DNA nanostructure-encoded fluorescent barcodes
Abstract
Identification and differentiation of various bioanalytes with high spatial and temporal resolution have spawned fluorescent barcode-based detection and imaging. DNA nanostructures with excellent structure programmability, addressability, and near-atomic structure accuracy have emerged as a versatile platform to develop fluorescent barcodes through finely controlled dye numbers, relative distances, and compositions. A variety of DNA fluorescent barcodes with distinguishable spectral colors, geometries and digitized fluorescence intensities have been constructed and favorably implemented in the field of bioimaging, multiplex bioassay, and information security. In this review, we first summarize the state of the art of self-assembled DNA nanostructures. Next, the utilization of DNA nanostructure to develop fluorescent barcodes and the photophysical properties of DNA-templated fluorophores are reviewed. Finally, the applications of DNA fluorescent barcodes for biosensing and imaging, and the challenges and outlook of DNA nanostructure-engineered fluorescent barcodes are discussed.
1 INTRODUCTION
High-throughput multianalyte analysis with high spatial and temporal resolution in complex mixtures is of great demand for the cell biology,[1, 2] drug discovery[3], and clinical diagnostic applications.[4, 5] In comparison to multiple independent analysis, multiple parameters of analytes in the mixtures can be collected and analyzed simultaneously with a multianalyte assay, which is facilitated to study the intermolecular interactions in real time with high accuracy. To achieve this goal, encoded labels that can be unambiguously distinguished are in high demand.
To date, a variety of encoding techniques have been developed, including graphical encoding,[6] chemical encoding,[7] electronic encoding,[8] physical encoding,[9] magnetic encoding,[10] and optical encoding.[11] Among these encoding techniques, optical encoding exhibits the highest design flexibility because of the availability of large number of luminescent materials and advances in fluorescence microscopy, which endows it with high encoding capacity and decoding speeds. For example, fluorescence microscopy has shown great advantage in simultaneously detecting and identifying multiple mRNAs in a single cell with fluorescence intensity-encoded barcodes.[12, 13] Generally, optical barcodes are encoded through mixing spectrally distinct fluorophores, ranging from genetically encodable fluorescent proteins,[14, 15] organic dyes,[12] to inorganic fluorescent nanoparticles.[17, 18] However, this method is mainly restricted by the overlap of the broad emission spectra of organic fluorophores, which limits spectral multiplexing capability to about four to five dyes.[19, 20]
To overcome these shortcomings, many materials are developed to improve encoding capacity of fluorescent barcodes.[6, 10, 16, 19, 21] Among them, DNA nanostructure with tailorable three-dimensional (3D) configuration, structure addressability, and near-atomic scale structure precision is an emerging material, which holds great potential to arrange fluorophores in predesigned patterns.[22, 23] Furthermore, with development of bioconjugation chemistry, various functional groups, such like small molecules,[24, 25] macromolecules,[26-28] or nanoparticles,[29-31] can be well arranged on DNA nanostructures at specific sites.[32-35] The present strategies using DNA nanostructures to encode fluorescent barcodes can be summarized into two directions, that is, DNA nanostructure geometry-encoded barcodes and quantitative fluorophore number-based intensity barcodes. These DNA nanostructure-based fluorescent barcodes have been widely applied in biosensing, tissue engineering, and regenerative medicine.[36-39]
In this review, we concentrate on the recent progress of DNA nanostructure-encoded fluorescent barcodes system and their applications in multiplex biosensing and imaging. We first introduce the origin of DNA nanotechnology and the design milestones of DNA nanotechnology in the past two decades. Then, we summarize the recent progress in encoding fluorescent barcodes with organic dyes and their applications in biosensing and imaging. Subsequently, we highlight the photophysical interactions of fluorophore oligomers at nanoconfined space on DNA nanostructure. Finally, we give a perspective of the future outlook about challenges and opportunities of DNA nanostructure-encoded fluorescence barcodes.
