Rational design for thermochromic luminescence in amorphous polystyrene films with bis-o-carborane-substituted enhanced conjugated molecule having aggregation-induced luminochromism
Abstract
We designed the triad molecule, bis-o-carborane-substituted bis(thienylethynyl)benzene, as a filler for realizing thermochromic luminescent behaviors based on conventional polymer films, such as polystyrene. From the optical measurements, it was found that the triad can show solid-state emission and dual-luminescent properties with variable intensity ratios depending on media. From the mechanistic studies including the experiments with the methyl-substituted model compound, it was revealed that dual emission should be originated from the locally excited and twisted intramolecular charge transfer states, and the latter emission band is significantly enhanced in the solid states. We prepared amorphous films containing variable concentrations of the triad with the spin-coating method and investigated optical properties. It was found that intensity ratios were drastically changed by altering the concentration of the triad. By increasing the proportion of the triad, aggregation occurred, and emission color was apparently varied through the changes in intensity ratios of the dual emission property. Based on the aggregation-induced luminochromic property of the triad, thermochromic luminescence was finally realized by heating the amorphous films. The rational design for obtaining thermochromic luminescent amorphous films is illustrated in this paper.
INTRODUCTION
As next generation of organic light-emitting diodes, film-type sensors are regarded as one of next goals. If vital signs, such as body temperature, vascular flow, and other metabolic factors, can be visualized at the surface of skins in real time with wearable sensors, this information would be helpful for establishing precise daily health care systems.[1] In industrial fields, visualization technologies for external stimuli are applicable to detection for damage to the products.[2] Therefore, many researchers have devoted much effort to develop luminochromic organic dyes with sensitive stimuli responsiveness. However, there are still several critical issues to be solved in the development of film-type sensors. In practical usages, these dyes are supposed to be used as paint or filler in the matrices. Most of luminescent organic dyes generally show poor emission in film. Due to intermolecular interaction, aggregation-caused quenching (ACQ) occurs. Therefore, luminescent dyes are commonly loaded onto transparent polymer matrices because of compatibility not only for suppressing ACQ but also for adding film-formability.[3-6] Thus, high level of solid-state luminescent properties are required for receiving sensitive responses toward external stimuli.
Commodity luminochromic behaviors are depended on drastic environmental changes during phase transitions between crystal‒amorphous and/or crystal polymorphs.[7-9] Therefore, specific film-preparation manners are necessary for growing environment-sensitive crystalline form.[10] Success strategies for preparing luminochromic films without restricted manners are to employ alkyl-branched polymers[11, 12] and elastomers[13] as a matrix. In the former materials, by altering degree of crystallinity at the side chains, microenvironmental changes around main–chain conjugations followed by luminochromic behaviors are inducible.[11, 12] In the latter matrices, dynamic regulation of luminescent properties with mechanical forces has been accomplished.[13] However, to obtain expected responses, extra treatments in material preparation, such as pre-annealing under specific conditions, are often necessary for setting ideal initial states. Therefore, it is still challenging to realize stimuli-responsive luminochromic behaviors with conventional amorphous polymers.
o-Carborane[14-19] is an icosahedral cluster composed of two carbon and 10 boron atoms and we have recently focused on an element-block, which is a minimum functional unit containing heteroatoms, for constructing not only solid-state luminescent dyes[20-26] but also stimuli-responsive luminochromic materials.[27-32] When bonded with π-conjugated molecules at the carbon, o-carborane works as an electron acceptor.[33] Therefore, intense emission can be observed from the intramolecular charge transfer (ICT) state from the series of aryl‒o-carborane dyads.[34, 35] It should be emphasized that the ICT emission can be often preserved even in the solid state.[36-39] Owing to steric hindrance of sphere carboranes, unfavorable molecular interaction should be disturbed. In the previous report, solid-state luminescent properties of bis-o-carborane-substituted bis(phenylethynyl)benzene triads were investigated.[40] Accordingly, intense emission can be detected both in the crystal and amorphous states. Moreover, emission color changes were observed during the transition from crystal to amorphous. Owing to the steric hindrances of o-carborane units, intermolecular interaction followed by ACQ can be suppressed. In most cases, even though emission can be obtained in crystal, ACQ is often induced in amorphous due to nonspecific intermolecular interactions. In contrast, it was shown that the bis-o-carborane substitution is effective to prevent emission annihilation even in the randomly condensed state and to realize luminochromic behaviors without losses of emission efficiency caused by molecular morphology changes in solid. On the basis of these results, we next aimed to realize stimuli-responsive luminochromic properties in the film state.
