Organic cocrystals: From high-performance molecular materials to multi-functional applications
Abstract
Advancements in organic electronics are propelling the development of new material systems, where organic materials stand out for their unique benefits, including tunability and cost-effectiveness. Organic single crystals stand out for their ordered structure and reduced defects, enhancing the understanding of the relationship between structure and performance. Organic cocrystal engineering builds upon these foundations, exploring intermolecular interactions within multicomponent-ordered crystalline materials to combine the inherent advantages of single-component crystals. However, the path to realizing the full potential of organic cocrystals is fraught with challenges, including structural mismatches, unclear cocrystallization mechanisms, and unpredictable property alterations, which complicate the effective cocrystallization between different molecules. To deepen the understanding of this promising area, this review introduces the mechanism of organic cocrystal formation, the various stacking modes, and different growth techniques, and highlights the advancements in cocrystal engineering for multifunctional applications. The goal is to provide comprehensive guidelines for the cocrystal engineering of high-performance molecular materials, thereby expanding the applications of organic cocrystals in the fields of optoelectronics, photothermal energy, and energy storage and conversion.
List of Abbreviations
-
- 9ACA
-
- 9-acetylanthracene
-
- ACQ
-
- aggregation-caused quenching
-
- AP
-
- aromatic-perfluoroaromatic
-
- BTC
-
- parallelogram-like cocrystal
-
- CT
-
- charge transfer
-
- DBTTF
-
- dibenzotetrathiafluoromanganese
-
- DCT
-
- degree of charge transfer
-
- DITFB
-
- 1,4-diiodotetrafluorobenzene
-
- DMA
-
- 9,10-dimethylanthracene
-
- DPTTA
-
- mesodiphenyl tetrathionene [2,1,2,1]
-
- ESR
-
- electron spin resonance
-
- F4TCNQ
-
- 2,3,5,6-tetrafluoro-7,7,8,8-tetracyanoquinodimethane
-
- FF
-
- fill factor
-
- HOMO
-
- highest occupied molecular orbital
-
- LUMO
-
- least unoccupied molecular orbital
-
- MOF
-
- metal-organic framework
-
- NDI
-
- naphthalene diamide
-
- NIR
-
- near-infrared
-
- NMR
-
- nuclear magnetic resonance
-
- NP
-
- nanoparticle
-
- OFN
-
- octafluoronaphthalene
-
- OPV
-
- organic photovoltaic
-
- ORTP
-
- organic room-temperature phosphorescence
-
- P5
-
- macrocyclic molecules
-
- PAH
-
- polycyclic aromatic hydrocarbon
-
- PCE
-
- power conversion efficiency
-
- PDI
-
- N,N'-bis(n-butyl)homophthalic acid diimide
-
- Pe
-
- perylene
-
- Phen
-
- phenanthrene
-
- PL
-
- photoluminescence
-
- PLQE
-
- photoluminescence quantum efficiency
-
- PLQY
-
- photoluminescence quantum yield
-
- POM
-
- polarized light microscopy
-
- PVT
-
- physical vapor transport
-
- Pyr
-
- pyrene
-
- QCl4
-
- p-chloranil
-
- RhB
-
- Rhodamine B
-
- STC
-
- styrylpyridine-TCNB
-
- SVA
-
- solvent vapor annealing
-
- TADF
-
- thermally activated delayed fluorescence
-
- TAPP
-
- 5,10,15,20-tetrakis(4-aminophenyl) porphyrin
-
- TC
-
- tetraphenylethylene
-
- TCNB
-
- 1,2,4,5-tetracyanobenzene
-
- TCNQ
-
- 7,7,8,8-tetracyanoquinodimethane
-
- TFP
-
- tetrafluorophenylene terephthalocyanide
-
- THF
-
- tetrahydrofuran
-
- TPA
-
- two-photon absorption
-
- TTF
-
- tetrathiafulvalene
1 INTRODUCTION
Organic materials exhibit advantages over inorganic materials in some areas, including their adjustable structures, solubility, scalability, cost-effective production, flexibility, and low weight. These characteristics are crucial for the design and creation of future generations of electronic devices.[1, 2] Organic single crystals, a specific category within the broader family of organic semiconductors, possess highly ordered structures, absence of grain boundaries, minimal defects, and excellent uniformity. This significantly reduces the negative effects of grain boundaries and impurities in organic thin films, making them ideal for high-performance devices.[3] Furthermore, the clear and definite crystal structures of organic single crystals enhance the understanding of the relationship between structure and performance, which is crucial for guiding the design and advancing the development of organic functional materials.
The understanding of organic single crystals lays the foundation for the field of organic cocrystal engineering, an extension of crystal engineering that focuses on studying intermolecular interactions within multicomponent-ordered crystalline materials. Organic cocrystal engineering, emerging significantly in 1973 with the discovery of the tetrathiafulvalene-7,7,8,8-tetracyanoquinodimethane (TTF-TCNQ) cocrystal, explores the potential to create functional materials with enhanced properties.[4] This discovery demonstrated that cocrystals could achieve higher electrical conductivity than any previously known organic compound at certain temperatures, propelling extensive research into cocrystals. This novel discovery highlighted how cocrystals can use the natural advantages of organic materials—like being lightweight, easy to process in solutions, and affordable. At the same time, they also benefit from the features of single crystals, such as orderly structures and improved performance. The special molecular stacking and aggregation structure of multi-component crystals can retain certain inherent properties of each component. Furthermore, these properties can be combined to exhibit novel properties and achieve multifunctional materials that are rare in single-component crystals, which offer a richer diversity, enabling more precise control over intermolecular forces and yielding a broader range of functionalities compared to single-component crystals, such as conductivity,[5] ferroelectricity,[6] bipolar charge transport,[7] light response,[8] luminescence,[9] stimulus-response properties,[10] etc.
However, the fascinating field of organic cocrystals faces significant challenges that slow its progression, including structural mismatches between constituent compounds, unclear cocrystallization mechanisms, and unpredictable changes in overall properties. Moreover, achieving effective cocrystallization between different molecules is difficult, as it relies heavily on the chosen preparation method, the structural compatibility between the co-assembly units, and the driving force behind the intermolecular assembly.[11] The complexities underscore the necessity of systematically exploring cocrystal engineering principles to guide the design and synthesis of molecular materials. Despite the abundance of documented research on organic semiconductor crystals, there is a lack of systematic reviews on building molecular materials from the perspective of cocrystal engineering. This gap in the literature highlights the need for a comprehensive analysis that can offer guidance and insights into the formation and properties of organic cocrystals. Thus, our review presents a comprehensive perspective specifically on organic cocrystals, an area that has not been thoroughly addressed in recent literature reviews.
As shown in Figure 1, in this review, we introduce the common donor-acceptor unit for co-assembly in the cocrystal and the mechanism of organic cocrystal formation, including charge transfer (CT), π-π, halogen bonding, hydrogen bonding interactions, and the aromatic-perfluoroaromatic (AP) interactions. Subsequently, we discuss the different stacking modes of organic cocrystals, which leads to a significant discrepancy in optoelectronic properties. Thereafter, we review current cocrystal growth techniques, including gas-phase, liquid-phase, and solid-phase methods. Finally, we explore the versatile applications of organic cocrystals and their transformative impact across various fields including optoelectronics, photothermal energy, biomaterials, stimuli-responsive materials, energy storage, and conversion. Furthermore, we provide a forward-looking analysis that outlines the challenges and future directions for scalable, high-performance applications in electronics and optoelectronics. This serves as a valuable guide for researchers entering the field of cocrystal engineering.
2 KEY COMPONENTS AND MECHANISM OF ORGANIC COCRYSTAL FORMATION
2.1 Key components of organic cocrystal
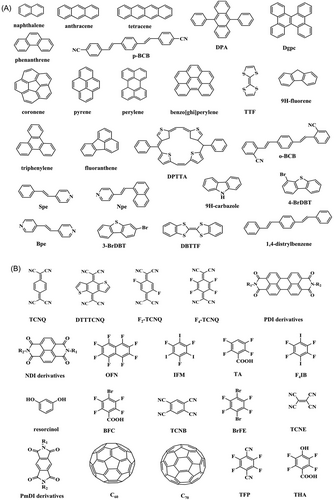
It is essential to consider the types of molecules that are commonly used as donors and acceptors in this context. Donor molecules can be categorized into four main groups:[12] n-type donors (neutral molecules with lone pairs of electrons), n−-type donors (anions with lone pairs of electrons), π-type donors (neutral molecules with π electrons), and σ donors (neutral molecules with σ electrons). n-type donors are neutral molecules (containing N, O, P, S, and halogen atoms) consisting of lone pairs of electrons, such as amines, alcohols, ethers, ketones, thiones, amides, anilines, nitrogen oxides, and phosphine oxides. n−-type donors are anions containing lone pairs of electrons, such as COO−, CN−, I−, and OH−. π-type donors are neutral molecules containing π electrons, such as aromatic compounds, unsaturated hydrocarbons, and their substitution products (substituents are electron-donating groups, e.g., alkyl, amino, and hydroxyl). σ donors are neutral molecules containing σ electrons, such as alkyl and aryl halides and esters.[13]
Electron acceptors are mainly divided into π-type electron acceptors, that is, aromatic compounds, and unsaturated hydrocarbon substitution products (substituents for the electron-withdrawing group, such as nitro and cyano). σ*-type electron acceptors are neutral molecules containing σ* empty orbitals, such as halogenated molecules (X–X), interhalogenated molecules (X–Y), and halogenated compounds (R–X).[14] The common donor and acceptor molecules used for growing organic cocrystals are summarized in Figure 2.
2.2 Mechanism of organic cocrystal formation
Organic cocrystals are fascinating structures formed by the interaction between molecules. Having gained an in-depth understanding of the types of molecules that act as donors and acceptors, we next explore how these molecules form cocrystals through specific interactions. It is crucial to understand the mechanism of cocrystal formation for successfully utilizing cocrystals. Figure 3 showcases different driving forces for organic cocrystal formation.
2.2.1 CT interaction
CT interaction is a key driving force that plays a crucial role in the formation of cocrystals. These interactions involve a specific amount of CT that occurs in a cocrystal between donor and acceptor molecules.