2 SELF-ASSEMBLY DNA NANOTECHNOLOGY
DNA is known as a natural carrier of genetic information and has been widely investigated as building materials to make well-defined nanostructures through self-assembly.[40, 41] With the rapid cost reduction of oligonucleotides syntheses and better understanding of DNA hybridization thermodynamics, researchers can construct DNA libraries with rational design in a bottom-up manner under the guidance of Watson-Crick base-pairing rules.[42-45]
In the last few decades, several strategies have been proposed to construct DNA nanostructures with high designability and complexity. In 1982, Seeman first proposed that DNA could be designed to construct DNA structure with certain morphology in vitro.[46] As shown in Figure 1A, four partially hybridized DNA strands with different amounts of sticky ends form a starting DNA nanostructure with a crisscross structure. This work opened possibility to design and fabricate complicated DNA structures using simple DNA nanostructures by sequence-specific hybridization process.
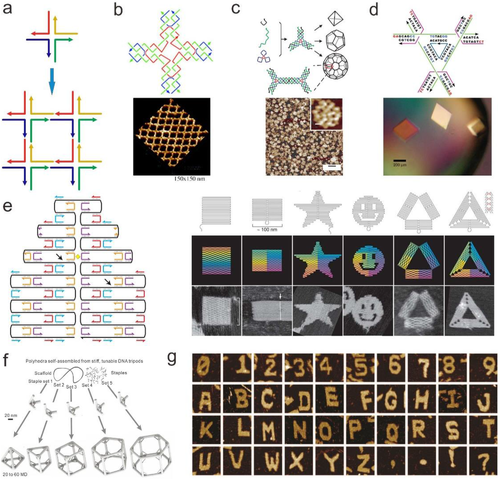
Up to date, a number of two-dimensional (2D) and 3D lattices assembled from small, repeating DNA nanostructure motifs have been reported (Figure 1B).[26, 47-50] Especially, polyhedral objects were also formed from fixed numbers of DNA junction motifs (Figure 1C).[51-55] On this basis, researchers constructed a 3D DNA assembly and applied it in the spatial arrangement of proteins for the first time [45]. This work presents a pioneering work in fulfilling Seeman's original vision of using 3D DNA lattices as scaffolds to achieve spatial organization of guest protein molecules (Figure 1B).[46] In 2009, Seeman's group fabricated more ordered 3D DNA crystals using sticky-end connection structure (Figure 1D).[49] 3D crystals of different sizes were obtained through triangle motifs design and assembly, proving the high spatial control of DNA nanostructure on 3D space.
In 2006, Rothemund reported the pioneering work of DNA origami technique. DNA origami involves the use of a very long single strand of DNA (7.3-kb long M13 phage genome) as the scaffold, which is held in a desired configuration through simple base-paring with hundreds of short “staple strand” oligonucleotides (30-80 nt each) (Figure 1E).[56] Compared to DNA motif assembly method, the DNA origami fabrication by simple strands mixing has lower requirement in strands ratio control. Hundreds of distinct staple strands are distinct in sequence, which empowers the fabricated DNA nanostructure with geometrical addressability. With this method, a large number of DNA nanostructures, including rectangles, triangles, five-pointed stars, and smiley faces, are produced in high yield.[56] Development in DNA origami design software further prompts the construction of 3D DNA origami structure. For example, twisted and curved 3D nanostructures have been constructed and increased their 3D complexity.[57-59] In addition, edge-to-edge base-stacking interactions and sequence-specific sticky end cohesion have adopted to construct huge-sized origami (Figure 1F).[25] These large DNA origamis were fastened with many cross-linkers that ensures the stability of DNA origami and promote their wide applications, such as genetic analysis,[60] viral sensing,[61] and cancer therapeutic.[62] More recently, Yin and co-workers synthesized a variety of 1D, 2D, and 3D DNA nanostructures from single-stranded DNA tiles (SSTs) (Figure 1G).[63] The platform is based on a series of interlocking local connections between SSTs. Collections of SSTs form 2D sheet or 3D block canvases can be selectively engraved to create different shapes and patterns by simply including or omitting specific SSTs.