By employing polymer matrices, we presumed that desired luminochromic behaviors might be observed by regulating molecular morphology as well as assembly in film. However, it is readily predictable that intermolecular interaction would be spoiled in polymer matrices due to intrinsic condensed morphology. Indeed, the previous triads[40] hardly showed luminochromism in polymer matrices even after annealing. Therefore, we intended to improve environmental sensitivity in the triad. According to previous works, it has been reported that environment sensitivity of the π-conjugated molecule can be enhanced by replacing benzene to thiophene.[10, 41] By enhancing molecular planarity and electronic density of the π-conjugation unit, stimuli-responsivity can be improved.[10] By applying this strategy in the bis-o-carborane-substituted triad molecules, we expected that highly sensitive environment-responsive luminophores can be obtained with luminochromic properties. To evaluate validity of this idea, we designed the thiophene-replaced triad molecules having dual o-carborane units.
We herein report thermochromic luminescent films composed of polystyrene (PS) containing bis-o-carborane-substituted bis(thienylethynyl)benzene, thio-CBH (Scheme 1). By introducing thienyl groups into the bis-o-carborane-substituted skeleton, enhancement of electronic interaction was expected between o-carboranes and the central conjugation unit for obtaining environmental sensitivity. We synthesized the triad and examined optical properties. Accordingly, not only solid-state emission but also the dual emission properties were observed. In particular, changes in intensity ratios followed by different luminescent color were detected under various measurement conditions. From the mechanistic studies including the data from the methyl-substituted model compound, the origins of each luminescent band were individually assigned. In the PS films, aggregation-induced luminochromism was detected by increasing the concentration of thio-CBH. Finally, based on this aggregation-induced luminochromic mechanism, thermochromic behaviors were realized with the PS-based amorphous films. Rational material designs are illustrated in this paper.
RESULTS AND DISCUSSION
Scheme 1 shows the synthetic route for thio-CBH. The two steps of the Sonogashira−Hagihara coupling reactions from 1,4-dibromo-2,5-diiodobenzene to 1,4-bis(2′-thiophenylethyn-1′-yl)-2,5-bis(2′-trimethylsilylethyn-1″-yl)benzene (2) followed by deprotection of trimethylsilyl groups with potassium carbonate afforded 1,4-diethynyl-2,5-bis(2′-thiophenylethyn-1′-yl)benzene (3).[40] Then, the treatment with decaborane (B10H14) in the presence of N,N-dimethylaniline and the successive regioselective alkyne-insertion reaction afforded thio-CBH. Similarly, thio-CBMe, in which the conformation is fixed at the twisted state, was also synthesized as a model compound for evaluating structure‒optical property relationships with the same manner. The structures of obtained triads were characterized by 1H, 11B, and 13C NMR spectroscopies and high-resolution mass analyses. From these data, we concluded that the desired products were obtained. The synthesized triads showed good solubility in conventional organic solvents, such as chloroform, dichloromethane, and tetrahydrofuran (THF) and were stable against moisture and air in both solution and solid states. Further, in comparison to thio-B, which does not have o-carborane units, higher thermal stability was observed from thio-CBH (Figure S1 and Table S1). Molecular tumbling should be suppressed at the conjugated unit by o-carboranes.