Mulliken's valence bond theory explains how molecules interact, especially in forming cocrystals.[15] According to Mulliken's theory, cocrystals are formed when the least unoccupied molecular orbital (LUMO) of a donor interacts with the highest occupied molecular orbital (HOMO) of an acceptor to create new molecular orbitals. The energy required for this process depends on various factors, including the ionization potential and electron affinity of molecules. When two molecules approach each other, their atomic orbitals merge to form molecular orbitals, which can be either bonding or antibonding depending on the phase alignment and energy relationship of the interacting atomic orbitals.
In cocrystals, the interaction between two unit molecules is important. Typically, the donor molecule possesses a relatively low-lying electron-rich molecular orbital, whereas the acceptor molecule has a comparatively high-lying electron-deficient molecular orbital. As these molecules come into proximity, their molecular orbitals interact, and new molecular orbitals are generated. Mulliken proposed that the wave function of a CT complex is a linear combination of nonbonded and bonded states, which involves the classical electrostatic attraction between the electron donor and acceptor and CT between them.
As we have discussed, n-type and n−-type donor molecules contain lone pair electrons, while π-type and σ-type donor molecules contain π and σ electrons. These electrons can be transferred to acceptor molecules with low electron affinity energies through intermolecular interactions. For example, π-type electron acceptors, such as aromatic compounds and unsaturated hydrocarbon derivatives, can interact with π-type or n-type donor molecules. On the other hand, σ*-type electron acceptors, such as halides and halogenated compounds, can engage with σ-type donor molecules.[16]
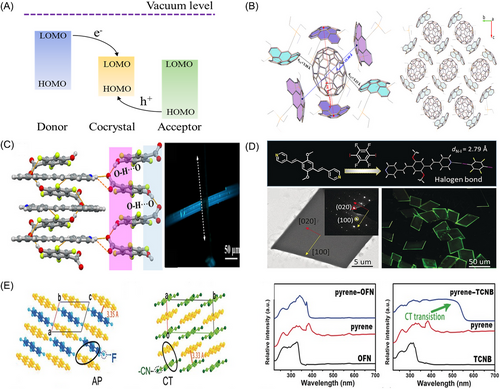
The degree of CT (DCT) depends on the electron affinity energy of the acceptor, the ionization energy of the donor, and the ionization energy of the donor-acceptor pair. A large DCT results in a cocrystal with strong ionic properties that adopts a disaggregated stacking pattern to stabilize its ionic state. Conversely, smaller DCTs (DCT < 0.5) typically yield neutral/quasineutral cocrystals.[17, 18] Modulating the DCT leads to an alteration in the cocrystal conductivity, consequently enabling the manifestation of diverse material properties, ranging from metallic, to insulating, semiconducting, and even extending to superconducting characteristics.[19] And in the CT cocrystal, the valence band will be close to the HOMO of the donor while the conduction band will relate more to the LUMO of the acceptor (Figure 3A).
CT in organic cocrystals can be qualitatively determined using electron spin resonance (ESR) spectroscopy,[22] polarized light microscopy (POM), and solid-state nuclear magnetic resonance (NMR).[23] The reactive radicals detected using ESR spectroscopy indicate CT properties in the ground state. ESR detects reactive radicals in cocrystals with a resonance signal close to g (2.0023), corresponding to free electrons. Solid-state NMR reveals changes in carbon atom environments, indicating CT shift states. The POM observations of the differences between the morphologies of cocrystal components help to examine cocrystal formation.[24]
2.2.2 π-π interactions
There are two types of π-π stacking:[29] the conventional interaction among aromatic rings and the structurally complementary curved π-π force, which exhibits a pronounced directionality. Characterized by van der Waals forces, these interactions promote dense packing of aromatic rings in aggregated forms. Notably, the intensity of π-π interactions greatly impacts the electronic and photoelectric conversion properties of organic substances. In the quest for novel semiconductor materials, minimizing the distance between π-stacked planes is vital, as closer packing tends to enhance electrical conductivity, as evidenced by studies.[20, 30] There are instances of π-π stacking between relatively electron-deficient and electron-rich molecules, which create the weak interactions that are typically observed between aromatic rings.[31]
The general design strategy for π–π interactions involves selecting molecules with strong electronic abilities, such as pyrene (Pyr)-TCNQ,[32] or donor-acceptor molecules with similar frameworks,[31] such as arene-perfluoro aromatic interactions.[28] These strategies contribute to optimizing the photoelectric properties of organic cocrystals. In a notable example, Gao et al. have made significant advancements in the design of novel fullerene bowl-shaped molecules. By carefully adjusting the size and arrangement of the functional groups on these molecules, they have optimized the intermolecular π-π interactions (Figure 3B). This strategic design approach has enabled the formation of cocrystal with C70 through strong concave-convex interactions. Importantly, these tailored interactions have not only facilitated the self-assembly of the fullerene molecules but also significantly improved their charge transport properties.[25]
2.2.3 Hydrogen bond interaction
The hydrogen bond (X-H···A) is one of the earliest studied interactions, and it is quite common in functional materials and biological systems. This bond plays a vital role in crystal engineering and has numerous applications because of its unique properties, such as stability and directionality, and its electrostatic nature.[33, 34] In organic cocrystals, element X typically includes C and O, and element Y commonly includes N, O, F, and S.[35] An obvious aspect of these cocrystals' luminescence properties is their tendency to form O-H···N hydrogen bonds, which is further modulated by parameters such as the number of proton donors and the substituent features (Figure 3C). Electronegativity plays a decisive role in defining the strength of hydrogen bonds, where a higher electronegativity of atoms X (proton donor) and A (proton acceptor) amplifies the hydrogen bond's attractive force. Consequently, manipulating the electronegativities of these atoms allows for tuning the electrostatic interaction's intensity, which directly governs the hydrogen bond's robustness.
In the locked-arm supramolecular ordering strategy, donor, and acceptor units with complementary rigid and flexible side chains are extended in 1D and 2D directions by forming complex hydrogen-bonding networks, which then form binary cocrystals under the synergistic effect of CT interactions.[36] Hydrogen bonding enhances ground-state CT interactions while maintaining the CT properties of the components and forming p/n-type two-channel organic semiconductor crystals.
The energy of the hydrogen bond is 0.25–40 kcal/mol.[37] The hydrogen bond strength depends not only on the proton-donating ability of C–H but also on the electron density of the p-donor. Thus, the following order is obtained: sp-CH > sp2-CH > sp3-CH; CHX3 > CH2X2 > CH3X (X, any electron-withdrawing atom or group).[38]
2.2.4 Halogen bond interaction
The halogen bond has definite directivity and strength, which is not only conducive for the reasonable and accurate prediction of crystal structures but also widely used in 1D, 2D, or 3D crystal construction.[30, 39] In addition, the migration of protons in a hydrogen-bonded cocrystal can induce ferroelectricity, which is uncommon in organic matter.[6, 40] This is because when the temperature drops below the Curie temperature, the space point group and hydrogen bond length change, proton migration occurs, and a stable dipole moment is generated, leading to ferroelectricity.[41] Examples of organic cocrystals in ferroelectric materials are described in detail in the subsequent sections.
The halogen bond is formed by the attraction of the electrophilic region of the R-X halogen atom to the nucleophilic region of Y-Z, in the form of R-Y…X-R, where R-X is the halogen bond donor and Y-Z is the halogen bond acceptor.[42, 43] The cocrystal containing halogen bonds is a σ-cocrystal, where lone pairs of electrons from electron-rich atoms (N, O, S, Se, I−, Br−, Cl−, and F−) transfer to the halogen atoms (I, Br, Cl, F) in the acceptor.[44]
Similar to the hydrogen bond, the halogen bond is strong, stable, and directional. However, it is hydrophobic and more sensitive to steric bulk or secondary interactions. The polarizability of the halogen atoms (I > Br > Cl > F) and the electron-withdrawing ability of the substituent are related to the strength of the halogen bond donor. The sp-hybridization modifies the strength of the halogen bond, and it generally follows this order: C(sp)-X > C(sp2)-X > C(sp3)-X.[45, 46] The high strength and directivity of halogen bonds can promote the construction of stable cocrystal supramolecular structures with various photoelectric properties.[47] For example, the organic molecules DPEpe and F4DIB, which constitute the DPEpe-F4DIB cocrystals, are united by halogen bonding (Figure 3D). This is evidenced by the normalized contact distance of RN⋅⋅⋅I = 0.79, which falls within the range indicative of a halogen bond. Furthermore, the bright green edges observed in the organic microcrystals signify a pronounced characteristic of a 2D optical waveguide.
2.2.5 AP interactions
In addition to serving as strong acceptors, these conjugated molecular donors, including anthracene and coronene, have the capability to form cocrystals with weak acceptors such as octafluoronaphthalene (OFN). This type of interaction is commonly referred to as the AP interaction.[48]
The AP interactions in the formation of organic cocrystals are a significant aspect, as discussed by Hu et al. These interactions, where perfluoroaromatics complex with aromatics or their derivatives in a 1:1 ratio, result in an almost parallel and alternating stacking pattern in the solid state.[28] Unlike CT interactions, AP interactions lack the characteristic absorption bands in the ultraviolet (UV) spectra, indicating a distinct mechanism that can be leveraged to generate novel optical and electronic properties through the pairing of AP components (Figure 3F).
Besides, Huang et al. demonstrated how AP interactions can be utilized to combat aggregation-caused quenching (ACQ), a common issue in CT interactions. By co-assembling the polycyclic aromatic hydrocarbon (PAH) chromophore with OFN, they were able to mitigate the ACQ effect.[49] The use of OFN as a molecular barrier effectively inhibits π-π stacking and other interactions among PAH chromophores, thereby minimizing luminescence quenching. This straightforward and cost-effective approach significantly enhances the photoluminescence quantum yields (PLQYs) in the solid state. In a related study, Ye et al. introduced a novel concept involving periodic molecular barrier arrays that can impede the transition from singlet to triplet states, ultimately achieving a nearly 100% PL quantum efficiency (PLQE) in π-conjugated organic cocrystals.[50] These results elucidate the underlying mechanism and open up an exciting new direction for the development of solid-state, highly efficient non-bluing organic luminescent materials, potentially overcoming the fundamental bottleneck in the advancement of organic optoelectronics and photonics.