3 DNA NANOTECHNOLOGY-ENABLED MULTIPLEXED ENCODING
In an optical multiplexed biosensing or bioimaging system, the multiplexed detection was achieved through labeling multianalytes with fluorescent barcodes that have distinguishable properties in color, lifetime, or intensity.[64] Many microscale materials have been well studied to prepare fluorescent barcodes.[65-67] However, owing to its microscale size, poor dispersity, and nonbiocompatibility, those barcodes are generally limited in practical applications.[68, 69]
The nanoscale particle size and sub-nanometer addressing resolution on DNA origami allows for placement of fluorophores at specific position and the consequent assembly of fluorescent arrays with predesigned spatial distribution. The programmability of DNA origami provides amounts of choices to construct 1D, 2D, or 3D fluorescent barcodes with different shapes and sizes. These merits make DNA origami an ideal candidate for constructing fluorescent barcodes at nanoscale. So far, intensity encoding and geometrical encoding are commonly adopted for current DNA nanostructure-based fluorescent barcodes design.[21, 70] For example, different numbers of fluorescent nucleic acids can be designed on DNA nanostructures to construct intensity-encoded barcodes.[68, 71-74] Using fluorescent probes with design distribution array, geometrical encoding can be constructed with DNA nanostructures.[65, 66, 75-78]
3.1 DNA nanostructure geometry encoded fluorescent barcodes
DNA origami offers a powerful platform to build robust geometry-encoded fluorescent barcodes by spatially placing fluorophores at the designed position.[79] The programmability and addressability of the DNA nanostructure allow researchers to anchor the organic fluorophores into a well-defined nanoarray, thus the capacity of the fluorescent barcodes is highly improved. With the lateral distance of fluorophore beyond the spatial resolution of adopted imaging methods (∼ 250 nm for diffraction-limited imaging, ∼25-40 nm for superresolution imaging), the specific fluorescence patterns are generated as geometrical barcodes.[70] The multiplexing capability of the geometrical barcodes can be enhanced by increasing the number of the spatially distinguishable fluorophores.
Yin and co-workers reported a geometrically encoded fluorescent tags utilizing DNA origami-based self-assembly (Figure 2A).[70] This DNA origami-based strategy enables constructing optical barcodes with length of 400-800 nm and producing 216 distinct barcodes through spatially controlling over the position of fluorophores (Alexa 488, Alexa 647, and Cy3) on the surface of a stiff DNA nanorod, and the barcodes can be decoded unambiguously using epifluorescence or total internal reflection fluorescence microscopy (Figure 2B). However, a relative larger DNA origami size is required for the geometrical barcodes design because of the diffraction limitation in fluorescence imaging, which restricted their applications in accurate detection application.
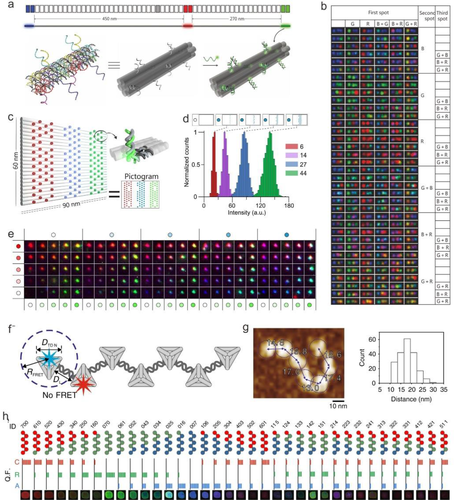
Recently, a new geometric barcoding strategy in combination with DNA-PAINT (point accumulation for imaging in nanoscale topography) technique have been reported. [80-83] DNA-PAINT is a superresolution imaging method that form images during the transient interactions between the fluorophore-labeled DNA strand (imaging strand) and the docking strand.[84] DNA-PAINT provides an alternate approach for quantitative analysis of targets by counting the stoichiometry of the docking sites that greatly extends spectral multiplexing capabilities under optical diffraction limit.[85] For example, Dai et al reported using a rectangular DNA origami with a square lattice pattern as superresolution geometric barcodes for multiplexed miRNAs profiling with highly sequence specificity.[86] In this work, DNA-PAINT can define the position of individual anchor sites immobilized DNA origami and generate the asymmetric pattern to identify and quantify the 8-plex miRNA simultaneously.