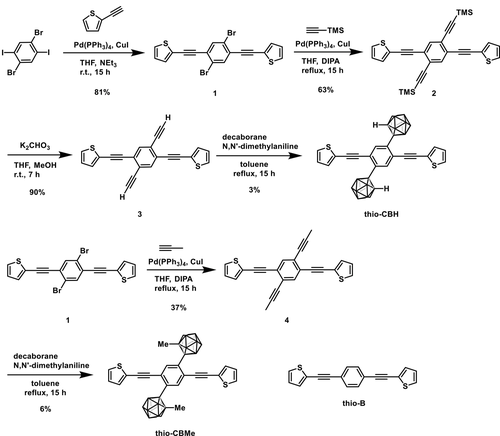
Optical measurements with thio-CBH were carried out in various media (Figure 1), and the photophysical data are summarized in Table 1. The most significant point in the photoluminescence (PL) spectra is the dual luminescent property composed of large and small emission bands with the peaks at 404 and 658 nm, respectively. It is known that the electron-accepting ability significantly depends on the molecular conformation between the π-plane and the direction of the C‒C bond in the o-carborane unit.[42, 43] Electron-donating and -accepting interaction between π-conjugation of the aryl moiety and o-carborane is minimized in the parallel conformation. Therefore, the emission band from the locally excited (LE) state at the aryl moiety, which is sensitive to ACQ, is often observable only in solution. Conversely, in the twisted conformation, the emission band, which is from the ICT state, can be induced originating from electron-donating and -accepting interaction. Significantly, as the ICT emission is insensitive to ACQ but sensitive to molecular tumbling followed by annihilation, broad emission bands with large Stokes shifts can be detected in solid. Therefore, many types of the aryl-modified o-carborane dyads exhibit the aggregation-induced emission (AIE) property.[27, 44-50] Correspondingly, intense emission was observed around 400 nm in solution thio-CBH, meanwhile significant emission band around 650 nm was obtained in aggregation and crystal. From the solvatochromic behavior (Figures S2 and S3 and Table S2) and longer lifetimes, it was suggested that the emission band in the longer wavelength region should be obtained from the ICT state. In particular, at 77 K, where molecular motions should be suppressed, this broad emission band disappeared, and instead the emission band was obtained only in the shorter wavelength region (Figure S4), indicating that the twisted intramolecular charge transfer (TICT) state should be formed after excitation.[51, 52] From these results, similarly to the previous studies on the aryl-modified o-carborane dyads, we concluded that the emission bands in shorter and longer wavelength regions should be attributable to emission from LE and TICT states, respectively.[42, 43] It should be noted that the peak wavelength was larger than those of the phenyl-substituted triads, suggesting that electronic interaction should be enhanced in thio-CBH by the thienyl substituents (Figures S5 and S15 and Table S3).[40] Moreover, from the comparison to thio-B, red-shifted optical bands were obtained, indicating that o-carborane should play a significant role in the construction of robust conjugation.
| λabs,max (nm) | λem,max1 (nm)c | λem,max2 (nm)c | τ11 (ns)d | τ12 (ns)d | τ21 (ns)d | τ22 (ns)d | χ12 | χ22 | ΦPLe | |
|---|---|---|---|---|---|---|---|---|---|---|
| THFa | 370 | 404 | 658 | 0.10 (68%) | 0.41 (32%) | 1.17 | 1.09 | 1.02 | 0.05 | |
| THF/H₂Oa, b | 369 | 411 | 603 | 0.67 (28%) | 0.36 (72%) | 11.42 | 1.08 | 1.08 | 0.11 | |
| Solid | 370 | 344 | 604 | 0.27 | 0.67 (31%) | 3.94 (69%) | 1.09 | 1.01 | 0.18 |
- a 1.0 × 10‒5 M.
- b Aggregated in THF/water = 1/99 vol%.
- c λex = λabs.
- d λex = 370 nm.
- e Determined as an absolute value.