Furthermore, Zhang et al. introduced the concept of solubility in AP cocrystal formation, highlighting its importance in the synthesis process.[51] The formation of the tetraphenylethylene (TC)-OFN cocrystal is achieved through a green grinding co-assembly technique. In this method, a solid mixture of TC and OFN is manually ground in the presence of a small amount of tetrahydrofuran (THF) as a catalyst. The low solubility of TC necessitates a substantial excess of OFN, which not only acts as a coformer but also functions as a solid solvent. The surplus OFN, along with the catalytic THF, facilitates the intercalation of OFN molecules into the TC phase. By acting as molecular barriers, OFN molecules prevent the close π-π stacking typical of pure TC crystals, disrupting their close-packing mode. The regular insertion of OFN molecules increases the intermolecular distance, effectively overcoming the ACQ effect observed in pure TC solids. This approach exemplifies how manipulating AP interactions can lead to the development of cocrystals with tailored properties.
In summary, investigating the link between intermolecular interactions, stacking structures, and functions is crucial for designing and developing organic cocrystals with enhanced properties. However, owing to the diversity of noncovalent intermolecular interactions and molecular structures, further in-depth investigations are required to develop more universal techniques.
3 STACKING MODE OF ORGANIC COCRYSTAL
The stacking modes of organic cocrystals are closely linked to their functional characteristics, which strongly influence their properties. The analysis of the structure-property connection and stacking structure are critical for the design and synthesis of functional cocrystals. The supramolecular stacking of organic cocrystals influences both their charge-transport performance and luminescence properties. The common stacking structures of organic cocrystals are illustrated in Figure 4.
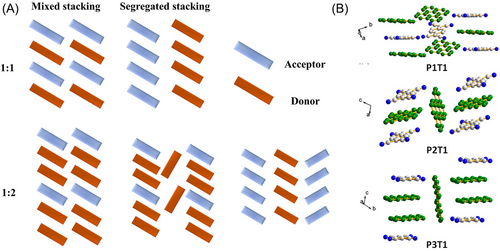
In most cocrystal systems, donor and acceptor molecules exhibit a stoichiometric ratio of 1:1, with mixed stacking and segregated stacking as the most common stacking modes. In a mixed-packing system, acceptor and donor molecules alternate along the optimal growth direction of the crystal, forming an A-D-A-D-A-D-A sequence. However, in this arrangement, adjacent donor or acceptor molecules do not directly interact with each other according to super-exchange coupling theory,[53] the nearest donor (or acceptor) molecule can utilize the adjacent acceptor (or donor) molecule as a bridge to form hybrid orbitals. This process is conducive to the generation of driving forces that facilitate the transport of holes or electrons.
In contrast, segregated stacking involves distinct stacking patterns for donor and acceptor molecules, and it typically exhibits A-A-A-A and D-D-D-D sequences in adjacent packing columns (Figure 4A).[54] This arrangement results in large intermolecular CT in the stacked cocrystals, thereby leading to high conductance at room temperature.[55] This difference in stacking modes highlights the versatility and complexity of cocrystal systems because it directly influences their electrical properties and potential applications in electronic devices.
There are two general stacking modes for 2:1 complexes: mixed stacking (-D-D-A- or -A-A-D-) and column stacking. A mixture of 1:2 complexes can be prepared by linking two or three donor units with two acceptors through a chemical modification strategy (Figure 4A). More compounds with different stoichiometric ratios can be prepared by adjusting the substituent groups or atoms to affect steric hindrance and adjust weak bond interactions In a system of Peand TCNQ,[52] the manipulation of the stoichiometric ratios of acceptors results in the formation of three distinct types of cocrystals: P1T1, P3T1, and P2T1 corresponding to ratios of 1:1, 3:1, and 2:1, respectively. P1T1 exhibits a typical stacking pattern with alternating Pe columns and acceptors. In contrast, P2T1 contains additional Pe inserted between two adjacent alternating columns. P3T1 exhibits a characteristic 3:1 stacking structure. These varying stoichiometric ratios lead to distinct chemical properties for each type. P1T1 functions as an n-type semiconductor, P2T1 displays bipolar charge transport characteristics, and P3T1 acts as a p-type semiconductor (Figure 4B). This variation in the semiconductor behavior may be attributed to the increase in the coupling between donors, which enhances the hole transport capacity. Furthermore, an increase in the distance between acceptors decreases the n-type charge transport performance of cocrystals.
Therefore, it is crucial to design suitable molecular structures and aggregates through cocrystal engineering. This can improve the mobility of charge carriers and luminous efficiency in organic systems. The effective combination is a key research direction for optimizing the performance of organic semiconductors.
4 ORGANIC COCRYSTAL GROWTH METHOD
There are three main methods for preparing organic cocrystals: liquid-phase, gas-phase, and solid-phase methods. As the growth mode significantly affects the properties of the composite and thus the performance of the composite device, selecting an appropriate crystal preparation method is a key factor in regulating the morphology and properties.
4.1 Gas phase method
In the 1990s, Kloc et al. pioneered the growth of organic crystals through physical vapor transport (PVT),[56] and since then, PVT has become one of the most common methods for preparing organic micro/nanocrystals. The gas-phase method is suitable for preparing cocrystals with poor solubility or large differences in the solubilities of component units.[57] Common gas-phase methods for preparing organic cocrystals are shown in Figure 5.
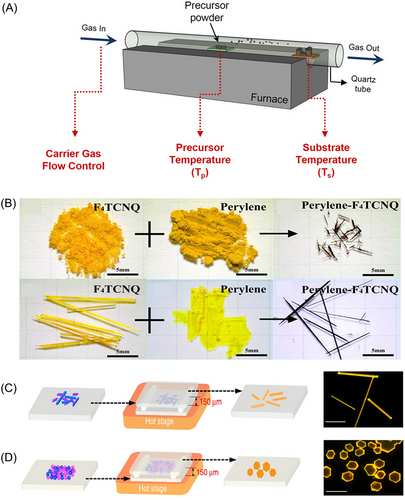
The equipment used in the PVT method consists of a vacuum pump, tubular furnace, quartz tube, temperature controllers, and gas path devices (Figure 5A). Under a flowing atmosphere of inert gas or vacuum, the original components in the high-temperature region sublimate and are subsequently transported to the low-temperature zone to form cocrystals.[58] It's important to carefully control factors like temperature, carrier gas, pressure, and raw material purity to regulate crystal growth.[56, 57, 59]
Hu et al. prepared cocrystals using different starting materials through a PVT method (Figure 5B).[20] Larger needle-like crystals were obtained from commercial, non-ground F4TCNQ. The largest crystals, over 1 cm long, were grown using large crystals obtained during the purification of F4TCNQ and Pe as starting materials.
Even though PVT provides high-quality crystals with controllable morphologies, it has drawbacks including low raw material utilization rates, the necessity for vacuum and carrier gases, and high cost. To address these limitations, Ye et al. developed a macro air sublimation method, which was an evolution of the PVT approach.[61] This method did not require a vacuum and was faster and more versatile compared to PVT. It was characterized by an ultrashort distance between a raw material and substrate. This allowed for crystal growth via a novel gas-melt crystal mechanism, which facilitated the preparation of high-quality single crystals. An example is red fluorene, which is an organic semiconductor with a mobility of 15–20 cm2 V−1 s−1. Additionally, they fabricated a fluoranthene-1,2,4,5-tetracyanobenzene (TCNB) cocrystal, where the simple mixing of donor molecules resulted in 1D needle-shaped crystals at 130°C, whereas a more thorough mixing and grinding with an acceptor led to 2D sheet crystals at 140°C (Figure 5C,D).[60]
4.2 Liquid-phase method
Compared with the gas-phase method, the liquid-phase approach provides advantages such as simplicity, cost-effectiveness, and enhanced controllability.[62] Common liquid-phase preparations of organic cocrystals are shown in Figure 6.
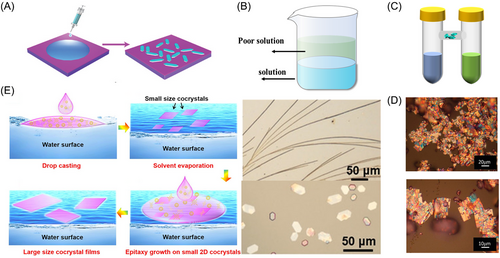
The parameters that influence the crystallization process are complex. Despite using identical molecular structures, different solvents, evaporation rates, temperatures, and concentrations can lead to significant variations in the packing configurations, morphologies, and characteristics of the resulting crystals. Several common solution-processing methods, including drop-casting, diffusion, and solution epitaxy, are explored.
Drop casting is a solution-processing technique used to grow crystals without external forces. It involves dropping a solution onto a substrate, allowing the material to assemble into crystals as the solvent evaporates (Figure 6A). This slow self-assembly process enables the formation of high-quality single crystals or crystal films prepared a mesodiphenyl tetrathionene [2,1,2,1] (DPTTA)-TCNQ organic cocrystal with bipolar charge transport performance using the drop-casting method.[63, 64] The hole mobility and electron mobility were 0.04 and 0.03 cm2 V−1 s−1, respectively. It should be noted that the solvents used in the volatilization process inevitably pollute the environment; therefore, the development of environmentally friendly solvents for organic cocrystal growth is a prospective work.[65]
In the reprecipitation method, two organic molecules are dissolved in a suitable solvent to create a concentrated mother liquor. A small quantity of this mother liquor is introduced into a large volume of a less suitable solvent to ensure a thorough mixing of the solvents (Figure 6B). Due to the predominance of the less suitable solvent, the solubility of the organic molecules decreases rapidly, leading to molecular aggregation, nucleation, and ultimately the precipitation of cocrystals. Li et al. used the reprecipitation method to create various cocrystal mixtures, including naphthalene, TCNB, and Pyr. Acetonitrile solutions (1:1 molar ratio) were injected into a water/ethanol mixture to create structured micro/nanotubes. They also developed a ternary cocrystal mixture by substituting Pyr with naphthalene.[69]
The diffusion method for cocrystal formation includes gas-phase and liquid-phase diffusion. In the gas-phase method, a mixture of donor and acceptor molecules is dissolved in a solvent and placed in a sample bottle inside a larger container filled with a low-boiling-point solvent. As the low-boiling-point solvent evaporates, its vapor diffuses into the sample bottle, leading to crystal growth and precipitation. Liquid-phase diffusion involves using H-shaped tubes to allow the diffusion and aggregation of molecules from different solutions containing either a donor or an acceptor.[70] Konarev et al.[71] prepared a DAN•C60(C6H6)3 cocrystal using the diffusion method. The crystals were obtained after 3 months of mutual diffusion. Although the cocrystals prepared using the diffusion method have a better crystal shape, the method requires considerable time compared with other methods and has certain requirements for donors, acceptors, and solvents. Therefore, this method has rarely been used to synthesize cocrystals (Figure 6C,D).
