Tumor Biology, Institut Curie, Paris), and Christophe Bovin, Sylvie Luby, and Jean-François Villerez (Department of Pathology, Institut Mutualiste Montsouris, Paris) for their technical assistance.