3.2 Intensity-based fluorescent barcodes
For intensity-based fluorescent barcodes, the most widely used strategy is adjusting the number of fluorophores on one DNA nanostructure. In comparison to size limitation confronted by geometrical barcodes, the intensity barcodes have higher design flexibility in consideration of numbers and spatial distribution of fluorophores.[21] However, with the increase of fluorophore number and decrease in intermolecular distance of fluorophores, unexpected Förster resonance energy transfer (FRET) or aggregation-induced fluorescence quenching can affect the fluorescence intensity and decrease the capacity of barcodes. Therefore, the precise position control of fluorophores on barcode is essentially important.[87]
Luo et al first proposed to develop the Y-DNA-templated barcodes.[72] By controlling the dye type and number precisely, five multicolor barcodes with different ratios of different dyes were built.[72] However, duo to the undesirable self-quenching and FRET effect, the capacity of the barcode was limited. Compared with small-sized Y-type DNA, DNA origami provides many modification sites, which is favorable for improving the capacity of barcodes. Woehrstein and co-workers designed 2D fluorescence arrays composed of three different fluorescent molecules (ATTO 647N, Cy3, and ATTO 488) on a rectangular DNA origami with dimensions of 90 × 60 nm2 to construct digitally adjustable ultra-fluorescent probes with sizes smaller than 100 nm (Figure 2C).[21] They found that the fluorescence brightness had a good linear relationship with the number of labeled fluorescent molecules, and thus proposed to obtain probes with different optical properties by digitally changing the number of fluorescent molecules. The number of fluorescent molecules was chosen at 0, 6, 14, 27, and 44 as five digitized fluorescence intensity states (Figure 2D). Combined with the difference in excitation and emission spectral characteristics of the three fluorescent molecules, 53 = 125 superprobes with different spectral and light intensity characteristics can be achieved (Figure 2E). However, synthesis of large size (> 100 nm) DNA origami often requires hundreds of single-stranded DNA, which restricts its applications. What is more, barcodes based on large size DNA nanostructures cannot satisfy the requirements of multiplex detection and imaging at single cells level.
Recently, Fan et al reported a facile method to create quantized fluorescence intensity barcodes using Cayley tree-like fractal DNA frameworks (FDFs).[88] The Cayley tree-like FDF is constructed through assembly of tetrahedral DNA nodes (edge length 20 bp). Compared to previous work based on large size DNA origamis or single-stranded-tile -based nanostructures (> 100 nm), the DNA scaffolds used in this work can minimize number of the DNA strands and optimize the modularity for multiplex analysis. Using 16 DNA strands, n-node (n = 53) structures can be constructed. To control the energy transfer between fluorophores, the lateral distance between two adjacent tetrahedral DNA nanostructures (TDNs) was designed to be 18.5 ± 3.8 nm (Figure 2F and G). Above all, these rigid-yet-flexible supermultiplex structures enable the fluorescence cross-talk restrained, and makes the encoded barcodes with quantized fluorescence states. By precise anchoring of three fluorophores (Cy5, ROX, and Alexa 488) on the FDFs, 36 encoding barcodes are created with distinct colors and intensities (Figure 2H).