In the hot-saturated solution cooling method, a solution is heated to a saturated state and then gradually cooled. During this cooling process, the solubility of the solute in the solvent decreases, leading to the precipitation of crystals. This method involves separately dissolving two organic compounds in a hot solvent, and then combining the hot solutions after saturation is reached. After evaporation of one-third of the solvent from the mixed solution, it is gradually cooled to room temperature. Finally, the solvent is decanted, and the mixture of the two organic molecules is isolated through washing. Konarev et al. prepared fullerene C60 molecular complexes with various donor compounds using this method, including substituted TTFs, aromatic hydrocarbons, and metalloporphyrins.[72]
In the solvent vapor annealing (SVA) method, the self-assembled structures that have already formed on the substrate are heated and annealed over an extended period in a particular solvent atmosphere. The molecular regularity and order of the microstructures and nanostructures are considerably improved because of the partial dissolution and recrystallization of the organic molecules in the saturated solvent atmosphere, which leads to the formation of high-quality crystals. This approach can produce multiple cocrystals that are challenging to successfully manufacture using solvent volatilization, even for organic compounds with extremely weak crystallinity. Park et al. used the SVA method to successfully create low-dimensional cocrystals of two oligostyrene-supported derivatives, which were used in microscale and nanoscale field-effect transistors.[73]
In the solution epitaxy method, a solid-phase material is precipitated from a solution and deposited on a crystal substrate to produce a single-crystal film, which is mainly used to prepare the crystal films required for photoelectric devices. Wang et al. prepared porphyrin/fullerene cocrystal films using this method.[74] They injected deionized water into a weighing flask and used the liquid-phase interface of water as the growth substrate for the organic cocrystal mixture. The configured ZnTPP–C60 mixed solution was placed on the water surface. The solution immediately spread out on the water surface and then gradually contracted under surface tension (Figure 6E). The organic solvent gradually evaporated, and cocrystals began to grow on the water surface. The sizes of the cocrystals ranged from tens to hundreds of micrometers. After the continuous addition of the mixed solution, long and small crystals were dissolved, and the remaining crystals were used as seed crystals to induce the growth of the crystalline film.
4.3 Solid-phase method
The solid-phase method mainly comprises mechanical grinding and solvent-assisted grinding. Both techniques are generally more simple than solvent evaporation; however, they face challenges in producing microcrystals and nanocrystals with regular morphologies.[75]
Mechanical grinding involves the pulverization of two raw material components of a cocrystal using tools such as a mortar, ball mill, or vibratory mill.[76] This process typically leads to a change in color, indicating the formation of organic cocrystals. Cocrystallization via grinding involves three stages: reconstruction, transition, and crystal disintegration. This process can be understood in terms of three types of mass transfer: molecular diffusion through cocrystal mixtures, gas phases, and amorphous phases. The interplay between these mechanisms and kinetic factors drives cocrystal formation.[77]
In contrast, solvent-assisted grinding incorporates a solvent to facilitate intermolecular diffusion and enhance molecular interaction forces, thus proving to be more effective than dry grinding.[78] This method can be used to successfully prepare systems that are difficult to produce via mechanical grinding (Figure 7A,B). The solid-phase method is efficient, rapid, and straightforward, thereby making it a valuable technique for pharmaceutical and materials science applications.[56, 79]
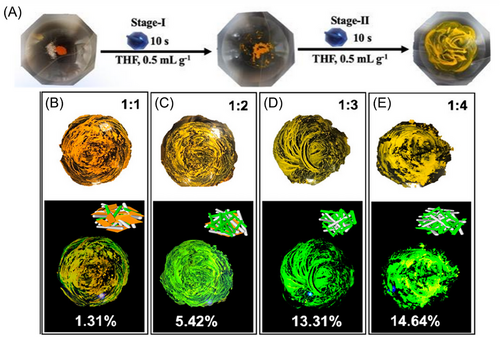
We have summarized the current methods for preparing organic cocrystals and evaluated the benefits and drawbacks of each method. Researchers can select the best approach for constructing crystal structures for various organic semiconductor systems to fabricate high-performance optoelectronic devices.
5 MULTIPLE APPLICATIONS
5.1 Electrical application
The development of electronics, which is integral to modern technology, has been a popular point of research for decades. This has led to the fabrication of electronic devices such as transistors, LEDs, solar cells, memory devices, and sensors.[80, 81] The wide range of devices has necessitated the development of advanced functional materials. Over the years, organic cocrystals have emerged as suitable candidates for electronic materials because of their low cost, ease of processing, and excellent electronic properties, which make them potentially useful in field-effect transistors.
5.1.1 Conductivity
Organic cocrystals show unique conductive properties when donor and acceptor molecules interact and the energy gap between the HOMO of the donor and LUMO of the acceptor decreases. This interaction facilitates electron excitation and transfer, resulting in partial CT and the formation of a mixed-valence band. This delocalization transforms organic cocrystals from insulators to semiconductors, conductors, and even superconductors. The application of organic cocrystal in electrical conductivity is shown in Figure 8.
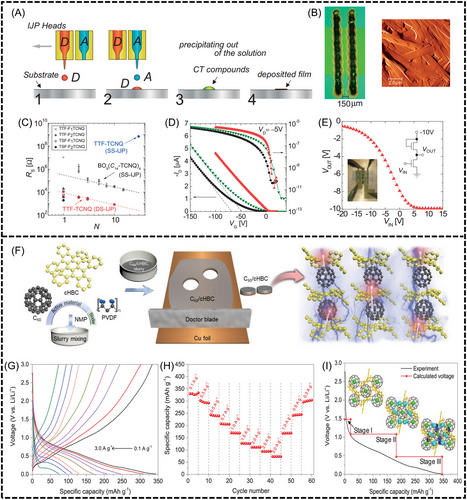
Rosenfeld et al. synthesized a TTF-TCNQ cocrystal using TTF as the donor and TCNQ as the acceptor in a 1:1 molar ratio.[5] Hiraoka et al. used inkjet printing to create a TTF-TCNQ thin film with an electrical conductivity of approximately 10 S/cm and used it in a pentaphene thin film transistor (Figure 8A–E).[82]
The exceptional conductivity of organic cocrystals opens new avenues for energy materials, particularly in the realm of batteries and photocatalysis. In the field of lithium battery cathodes, Zheng et al. demonstrated that cocrystals could significantly enhance the electrochemical performance of energy storage devices.[84] They prepared CN-1 and CN-3 cocrystals using a simple solution centrifugation method with naphthalene diamide (NDI) derivatives as the acceptors and coronene as the donor. The resultant cocrystal-based cathode showed a specific capacity of 230 mA h g−1 and maintained good cycling stability. Park et al. further demonstrated the potential of cocrystals in lithium-ion batteries, using a C60 and cHBC cocrystal as an anode material (Figure 8F–I). They achieved a reversible capacity of 330 mA h g−1 with 80% retention after 600 cycles.[83] These studies highlight the important role of organic cocrystals in energy storage solutions.
5.1.2 Charge transport
Cocrystal engineering provides a practical and simple strategy for systematically controlling the operation mode of transistors (ambipolar, p-type, or n-type) by modifying their components. The design and synthesis of materials have made significant advancements in achieving high-performance charge transport in organic materials for applications in basic electronic devices.[85, 86] The application of organic cocrystal in charge transport is shown in Figure 9.
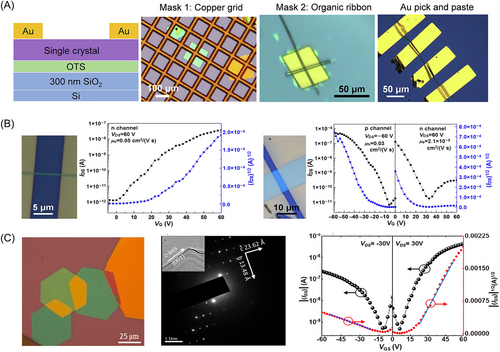
The method for constructing an organic cocrystal field effect transistor is shown in Figure 9A. The development of ambipolar charge-transporting cocrystals, as reported in 2004, has gathered significant attention, and it is a pivotal point in the exploration of cocrystal properties.[87] Researchers have made substantial progress in the development of cocrystals with ambipolar charge-transport characteristics. Donors such as TTF derivatives and acceptors such as TCNQ and TCNQ-like derivatives have been instrumental in achieving ambipolar charge-transport properties in cocrystals.
For instance, a team led by Zhu and Hu synthesized a cocrystal named DPTTA-DTTCNQ in 2014 with electron and hole mobilities of 0.24 and 0.77 cm2 V−1 s−1, respectively, and the device mobility did not decay significantly after 6 months of storage in the air atmosphere. Crystal structure analysis and quantum simulations showed that electronic coupling and super-exchange interactions simultaneously existed in this cocrystal and formed a quasi-2D electron and hole transport network, which was an effective model that revealed the internal mechanism for efficient carrier transport.[89] In 2016, the team developed DPTTA-F2TCNQ cocrystals with electron and hole mobilities of 1.57 and 0.47 cm2 V−1 s−1, respectively, which were the highest values observed in complex systems.[68] Zhu et al.’s study on perylene (Pe) and TCNQ co-crystals shows that cocrystal type and shape depend on the concentrations of donor and acceptor molecules.[88] Higher Pe levels lead to 3:1 cocrystals (P3T1), and lower levels result in 1:1 co-crystals (P1T1). P1T1 forms n-type wires with electron mobility of 0.05 cm2 V−1 s−1, and P3T1 forms ambipolar blocks with a high hole mobility of 0.03 cm2 V−1 s−1 and a lower electron mobility of 2.1 × 10−5 cm2 V−1 s−1 in the same conditions (Figure 9B).