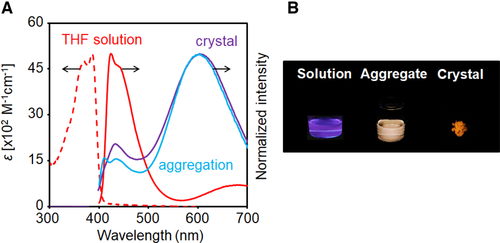
In the solid state, the ICT emission can be observed from thio-CBMe, in which molecular conformation should be fixed at the twisted state. The broad emission bands with large degrees of Stokes shifts were observed not only in solid but also in solution (Figure 2A and Table 2). Bathochromic shifts were inducible in the PL spectra by using polar solvents (Figures S2 and S3 and Table S5), while almost same spectra were obtained under the frozen condition (Figure S7). It is because thio-CBMe forms the twisted state because of the steric hindrance of the methyl substituent at the o-carborane unit. Therefore, the emission band from the ICT state with the AIE character should be exhibited in all media. Thus, similar shapes of PL spectra were obtained. Regarding peak positions in the spectra, it is assumed that lower polar circumstances than that in THF could be generated in the molecular condensation with o-carborane triads. Thus, blue shifts in the spectra could be observed from the solid samples. As large difference in the peak wavelength was observed between aggregation and crystalline samples, we investigated mechanochromic properties of the crystal powder (Figure 2B). As we expected, the red shift by 33 nm corresponded to emission color change was observed after grinding (Figure 2C). Environmental changes induced by collapse of regular structures should influence electronic properties of the conjugation unit (Figure S8). Additionally, reversible processes were confirmed by heating and vapor fuming with chloroform to facilitate recrystallization (Figure 2D and Figure S9). These data indicate that the emission bands from ICT state have environmental sensitivity as well as resistance to ACQ. In the case of thio-CBH in solution, the fast decay processes were observed, suggesting that molecular motions should occur in the excited state (Tables 1 and 2). Furthermore, shorter lifetimes were obtained compared to those from thio-CBMe in solid. This fact supports the TICT mechanism and means that molecular motions would be restricted in thio-CBMe because of steric hindrance between the methyl groups to the π-conjugated moiety.
| λabs,max (nm) | λem,max (nm)c | τ1 (ns)d | τ2 (ns)d | χ2 | ΦPLe | |
|---|---|---|---|---|---|---|
| THFa | 395 | 628 | 3.40 | 1.09 | 0.11 | |
| THF/H₂Oa, b | 405 | 608 | 4.74 (34%) | 10.9 (66%) | 1.11 | 0.15 |
| Crystal | 395 | 557 | 3.66 (29%) | 6.59 (71%) | 1.19 | 0.26 |
| Ground | 395 | 590 | 3.42 (41%) | 8.22 (59%) | 1.03 | 0.19 |
- a 1.0 × 10‒5 M.
- b Aggregated in THF/water = 1/99 vol%.
- c λex = λabs,max.
- d λex = 370 nm.
- e Determined as an absolute value.
Optical properties in the PS matrix were examined with a spin-coating film containing variable concentrations of thio-CBH (Figure 3 and Table S6). Significant peak shifts were hardly observed in the absorption spectra, representing constant electronic structures obtained in the ground state. Interestingly, drastic changes were detected in the PL spectra. In the presence of 10 wt% thio-CBH, the emission band from the LE state was mainly observed, whereas the intensity from the TICT state increased by increasing the triad concentration. From the surface observations with scanning electron microscopy (SEM), significant morphology changes were detected by loading thio-CBH in the PS matrices (Figure S10). Accordingly, aggregation was observed in the film with 10 wt% triad. In particular, by increasing the concentration, crystallization could proceed in the polymer matrix in the 60 wt% film. In the normalized emission spectra, almost same shapes of the emission bands were observed between the films with 20 wt%, 30 wt%, 40 wt%, and 50 wt%. It is implied that aggregation could be formed below 20 wt%, and crystallization might be facilitated by adding the triad over 30 wt%. Corresponding to these morphology changes, emission color should be altered. By changing the type of polymers, such as poly(methyl methacrylate) (PMMA) and polyvinylchloride (PVC), concentration-dependent color changes were hardly observed (Figure S11). Due to low compatibility, aggregation of thio-CBH should form even in the lower concentrations in PMMA and PVC matrices, followed by intense emission from the TICT state from 30 wt% films. Thus, concentration dependency of the intensity ratios could be hardly observed.