4 DNA NANOTECHNOLOGY-ENGINEERED DYE AGGREGATES
DNA structures can serve as a rigid scaffold to arrange dye molecules with precise control of stoichiometry and dye-dye interactions. It was well known that photophysical properties of dye arrays are sensitive to the distance of two neighboring dyes. For example, Tinnefeld et al demonstrated that the strong quenching of intensity and lifetime occurred when the relative distance of organic dyes was below 2 nm.[89] To study the distance effect on fluorescent properties of dye arrays, they used a 2D rectangular DNA origami as a scaffold to arrange ATTO 647N dyes with single base pair resolution. Due to dynamic quenching and static quenching in H-type dimer, halved lifetime and a 10-fold decrease of intensity were observed when the relative distance of dyes are fixed within 7 bps. When two dyes are separate with distance > 5 nm, fluorescence intensity increases linearly with the number of organic dyes, which have been demonstrated in previous work.[21, 88] In another recent work, Cayley tree-like FDFs are used as structural basis to anchor organic fluorophores (Alexa 488, ROX, and Cy5) with distance of ∼ 4 nm.[81] Compared to the monomeric TDN modified with a single Alexa 488, the fluorescence probes with the dense arrangement (four fluorophores per DNA framework) displayed ∼24-fold increase in terms of fluorescence intensity. The experimental data indicated that the self-quenching was avoided in case of ∼4 nm interspace. Besides, with an interfluorophore distance of >10 nm between three type of fluorophores (Cy5, ROX, and Alexa 488), the FRET quenching effect was restricted, resulting the linear relations between fluorescence intensity and dye number in each channel.
Recently, Vale et al focus on the optical properties of dye aggregates with an interfluorophore distance ranging from 2 to 5 nm (Figure 3A).[90] Compared with a single organic dye (Cy3), six-Cy3 modified DNA FluoroCube exhibits ∼54-fold higher lifetime and ∼43-fold photons (Figure 3B). Further studies replacing Cy3 with other dyes, such as ATTO 488, ATTO 565, ATTO 647N, Cy3N, and Cy5, showed the similar nonlinear photostable increase. The increased photostability was proposed to be resulted from the direct dye interactions and resonant energy transfer between individual dyes.
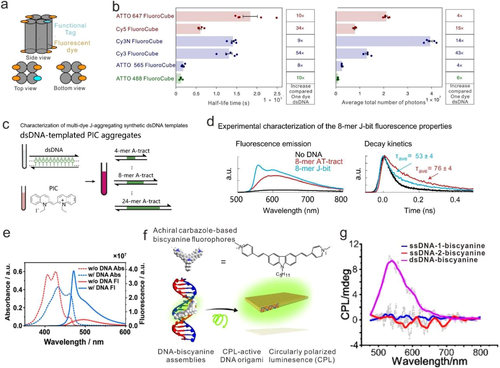
Except for chemical modification process, some fluorophores can bind to DNA directly. Bathe and coworkers present a bottom-up strategy to program the spatial organization of densely packed, discrete clusters of cyanine dye aggregates with tunable absorption spectra (Figure 3C).[91] The dye aggregates shown strongly coupled exciton dynamics in a similar way as natural light-harvesting systems. The prepared DNA templated exciton circuits exhibit excitonic transport properties with prominent circular dichroism, superradiance, and fast delocalized exciton transfer (Figure 3D). More recently, Yan's group reported that a benzothiazole cyanine dye, K21, can bind to minor groove of DNA duplex and form J aggregates (Figure 3E).[92] The strong intermolecular excitonic coupling can mediate long distance energy transfer along the DNA template.
DNA can also be used as a chiral template to transfer the chirality to dye aggregates. Ding et al describes a novel strategy to build biscyanine fluorophores assemblies with circularly polarized luminescence (CPL) activity, using chiral deoxyribonucleic acid (DNA) molecules as the template (Figure 3F and G).[93] The significant blueshift of absorption and strong emission of dyes was ascribed to the restriction of intramolecular rotation (RIR) effect. Interestingly, this dye-DNA complex exhibits the reversible CPL responses during cyclic thermal treatments.