Gao et al. successfully synthesized a cocrystal of C70:Buckybowl (Figure 9C), which exhibited ambipolar transport characteristics with electron and hole mobilities of up to 0.40 and 0.07 cm2 V–1 s–1, respectively. This study represents the first device application of a functionalized C70 fragment, showcasing the high potential of buckybowls for the development of novel semiconductors in organic devices. Additionally, it highlights their potential for future advancements in solution-phase fullerene chemistry.
Hu et al. introduced a novel method for creating high-performance bipolar organic cocrystals. They synthesized ZnPc-F16CuPc cocrystals by pairing molecules with closely aligned π-conjugated backbones. The resulting ZnPc-F16CuPc cocrystals demonstrated remarkable field-effect mobilities of 0.68 cm2 V−1 s−1 for electrons and 1.64 cm2 V−1 s−1 for holes.[90] This approach leveraged geometric and electronic factors to facilitate the formation of high-quality microcrystals and nanocrystals with high field-effect mobility for electrons and holes. These advancements in ambipolar charge-transport properties and the ability to control transistor operation modes demonstrate the versatility and potential applications of organic cocrystals in the field of organic circuits.
Overall, cocrystallization can be used to adjust the bandgaps of semiconductors to facilitate energy matching between the frontier orbitals of cocrystals and the work function of injected electrodes, which is beneficial for efficient charge injection to improve the performance of organic field-effect transistors.
5.1.3 Ferroelectricity property
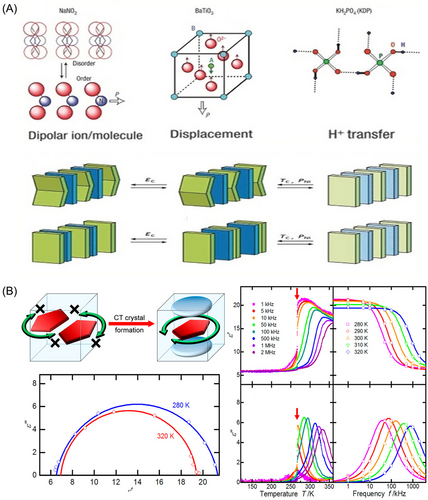
Inorganic ferroelectrics form through mechanisms like i) Order-disorder with polar molecules or ions creating spontaneous polarization at temperatures below the Curie point, and ii) Displacive, where ion displacement leads to permanent polarization due to structural instability. Organic ferroelectrics, despite their scarcity and limitations to polymers like polyvinylidene fluoride and compounds like thiourea, have seen new exploration through co-crystallization, enabling tailored properties via non-covalent interactions. Organic ferroelectrics often involve iii) Proton transfer in hydrogen-bonded systems like KDP, where polarization reversal occurs with proton movement. Supramolecular ferroelectrics, including charge-transfer complexes, form ferroelectricity through distorted stacking, leading to one-dimensional chains with alternating donor-acceptor pairs that can switch polarity under an external field (Figure 10A).[91]
Recent research has identified it as a method for obtaining organic materials with promising near-room-temperature ferroelectric properties. Cocrystals based on intermolecular hydrogen bonds have demonstrated ferroelectric behavior.[92, 93] Organic ferroelectric cocrystals usually contain CT processes. The CT strategy for cocrystal formation, exemplified by TTF and p-chloranil (QCl4) cocrystals, leads to π-stacked columns of alternating donor and acceptor molecules in one dimension. The arrangement of molecules in the crystal lattice can result in either a ferroelectric or an antiferroelectric state, depending on the 3D arrangement of polar chains. TTF-QCl4 exhibits both ordering style, and its dielectric properties can be controlled through chemical and physical modifications.[94]
Researchers in this field have also explored cocrystals with hydrogen bonding and CT forces. Tayi et al. studied three cocrystals prepared using homotetramethylene diimide as the acceptor and naphthalene, Pyr, and TTF as donors.[6] The cocrystals exhibited ferroelectric behavior at 5–400 K and polarization hysteresis was observed at room temperature, with residual polarization exceeding 1 µC/cm. The cocrystal with a ratio of 1:4 demonstrated large polarization at low temperatures, which was the highest value reported for organic cocrystal ferroelectrics. Researchers speculated that this was due to the CT process in the cocrystal and proton dynamics.
5.1.4 Dielectric properties
Researchers have investigated the dielectric properties of cocrystals and their responses to temperature changes. Inabe et al. examined the dielectric response, phase transition process, and molecular rotation of a cocrystal consisting of a range of thickened aromatic hydrocarbon donors and a TBPA acceptor molecule at different temperatures.[95] The single crystal with the TBPA acceptor molecule did not exhibit dielectric response properties, whereas the cocrystal consisting of the same components showed significant molecular reorientation and dielectric response phenomena (Figure 10B). Additionally, a phase transition in one of the crystals led to an abrupt change in the dielectric constant of the cocrystal at the phase transition temperature. This study suggested that cocrystals had the potential to become a class of molecular dielectrics, thereby expanding the range of dielectric-responsive materials.
In a word, organic cocrystals have garnered significant interest for their potential applications in electrical fields due to their unique molecular arrangements and tunable electronic structures. Their conductivity can be enhanced through molecular design, optimizing CT between molecules. Cocrystals with bipolar transport properties support the movement of both electrons and holes, crucial for high-performance electronic devices like transistors and diodes. Certain organic cocrystals exhibit ferroelectricity, enabling them to maintain polarization states even after the removal of an electric field, opening avenues for non-volatile memory devices and sensors. Additionally, their dielectric properties can be modulated by altering molecular polarity and interactions, making them promising for applications in capacitors and insulating materials. The exploration of organic cocrystals continues to pave the way for innovative electrical applications, with ongoing research promising advancements in material performance and device development.
5.2 Photoresponse
Organic materials used in photoelectric applications exhibit high sensitivity and selectivity for light absorption, efficient light generation, and photoconductivity across a wide range of wavelengths. Organic cocrystals, not only serve as excellent semiconductors but also respond effectively to light. Figures 11 and 12 depict the application of organic cocrystal in photoresponse.
5.2.1 Photoelectric detection
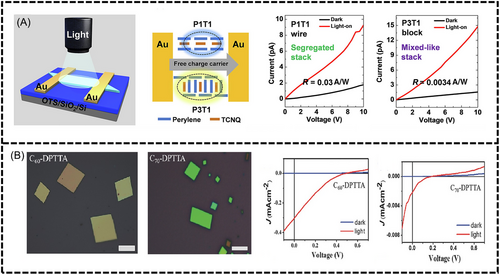
Current research on organic cocrystal-based detectors has focused on UV and visible detectors. In these cocrystal complexes, intermolecular interactions create a new CT state between a donor and acceptor, leading to a red-shift in absorption. The strength of the CT interaction determines the absorption band shift, making it possible to achieve optical responses in the infrared or near-infrared (NIR) regions. Hu et al.[96] prepared a 9,10-diphenylethynylanthracene-homophthalic diimide cocrystal using the solution self-assembly method and used it to fabricate photoresponsive transistor devices. These devices exhibited a photoresponsivity (Rmax) of 1.67 × 103 A/W, and detection degree (D*max) of 2.06 × 1013 Jones, which demonstrated the high potential of organic cocrystals for photodetector applications Zhu et al. prepared P1T1 and P3T1 cocrystals by adjusting the concentration of Pe and TCNQ in acetonitrile solution in a controlled manner.[88] Further morphological and structural characterization showed that the P1T1 complexes self-assembled into linear cocrystals by segregated stacking, while the P3T1 complexes aggregated into massive crystals by mixed stacking (Figure 11A). The results indicate that the molecular stacking structure of the CT cocrystal largely affects the charge transport behavior and photoresponsivity.
5.2.2 Organic photovoltaic
Energy scarcity is a significant limitation for rapid economic growth in the modern era. Organic photovoltaic (OPV) devices are novel energy-collection devices that have advanced rapidly. However, there is ongoing research on enhancing the photoelectrical conversion efficiency of OPV devices. Organic cocrystals show promise in this regard because of their ability to enhance exciton diffusion, dissociation, and transport.
Kochi et al. demonstrated that the CT excitons generated in TCNB-based cocrystals could act as highly localized excited ion-pair states, potentially leading to efficient photovoltaic devices. In certain cocrystals, the yield of carrier dissociation is surprisingly close to 50%, implying that organic cocrystals may function as efficient photovoltaic devices.[98] Zhang et al. prepare C60/C70-DPTTA cocrystal. The C60-DPTTA co-crystal solar cell had an open-circuit voltage (VOC) of 0.48 V, short-circuit current density (JSC) of 0.3 mA/cm2, and fill factor (FF) of 0.183, leading to a power conversion efficiency (PCE) of 0.27%. Conversely, the C70-DPTTA co-crystal solar cell showed a VOC of 0.17 V, JSC of 0.002 mA/cm2, and FF of 0.157, resulting in a significantly lower PCE of 0.0005% (Figure 11B). The power conversion efficiency of C60-DPTTA is 540 times greater than C70-DPTTA, primarily attributed to a substantial disparity in charge recombination efficiency.[97]
5.2.3 Photocatalysis
A donor-acceptor system facilitates the spontaneous transfer of charges from a donor to an acceptor, thereby enhancing exciton separation in organic photocatalysts. Excitons undergo a gradual electron transfer process, resulting in the formation of a charge-separated state. This state prevents their recombination and complexation with the ground state, leading to the generation of more photogenerated carriers that actively participate in the photocatalytic reaction. Consequently, the creation of cocrystals emerges as a logical approach to efficiently generate and separate photogenerated carriers.