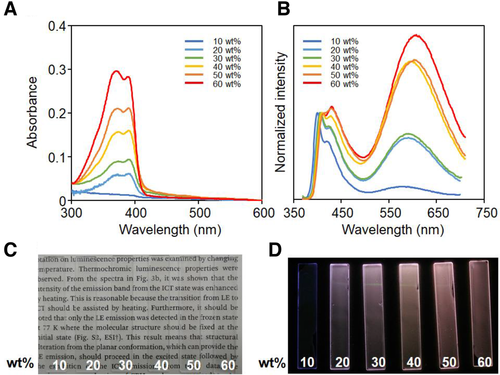
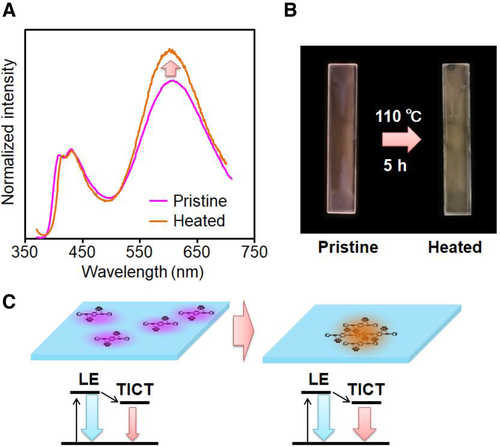
Finally, we monitored emission property changes of the films during heating (Figure 4). From differential scanning calorimetry, glass transition temperatures (Tg) of all PS films in the absence and presence of thio-CBH (60 wt%) were detected at 90°C (Figure S12). Therefore, the films were annealed at 110°C for 5 h, and their optical properties were compared. In the film containing 10 wt% thio-CBH, almost same spectra were obtained before and after heating (Figure S13), and the film containing 60 wt% thio-CBH showed a different shape of the spectrum (Figure 4 and Table 3). The band attributable to emission from the TICT state was enhanced by heating, and correspondingly emission color was altered. From the transmittance measurements, significant changes were hardly detected after heating (Figure S14 and Table S7), meanwhile from the SEM observations, inhomogeneity was enhanced by heating (Figure S10). This result implies that thermochromism should be caused not by thermal reactions at the molecular level but by morphology changes. In other words, aggregation of thio-CBH should be facilitated by the heating treatment, leading to enhancement of the emission band from the TICT state. Finally, the emission color change through the aggregation-induced luminochromic mechanism should be obtained after heating. By molecular assembly, quenching processes of the TICT emission through C‒C elongation could be suppressed. As a result, the emission band from TICT state should be enhanced. By incubating at 80°C, which is lower than Tg, significant changes were hardly observed with the film containing 60 wt% thio-CBH. It is likely that loaded molecules should be entangled by polymer chains in amorphous matrices. To widely apply this system as a thermometer in various scenes, it is essential to control transition temperature. It is, for example, assumed that luminochromic behaviors could be exhibited in the lower temperature region by using the polymer matrices with lower Tg and appropriate miscibility to the triad.
CONCLUSION
Based on the idea for improving environmental sensitivity, we designed and synthesized thienyl-substituted conjugated compound. The synthesized triad exhibited solid-state dual emission, and especially intensity ratios varied in the different media. Furthermore, in the PS film, luminescent color changes originating from ratiometric alteration of dual emission properties were detected by aggregation formation. Finally, thermochromic luminescent behaviors were achieved with the triad-loaded amorphous films through aggregation-induced luminochromism. As most of stimuli-responsive luminochromic dyes still suffer from low film formability, it can be said that there is still difficulty in the practical usage of conventional dyes as paint- and film-type chemosensors. Our study describes one of the strategies for application of new functional organic dyes as well as commodity ones to advanced optical sensors.
ACKNOWLEDGMENTS
This work was supported by the Nakatani Foundation (for Kazuo Tanaka) and by a Grant-in-aid of the Ministry of Education, Culture, Sports, Science, and Technology, Japan for Scientific Research (B) (JP21H02001) and for Scientific Research on Innovative Areas “New Polymeric Materials Based on Element-Blocks (No.2401)” (JP24102013).
CONFLICT OF INTEREST
The authors declare that is no conflict of interest.