5 APPLICATION OF DNA NANOSTRUCTURE-ENGINEERED FLUORESCENT BARCODES
Multiplexed detection of biomolecule has substantially significance in many areas, such as gene expression profiling, drug discovery, and clinical diagnostics.[1-5] Lin et al proposed a multiplexed detection of multiple DNA sequences and aptamer binding molecules based on a fluorescently encoded suspension array (Figure 4A and B).[94] The suspension arrays were constructed with three tiles including two kinds of encoding tiles and one detection tile. For multiplexed target detection, the targets can competitively bind with the dye-modified DNA on the detection tile and depart it from the detection tile, leading to the intensity decrease. To test the multiple detection capacity (DNA oligos, protein, and a small molecule) of the combinatorial encoding arrays, four types of arrays (3R0G3B, 3R0G, 2R1G, and 1R2G) were used. Upon the addition of targets in different combinations, the corresponding color changes of the encoding arrays were obtained, revealing that this encoding method processes excellent specificity for multiplex detection. As shown in Figure 4B (down row), the existence of the target 5 (human R-thrombin) and target 6 (ATP) leads to corresponding color changes individually or in a mixture, while no color change was observed in the presence of BSA. However, the nonspecific binding may cause false positive signal in multiplexed detection. Luo et al reported using Y-DNA to construct intensity-based fluorescent barcodes, which was further utilized to detect several pathogens with high specificity (Figure 4C).[72] By controlling the exact ratio (4:1, 2:1, 1:1, 1:2, and 1:4) of two distinct dyes (Alexa Fluor 488 and BODIPY 630/650), five fluorescent barcodes were designed. The applications in multiplexed detection of the DNA from several pathogens were tested with fluorescence microscopy and dot blotting (Figure 4D). However, aggregation-induced fluorescence quenching and the low encoding capacity restrict its applications.
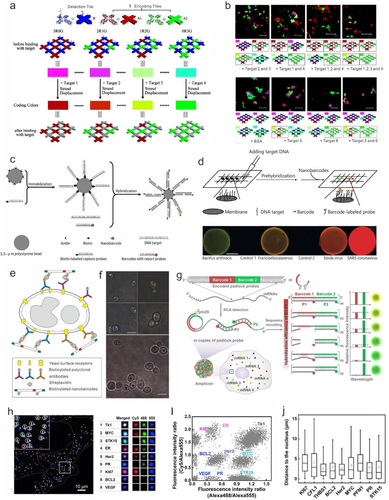
Probing biomolecules on the cell surface or inside of cells plays a vital role in the field of disease diagnostics.[95] With the modification of antibodies or aptamers, the functional barcodes can specifically bind with target biomolecules on cell surface and serve as the multiplexed imaging tag.[36] Yin et al proposed a method to tag yeast surface receptors using geometrical fluorescent barcodes (Figure 4E and F).[70] Under total internal reflection fluorescent microscope (TIRFM), the GRG (green//red/green) barcode was used for wild-type Candida albicans yeast imaging. The barcodes used in this work can maintain their structural integrity when incubated with cells.