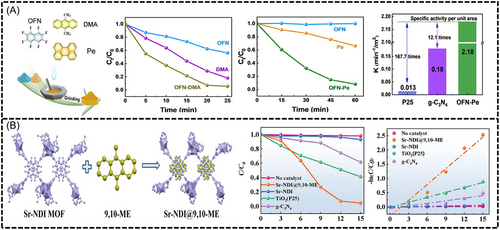
Wang et al. prepared cocrystals of OFN-9,10-dimethylanthracene (DMA) and OFN-Pe in large quantities using liquid-assisted milling.[99] These molecular cocrystals showed AP interactions between donor and acceptor molecules (Figure 12A).[99] This cocrystal can degrade over 95% of Rhodamine B (RhB) within just 20 min under broad-spectrum illumination (300–780 nm), significantly compassing its individual components, which only achieve degradation efficiencies of 44% and 80%, respectively under the same conditions. The reaction rate constants (k) for OFN, DMA, and the OFN-DMA composite are 0.023, 0.068, and 0.12 min⁻¹, respectively. Moreover, the specific activity per unit area (K) of the OFN-DMA co-crystals is 167.7 times greater than that of TiO2 and 12.1 times greater than g-C3N4, underscoring the effectiveness of molecular co-crystallization engineering in enhancing photocatalytic performance.
Liu et al. developed a novel metal-organic framework (MOF) self-assembled cocrystalline material by embedding an electron-rich guest molecule (DMA into electron-deficient strontium–NDI (Sr-NDI).[100] The unique porous structure of this material was conducive to increasing the interfacial area for photocatalytic reactions. Each Sr2+ ion is coordinated by six oxygen atoms of the ligand and two oxygen atoms of N,N-dimethylformamide. Two guest molecules are integrated into adjacent ligands via π–π stacking, resulting in an orderly ··· A ··· D ··· D ··· A ··· structure with molecule spacing of approximately 3.45 Å (Figure 12B). In photocatalytic tests, the MOF cocrystal degraded over 95.31% of RhB in just 15 min, while the original MOF managed only 6.93% degradation under similar conditions.
5.3 Photothermal properties
Organic photothermal materials have gathered significant attention owing to their potential applications in solar-powered desalination and thermoelectricity. Cocrystals represent a new generation of highly promising high-performance photothermal materials, which are characterized by excellent CT properties that effectively narrow the HOMO-LUMO band gap. Figure 13 illustrates the diverse applications of cocrystals in thermal properties.
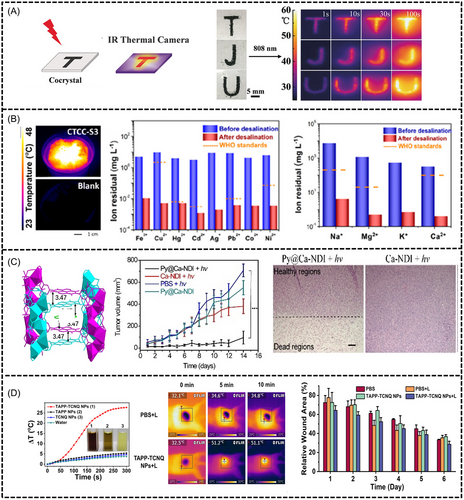
5.3.1 Photothermal imaging
Wang et al. developed a cocrystal from dibenzotetrathiafluoromanganese (DBTTF) and TCNB to investigate its use in photothermal imaging.[23] This photothermal material, when irradiated with NIR light, exhibits a substantial temperature increase, monitored using an infrared thermographic camera (Figure 13A). They prepared a cocrystal pattern “TJU”, which progressively brightened under prolonged exposure to NIR light (808 nm, 0.7 W/cm2).
Zhuo et al. introduced a remarkable large-area photothermal nanofiber membrane designed for photothermal imaging through electrospinning.[101] The nanofibers utilized the CF–PU CT cocrystal, which was derived from coronene and F4TCNQ, and exhibited a notable increase in temperature from 26 to 52°C in only 250 s under 808 nm laser irradiation. In addition, they showed a high NIR PTC efficiency of 53.7%. These findings highlighted the significant potential of CF-PU nanofibers in NIR photothermal imaging.
5.3.2 Solar water evaporation device
Wang et al. strategically reduced the intermolecular interactions and enhanced the internal motion of aggregated state molecules, which was crucial for increasing the photothermal conversion efficiency. They constructed an integrated device for water-to-electricity coproduction by utilizing a dendritic triphenylamine derivative as the donor and heterocyclic QCN as the electron acceptor. This resulted in a remarkable water evaporation rate of 0.94 kg m−2 h−1 under simulated sunlight irradiation, which demonstrated the potential of organic photothermal materials in desalination, waste heat, and power generation applications.[10]
In a related study, Tang et al. synthesized BTPPA derivatives (BTPAA, BTPEA, and BTPPA) with a D-A structure, where the diazole fragment BTT and triphenylamine derivatives acted as powerful electron acceptor and donor units, respectively.[102] They achieved a high photothermal conversion efficiency of 60% under laser irradiation at 660 nm and 0.3 W/cm2, which demonstrated the potential of customized molecular structures for optimizing photothermal properties.
Tian et al. introduced TTF-TCNQ cocrystals (CTCC-S3) for solar desalination and water purification.[103] In their study, CTCC-S3 cocrystals were floated on water. After 30 min of solar simulator irradiation at 1 Sun intensity, CTCC-S3 reached 47.4°C, inducing rapid steam generation (Figure 13B). The device showed a solar-water evaporation conversion efficiency of 90% under irradiation of the Sun, which corresponded to a water evaporation rate of 1.67 kg m−2 h−1. MB-polluted water turned colorless, confirming dye removal. Solar evaporation effectively purified E. coli contamination and removed heavy metal ions (Fe3+, Cu2+, Hg2+, Cd2+, Ag+, Pb2+, Co2+, Ni2+, Zn2+) with ∼99.8% efficiency. The system also achieved ∼99.9% removal of ions (Na+, Mg2+, K+, Ca2+) in seawater, meeting the World Health Organization standards.
5.3.3 Biological applications
Duan et al. constructed MOF cocrystals with electron-deficient NDI as the acceptor and Pyr as the donor. The duplex intercalation structure and high-density group distribution of the MOFs contributed to enhanced and stabilized CT, resulting in a remarkable photothermal conversion efficiency of 41.8%. They also reported the development of a host-guest MOF (Pyr@Ca-NDI) cocrystal for in vivo photothermal cancer treatment. This electron-deficient MOF was composed of NDI ligands and Ca2+ metal nodes, and it exhibited significant efficacy in restraining tumor growth, as confirmed by a reduction in tumor volume and histological analysis. This highlighted the potential of MOF cocrystals for biomedical applications.
Zeng et al. synthesized the host Ca-NDI naphthalene diimide MOF using a modified solvothermal reaction, employing N,N'-bis(5-isophthalic acid) naphthalene diimide as the ligand and Ca2+ as the metal ion nodes.[104] The Pyr molecule within this framework is self-assembled in parallel to the nearest NDI unit at a distance of 3.47 Å. Post-treatment histological analysis using hematoxylin and eosin of tumor tissues demonstrated a distinct margin between normal and severely necrotic areas in the Py@Ca-NDI + hv group, which reveals the potential application of light-induced cancer photothermal therapy of MOF cocrystal (Figure 13C).
Wu et al. synthesized 5,10,15,20-tetrakis(4-aminophenyl) porphyrin (TAPP)-TCNQ nanoparticle (NP) cocrystals and found that under 808 nm laser irradiation, the temperature of the TAPP-TCNQ NPs could reach 28°C after 5 min, while the temperature of pristine TAPP NPs and TCNQ NPs changed only slightly.[105] The antibacterial ability of TAPP-TCNQ NPs was investigated in vivo using a wound infection model in female Kunming mice. The temperature of the infection areas of the mice in the TAPP-TCNQ NPs + L group could reach 51°C in 5 min and stay above 50°C within 10 min, while the temperature changes of those in the control group were negligible. The mice in the TAPP-TCNQ NPs + L group exhibited a better wound-healing effect compared to the other groups (Figure 13D).
5.3.4 Other photothermal applications
Li et al. developed a TMPD-TCBQ cocrystal through self-assembly and revealed a unique application for laser ignition under NIR laser irradiation.[107] This cocrystal exhibited high-temperature performance and remote triggering capabilities at distances of up to 4 m. The incorporation of TMPD-TCBQ in a transparent polypropylene tube coupled with 808 nm laser irradiation showed excellent penetration, indicating potential exothermic reactions even when encapsulated. Additionally, the mixture of KNO3 and TMPD–TCBQ showed rapid deflagration under 808 nm laser irradiation, thereby highlighting the efficacy of the cocrystal as a low-power NIR laser-responsive initiator. This innovative strategy not only allowed for the control of absorption through self-assembly but also provided photo-thermal conversion materials for diverse applications to meet evolving demands in laser ignition.
Huang et al. introduced the concept of photo thermoelectric conversion using organic cocrystals.[103] The assembly of DBTTF, TTF, TCNB, and TCNQ resulted in the formation of organic cocrystals (DTC, TBC, and TQC), which exhibited effective power generation when combined with thermoelectric modules. Under the illumination of the sun, this combination demonstrated an outstanding open circuit voltage (0.427 V) and output power density (2.21 W/m2), which were higher than those of typical photothermal conversion materials. The integration of organic cocrystals with high-output-power-density thermoelectric devices has the potential to emerge as a viable solar-based photo thermoelectric conversion technology, which is particularly suitable for portable power production in remote rural locations.
In summary, with notable NIR absorption and low toxicity, cocrystals have the potential to be used in biological therapies by seamlessly integrating photothermal imaging with treatments for enhanced precision. Organic cocrystals with broad solar spectrum absorption combined with superior interface materials can be used for efficient seawater desalination. Their versatility extends to photothermal-to-thermoelectric conversion, thereby providing a novel perspective on eco-friendly electricity generation. Cocrystals present multifaceted solutions and show adaptability and potential for use in various fields.
5.4 Optical properties
Organic cocrystals have demonstrated significant potential in various optical applications, such as optical waveguide phenomena, photoemission, room temperature phosphorescence, two-photon absorption properties, and thermally activated delayed fluorescence (TADF). These applications open new avenues for integrated photonics, optoelectronic devices, and fluorescence modulation, showcasing the versatility and promising future of organic cocrystals in the field of optical materials.[108] Figures 14 and 15 showcase the diverse applications of cocrystals in optical properties.