Taking advantage of high capacity of DNA nanostructures-encoded barcodes, visualizing RNA in vitro can be achieved, which is critical to the gene expression and reveal disease mechanism.[86] However, the large size of the most existing barcodes is not suitable for the detection of diverse molecules inside cells. More recently, Li et al introduced a DNA-sequence-encoded fluorescence barcoding method, termed sequence-encoded amplicon (SeqEA) that was used for multiplexed imaging of RNAs in single cells at single molecule level (Figure 4G).[96] In the SegEA platform, encoded padlock probe is designed with two components, including recognition (R) and barcode (B1 and B2) modules. The recognition section can recognize the target RNA, which will initiate rolling circle amplification RCA and create a long amplicon. And the barcode section B1 and B2 can hybridize with P1 and P2 detection probes, which is labeled with two or three kinds of fluorophores. The fluorescence intensity of P1 probe is determined by the hybridization efficiency of B1-P1, which was modulated by precise control of the sequence of B1 module. Thus, amplicons with different fluorescence signals can be served as barcodes for multiplex RNAs detection. Nine target mRNAs related to breast cancer can be identified by the encoded padlock probes (Figure 4H and I). Moreover, the distributions of nine mRNAs in single cells have obvious difference (Figure 4J). For example, the mRNAs THBS1 and Hers2 tend to be close to the nucleus. This SegEA-enabled single-cell RNA imaging technology hold great potential in gene expression study and disease diagnostics.
6 SUMMARY AND OUTLOOK
DNA nanotechnology has enabled the quantitative organization of the organic dye with near-atomic precision, and DNA nanostructures have become one of the widely used platform to construct fluorescent barcodes. Compared to conventional labels, DNA nanostructure-based fluorescence barcodes have many advantages: (a) the size of the DNA barcodes (< 800 nm) is smaller than the existing geometry-encoded fluorescent barcodes (range 1-100 μm). The submicrometer dimensions make them potentially useful as in situ single-molecule imaging probes. (b) Compared to other fluorescent barcodes, the synthesis and purification of the DNA nanostructure-enabled barcodes eliminated the requirement of enzymatic reaction, genomic engineering, photolithography, electrochemical etching, or microfluidic devices, and is easy to perform in a typical biochemistry laboratory. (c) The robustness and programmability of the strategy based on DNA origami enables a high multiplexing capability. (d) DNA nanostructure-enabled barcoding system processes outstanding biocompatibility, allowing the barcodes to tag specific biological samples and serve as multiplexed in situ cell imaging probe.[97, 98]
Some challenges are still present to realize the full potential of the DNA nanostructure-based fluorescent barcodes. First, because DNA nanostructures are built under the guidance of Watson-Crick base pairing rules, a minor alteration in the ambient environment may result in the disassembly of DNA nanostructures. Therefore, chemical protections are needed to protect the self-assembled DNA nanostructures from being degraded during its in vivo applications. Recently, certain photoactive or light actuated cross-linker strategies have been used to covalently cross-link the DNA.[99, 100] Second, crowding of multiple barcodes may render an individual barcode unresolvable. Although some barcodes are potentially suitable for in situ labeling of multiple cell types (each carrying distinct surface biomarkers), it is generally too bulky to detect many subcellular identities in a single cell. This challenge could be addressed by designing new fluorescent barcodes with smaller size and stable structures.
In conclusion, DNA nanostructures have extraordinary advantages in constructing fluorescent barcodes with near-atomic scale structure precision, tailorable sizes and shapes, and quantitative colors and intensity, and thus are expected to promote the rapid development of bioimaging, multiplex bioassay, and information security.
ACKNOWLEDGMENTS
This work was financially supported by the National Key R&D Program of China (2016YFA0201200), the National Natural Science Foundation of China (91953106, 21904087, 21904060, and 21834007), and the Shanghai Municipal Science and Technology Commission (19Z111030000 and 19ZR1474600).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Biographies

Tingting Zhai is currently a postdoctoral fellow at Shanghai Jiao Tong University. She received her bachelor's degree from Hunan University at 2013 and Ph.D. degree from Nanjing University at 2018. Her current scientific interests focus on the design and synthesis of DNA nanostructure-based fluorescent barcodes for biosensing and bioimaging.

Jianlei Shen obtained his Ph.D. degree (2013-2016) from Shanghai Institute of Applied Physics (SINAP), Chinese Academy of Sciences (CAS). From 2016 to 2018, he was a postdoctoral fellow at South China University of Technology. He is currently an assistant professor at the School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University. His research interests include design and biochemical applications of DNA-based nanoprobes.