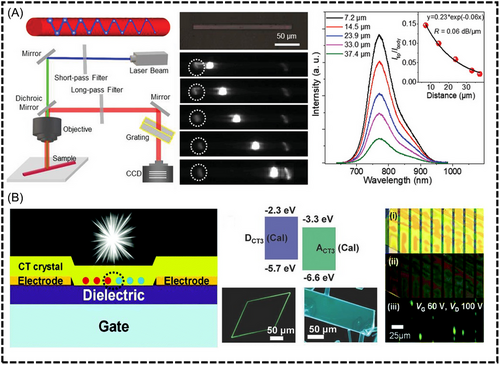
5.4.1 Optical waveguide
The optical waveguide phenomenon refers to the guided propagation of light within a dielectric structure, and it is a pivotal aspect of integrated photonics. This structure typically comprises a core with a high refractive index surrounded by a cladding region with a low refractive index, which confines light through total internal reflection.[109] Unlike inorganic materials, organic materials provide diversity, cost-effectiveness, and scalability for large-scale preparation. Micro/nanoscale binary crystals, which serve as 1D and 2D optical waveguides and logic devices, expand the range of materials, reduce the energy consumption in integrated photonics, and accelerate advancements in optoelectronics.[110]
Zhuo et al. showed that a cocrystal (DPEpe–F4DIB) based on halide bond interactions exhibited asymmetric optical waveguide performance.[27] Unidirectional internal reflection, which was attributed to asymmetric crystal stacking, resulted in optical loss coefficients of 0.0346 and 0.0894 dB/mm along the (001) crystal plane, showcasing the potential of organic cocrystals in microoptical logic gates. In another research work, he successfully synthesized a NIR emissive CT cocrystal TP-F4TCNQ (Figure 14A), which exhibited a maximum PL peak at 770 nm, a PLQY of 5.4%, and a radiation constant (kf) of 0.0048 ns−1. The TP-F4TCNQ single-crystalline microwires prepared in this study demonstrated active optical waveguiding properties in the NIR region,[27] with a low optical-loss coefficient of 0.060 dB/µm.
Zhao et al. explored the effect of concentration on cocrystal properties.[111] Two types of cocrystals with different molecular orientations and nanophotonic transmission characteristics were synthesized. M-DFC cocrystals displayed anisotropic photon propagation behavior along the first, second, and third directions of the crystal at 140/520/540 dB/cm, whereas the T-DFC cocrystal exhibited isotropic reabsorption waveguide loss along the A, B, and C directions of the crystal at 1040/1150/1080 dB/cm, highlighting the tunability of optical waveguide properties in organic cocrystals.
Moreover, Zhu et al. fabricated “cocrystal waveguide couplers” using Npe-TCNB and Bpe-IFB cocrystals.[112] These structures, which were designed for energy transfer, facilitated the construction of photonic “AND” and “OR” gates and had the capacity for implementing complex optical functions. This provided a basis for advanced photonic materials and device technologies. This collective research highlights the potential of organic cocrystals in shaping the future of integrated photonics and optical device applications.
5.4.2 Photoemission
The recent advancement of organic solid fluorescent materials has expanded their use in sensors, multicolor displays, and anticounterfeiting materials. Adjusting the fluorescence characteristics of these materials for practical applications is an active area of research. Molecular aggregation in dispersed fluorescent materials can weaken or eliminate their fluorescence, posing challenges for practical use. Understanding the emission properties depends on both the chemical structure of the molecules and their accumulation in the solid state.[115]
Cocrystal engineering has emerged as a valuable approach for enhancing the fluorescence properties of materials. Zhu et al. prepared 1,2-di(4-pyridyl)ethylene and TCNB molecules that self-assembled into parallelogram-like cocrystals (BTCs).[116] Although 2D crystal morphology is ideal for anisotropy studies, researchers have observed that the photon propagation properties in a single BTC are independent of molecular stacking and orientation, indicating the versatility of cocrystal structures.
Further advancements in modulating optical emission were explored by Wu et al., who synthesized (TCNB)x(OFN)1-x(coronene)-doped cocrystals.[117] They utilized CT-induced self-assembly and observed a shift in the emission color from blue to orange as the TCNB doping concentration increased. This demonstrated the potential for optical emission modulation in organic cocrystals. In addition, Zhang et al. successfully modified the intrinsic cocrystals BNT-TCNB by employing a donor/acceptor bipolar doping strategy to create ternary cocrystals doped with BCZ and TCNQ with tunable luminescent colors, lifetimes, and quantum yields.[118] These cocrystals exhibit luminescence tunability from yellow-green to red and high photothermal conversion efficiencies.
Sang et al. demonstrated the first CT-cocrystal-based organic light-emitting transistor device, which achieved a high external quantum efficiency (EQE) of 1.5% in the pristine device structure (Figure 14B).[114] The head-to-tail dipolar alignment of the complementary DCT3–ACT3 pair promoted the formation of a highly uniform and flexible 2D-type supramolecular structure. This distinctive assembly significantly influenced the photophysical properties, resulting in ambipolar characteristics within the 2:1 D:A stoichiometry. The CT3 system exhibited bright luminescence (ΦF = 60%) and ambipolar transport (µh and µe of = 10−4 cm2 V−1 s−1).
Zhang et al. precisely controlled the multicolor PL behavior of microcrystals through CT interactions.[119] The interaction between the electron donor (CzC6Cz) and acceptor (FA) resulted in significant redshifts of 56, 104, and 221 nm in fluorescence emissions. This demonstrated the potential for manipulating the PL behavior of microcrystals through CT interactions involving two components.
Yu et al. pointed out that the emission behavior of cocrystals can be adjusted by applying mechanical force, as this leads to changes in intermolecular interactions and stacking patterns. Due to the phase transition caused by the grinding process, the cocrystals composed of donor and acceptor exhibit a blue-shifted emission under anisotropic grinding. In contrast, under isotropic pressure, the cocrystals exhibit a red-shifted emission due to tighter molecular stacking.[115]
These collective advancements represent the dynamic landscape of research on organic solid fluorescent materials and provide insights into their diverse applications and the potential for further innovations in fluorescence modulation and control.
5.4.3 Room temperature phosphorescence
Organic room-temperature phosphorescent materials have long exciton lifetimes and high utilization, making them valuable for screen display, lighting, information encryption, anticounterfeiting, bioimaging, and photodynamic therapy. Researchers have achieved room-temperature phosphorescence through molecular design and materials engineering, focusing on improving efficiency, and lifetime, and exploring photophysical behavior. Strategies such as introducing heavy atoms, forming host-guest materials, constructing hydrogen aggregates, forming hydrogen bonds, and designing donor-acceptor structures have led to highly efficient organic room-temperature phosphorescent materials with long lifetimes.
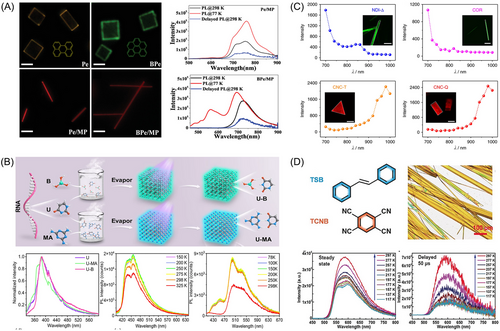
Gao et al. designed and synthesized a Pyr-MP organic cocrystal through solution self-assembly (Figure 15A). This crystal exhibited NIR phosphorescence with a peak at 730 nm and a PLQY of 34.2%.[120] The singlet states of MP molecules with an intermediate energy level effectively bridged the singlet and triplet states of Pyr molecules, leading to efficient NIR phosphorescence. It should be noted that the loose packing pattern of MP allows for versatile assembly with various aromatic molecules, ranging from ternary to six-membered benzene rings and even larger benzene skeletons. Unlike conventional cocrystal systems, cocrystal engineering based on MP molecules with weak π-π interactions eliminates the limitations related to molecular selection, uncertain assembly behavior, and unpredictable properties. This study provides valuable insights into the development of novel NIR-phosphorescent materials using a universal strategy.
Abe et al. explored the potential of halogen-bonding-based cocrystals by examining the connection between crystal stacking arrangements and organic room-temperature phosphorescence (ORTP) characteristics in binary cocrystals.[124] These cocrystals were composed of 1,4-diiodotetrafluorobenzene (DITFB) and various PAHs such as phenanthrene (Phen), chrylene, and Pyr. To explore the impact of the crystal structure on PLQYs, a Phen-DITFB binary cocrystal was created by partially introducing Pyr into the ternary cocrystal. The stacking motifs in the ternary cocrystal inherited σ-hole–π interactions from the Phen-DITFB cocrystal, with Phen sites randomly substituted with Pyr molecules. In this scenario, the ORTP emission originated from Pyr, with the maximum PLQY exceeding 20%, which was attributed to the suppression of nonradiative decay by the altered crystal stacking pattern.
David et al. employed a co-assembly strategy based on strong hydrogen bonding interactions to successfully prepare nucleic acid base-based photofunctional cocrystal materials with excellent phosphorescent properties.[121] These materials exhibit stable triplet and high-temperature phosphorescent properties, and can effectively generate long-lived triplet and single-linear states of oxygen. This discovery not only provides a new strategy for the preparation of high-performance phosphorescent materials but also opens up new avenues for information security and antimicrobial applications (Figure 14B).
5.4.4 Two-photon absorption properties
Two-photon-excited fluorescence and single-photon-excited fluorescence are different in terms of the simultaneous interaction of two photons in the former, which presents a fluorescence spectrum intensity with a quadratic relationship with the excited optical density. In contrast, single-photon-excited fluorescence exhibits a linear relationship with the excited optical density. The applications of two-photon-excited fluorescence are widespread and encompass fields such as two-photon microscopy, microfabrication, 3D data storage, optical power limiting, up-conversion laser technology, and photodynamic therapy.
Wang et al. synthesized two cocrystals using a naphthalenediimide-based triangular macrocycle and coronene.[122] These co-crystals exhibited distinct optical properties. The triangle-shaped co-crystal emitted deep-red fluorescence, while the quadrangle-shaped co-crystal displayed deep-red and NIR emission at 668 nm, which was a significant red shift compared to the precursors (Figure 14C). This red shift was attributed to intermolecular CT interactions within the co-crystals. Furthermore, both co-crystals demonstrated higher calculated two-photon absorption cross-sections compared to their individual constituents.
Sun et al. explored the synthesis of styrylpyridine-TCNB cocrystals (STC) using 4-styrylpyridine as the donor and TCNB as the acceptor, resulting in light-yellow cocrystals.[125] These cocrystals demonstrated a distinctive two-photon absorption (TPA) effect attributed to donor-acceptor stacking interactions, even though each material was TPA-free. This pioneering achievement marked the first realization of TPA through the cocrystallization of two TPA-free components. The ordered arrangement of the cocrystals enhanced the CT interactions, which were validated through spectroscopic analysis. The electronic communication facilitated by π conjugation in the supramolecular pair elucidated the TPA properties of the STC cocrystals arising from CT interactions. This innovative cocrystallization strategy provides a basis for modifying fluorescent properties through one-photon and two-photon processes, potentially opening avenues for groundbreaking applications in next-generation optoelectronic devices.
5.4.5 Thermally activated delayed fluorescence
Sun et al. investigated a 1:2 cocrystal composed of TCNB and trans-1,2-diphenylethylene and examined TADF.[123] They employed analytical techniques, including absorption spectroscopy, variable-temperature steady-state and time-resolved PL spectroscopy, single-crystal X-ray diffraction, and first-principles computations, to clarify the intricacies of this cocrystal system (Figure 14D). Their findings revealed that within the cocrystals, intermolecular interactions played a pivotal role in diminishing the singlet-triplet energy gap, thereby facilitating reverse intersystem crossing for TADF. These results can not only improve the understanding of TADF mechanisms but also provide a basis for innovative designs and advancements in TADF materials.
5.5 Stimulus-responsive material
The transformative nature of organic cocrystals under various stimuli, such as temperature, pressure, and solvents, induces changes in molecular interactions and stacking structures and affects their bulk properties. These stimulus-responsive cocrystals have considerable potential for applications in sensors, memory chips, anticounterfeiting, and data storage. Figure 15 highlights the various stimuli-responsive behaviors of different cocrystals.
Wang et al.[126] explored blue luminescent 9-acetylanthracene (9ACA)-tetrafluorophenylene terephthalocyanide (TFP) cocrystals formed through the self-assembly of 9ACA and TFP. These cocrystals exhibited a continuous redshift in the luminescent bands as pressure increased, and the color and luminescent bands returned to their initial states when pressure decreased.
Yan et al.[26] investigated the molecular cocrystals formed by 4-(1-naphthylvinyl)pyridine (NVP) with different coassembled units that exhibited distinct photostimulant-responsive behaviors (Figure 16A). Under UV irradiation, the cocrystal formed using 4-(1-naphthylvinyl)pyridine and 2,3,5,6-tetrafluoro-benzoic acid (TA) showed rapid macroscopic jumping and movement, whereas the cocrystal formed using 4-(1-naphthylvinyl)pyridine and 2,3,5,6-tetrafluoro-4-hydroxybenzoic acid remained intact after illumination and exhibited unique emission properties under laser irradiation.
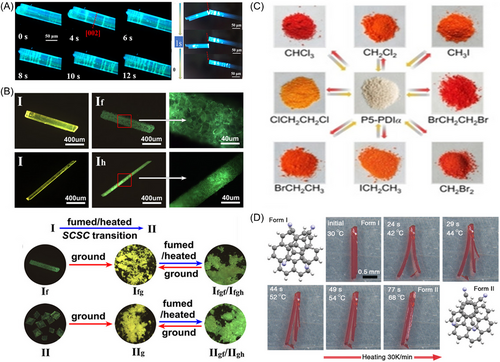
Liu et al. discovered a multi-stimuli-triggered phase transition between two luminescent cocrystal polymorphs.[127] By exposing the yellow-emitting polymorph I to THF vapor-fuming or heating, it transformed into the green-emitting polymorph II. The researchers also found that grinding the polymorph II restored the initial yellow emission (Figure 16B).
Li et al.[128] utilized macrocyclic molecules (P5) as donors and N,N'-bis(n-butyl)homophthalic acid diimide (PDI) as an acceptor to creating P5-PDI MCCs. The removal of solvent molecules led to a vapor-induced color change and crystalline phase structural transition, resulting in selective chromogenic properties toward volatile organic compounds (Figure 16C).
Liu et al. observed that coronene-TCNB cocrystals exhibited remarkable self-healing behavior and underwent macroscopic shape changes when heated at 30°C/min (Figure 16D).[129] The crystal initially split by bursting at one end at around 40°C, but gradually self-healed between 50°C and 70°C. As a result, the macroscopic integrity of the crystal was almost entirely restored. This recovery persisted even after the crystal had cooled to room temperature.
Wang et al.[130] cocrystallized FSBO and TCB and demonstrated their unique applications in UV-responsive paintings. No significant changes in the paintings were observed under daylight conditions. However, under UV light, clear outlines of green mice and yellow cheese were observed. After trifluoroacetic acid was sprayed on the painting, the green mouse turned blue and the yellow cheese disappeared immediately. The application of trifluoroacetic acid resulted in an acid-base stimulation response, highlighting the potential of organic cocrystals in information anticounterfeiting applications.
These studies collectively emphasize the diverse stimuli-responsive behaviors of organic cocrystals and their potential applications in a wide range of fields.
5.5.1 Other application
The presence of unpaired and ordered electron spins within p-orbitals can lead to strong interactions, potentially aligning the spins and resulting in ferromagnetic properties, particularly in materials that contain heteroatoms. However, if the spin alignment is not possible, these materials may default to antiferromagnetic ordering or exhibit paramagnetic behavior.[131]
The recent strides in this area have significantly motivated researchers to explore the magnetic properties of organic cocrystals. Cocrystal engineering presents a unique opportunity to fine-tune the multifunctional properties of these materials. For instance, Yang et al. have demonstrated that Pyr-F4TCNQ cocrystals, under the influence of a horizontal electric field, generate a notably higher magnetoelectric coupling coefficient compared to that induced by a perpendicular electric field.[132] This disparity is attributed to charge-transfer interactions and the anisotropic arrangement of molecules along the perpendicular axis, leading to anisotropic magnetoelectric coupling at room temperature. Such findings are particularly promising for the development of multiferroic memory devices.
Furthermore, these Pyr-F4TCNQ cocrystals have unveiled additional intriguing properties, such as magneto-optical and Cotton-Mouton effects. These effects refer to the interplay between optical and magnetic properties, offering new avenues for the integration of magnetic and optical functionalities in a single material system. The discovery of these effects in organic cocrystals not only expands the scope of their potential applications but also underscores the vast potential of cocrystal engineering in creating materials with tailored properties for advanced technological applications.
6 CONCLUSION AND OUTLOOK
This review comprehensively discusses the recent progress in organic donor and acceptor cocrystals, focusing on their assembly through noncovalent interactions such as hydrogen bonding, halogen bonding, CT, π-π stacking, and AP interaction. It also examines the influence of external factors such as different growth methods (solution, vapor-phase, and mechanochemical techniques), which are crucial for achieving the high-quality crystallization that is necessary for high-performance cocrystals. This review presents examples of the unique optical, electrical, and thermal properties of these cocrystals, thereby highlighting their potential for creating functional materials.
- 1)
Uncontrolled preparation of organic cocrystals
- 2)
Unpredictable structural transformations
- 3)
Unclear relationship between aggregated states structure and properties of cocrystal
- 4)
Limited diversity for organic cocrystal
Despite the progress made in binary organic cocrystals, there is a pressing need to explore and develop ternary and higher-order cocrystals to significantly enhance the structural and functional diversity of organic cocrystals. By introducing additional molecular species into the cocrystal lattice, we can create a richer variety of cocrystals with unique and tailored properties. The current landscape of reported organic cocrystals, predominantly consisting of two-component systems, underscores the necessity for a more concerted effort to discover and synthesize multicomponent cocrystals, thereby unlocking a broader spectrum of applications and capabilities in the field of organic materials science.
To facilitate the development of such fascinating materials, researchers need to consider the following possible directions. Firstly, the preparation of large-area co-crystalline films is essential for the practical application of organic cocrystals. Techniques such as solution processing, vapor deposition, and other methods should be optimized to achieve uniform and defect-free films over large areas. Additionally, patterning techniques of cocrystals are crucial for integrating these materials into electronic and optoelectronic devices. Advanced lithography, inkjet printing, and template-assisted methods can be explored to create precise and customized patterns. Furthermore, the exploration of flexible co-crystalline materials opens up new horizons for wearable electronics and implantable devices. Exploring the crystals that maintain their structural integrity and properties even under bending or stretching can pave the way for the development of bendable displays and conformable sensors.
In summary, ongoing research holds promise for the innovative applications of organic cocrystals. The journey towards these breakthroughs will require a concerted effort in material synthesis, device fabrication, and systems integration, but the rewards are well worth the pursuit.
ACKNOWLEDGMENTS
This research was supported by the National Key R&D Program of China (Grant No. 2022YFB3603804) and the Natural Science Foundation of Shanghai (Grant No. 22ZR1407800).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Biographies

Yuqing Ding is a Ph.D. candidate in materials science at the Department of Materials Science at Fudan University. She received her B.S. degree in materials science and engineering from Yunnan University in 2023. Her research interests include the fabrication and application of organic synaptic transistors.

Yan Zhao received his B.S. degree in Chemistry from Shandong University in 2008 and a Ph.D. degree in Physical Chemistry from the Institute of Chemistry, Chinese Academy of Sciences (CAS) in 2014. He is now an Associate Professor at the Department of Materials Sciences, at Fudan University. His research focuses on the processing of organic/polymer semiconductors for field-effect transistors, organic circuits, and organic sensors.

Yunqi Liu graduated in 1975 from the Department of Chemistry, at Nanjing University, and received a Doctorate from Tokyo Institute of Technology, Japan, in 1991. Presently, he is a Professor at the Institute of Chemistry, CAS. He is also a Professor at the Department of Materials Sciences, at Fudan University. His research interests include molecular materials and devices.




