DNA nanostructures prevent the formation of and convert toxic amyloid proteospecies into cytocompatible and biodegradable spherical complexes
Abstract
The deposition of insoluble proteinaceous aggregates in the form of amyloid fibrils within the extracellular space of tissues is associated with numerous diseases. The development of molecular approaches to arrest amyloid formation and prevent cellular degeneration remains very challenging due to the complexity of the process of protein aggregation, which encompasses an infinite array of conformations and quaternary structures. Polyanionic biopolymers, such as glycosaminoglycans and RNAs, have been shown to modulate the self-assembly of amyloidogenic polypeptides and to reduce the toxicity induced by the formation of oligomeric and/or pre-fibrillar proteospecies. This study evaluates the effects of double-stranded DNA (dsDNA) nanostructures (1D, 2D, and 3D) on amyloid self-assembly, fibril disaggregation, and the cytotoxicity associated with amyloidogenesis. Using the islet amyloid polypeptide (IAPP) whose pancreatic accumulation is the hallmark of type 2 diabetes, it was observed that dsDNA nanostructures inhibit amyloid formation by inducing the formation of spherical complexes in which the peptide adopts a random coil conformation. Interestingly, the DNA nanostructures showed a persistent ability to disassemble enzymatically and thermodynamically stable amyloid fibrils into nanoscale DNA/IAPP entities that are fully compatible with β-pancreatic cells and are biodegradable by proteolysis. Notably, dsDNA nanostructures avidly trapped highly toxic soluble oligomeric species in complete cell culture media and converted them into non-toxic binary complexes. Overall, these results expose the potent modulatory effects of dsDNA on amyloidogenic pathways, and these DNA nanoscaffolds could be used as a source of inspiration for the design of molecules to fight amyloid-related disorders.
1 INTRODUCTION
Protein aggregation into ordered amyloid structures and subsequent tissue deposition are associated with numerous human diseases, including Alzheimer's disease, systemic amyloidoses, and type 2 diabetes (T2D).[1, 2] While the protein precursors that misfold into insoluble amyloids share neither sequence nor native structure homology, the resulting unbranched fibrils share the stable cross-β-sheet quaternary conformation, in which β-strands run perpendicular to the fibril axis.[2, 3] While the amyloid hypothesis (i.e., that protein aggregation causes tissue degeneration) is supported by compelling clinical evidence, the development of therapeutic approaches that interfere with the amyloidogenic cascade and prevent the resulting pathology is still in its infancy. Among strategies that have been used to circumvent amyloidogenesis, the stabilization of the protein's native state appears to be the most promising approach. This has been exemplified by the clinically-approved drug Tafamidis, which arrests transthyretin aggregation by inhibiting the dissociation of the tetramer into misfolding-prone monomers.[4] Nonetheless, this strategy is based on pharmacological chaperoning and is difficult to apply to amyloidogenic polypeptides that are intrinsically disordered, such as the amyloid-β (Aβ) peptide and α-synuclein. Besides, while the inhibition of amyloid growth and/or the destabilization of the cross-β-sheet quaternary structure by small molecules, such as natural polyphenols[5] and short peptides with self-recognition motifs[6, 7] have shown interesting efficacy in vitro, their clinical translation remains elusive. More recently, passive immunotherapies based on antibodies that bind and remove amyloid deposits have been introduced into the clinics,[8-10] albeit their efficacy in improving the quality of life of patients has been somewhat limited and safety concerns have been raised.[11] Thus, it is still critical to identify (bio)molecules that can interact with the large ensemble of proteospecies generated throughout the amyloidogenic cascade and convert them into non-toxic structures that can be cleared by the host organism.
A diversity of natural products, macromolecules, synthetic polymers, multifunctional supramolecular structures, and nanoparticles have been reported to mitigate or reverse amyloid toxicity by inhibiting amyloid formation and/or by stimulating the degradation of amyloid fibrils in different disease models.[12] In particular, several highly charged macromolecules have been created based on biopolymers, and their effect on protein aggregation and amyloid formation was evaluated.[12] Among these, polyanionic biopolymers, such as glycosaminoglycans (GAGs), RNAs, and dendrimers, have been shown to promote the self-assembly of amyloidogenic proteins and to reduce the toxicity associated with the formation of oligomeric and/or pre-fibrillar species.[13-16] However, GAGs are well-known to be associated with virtually all amyloids extracted from patients, where they contribute to the accumulation and persistence of protein deposits.[17] Interestingly, short single-stranded DNA (ssDNA) sequences were reported to not only inhibit Aβ peptide self-assembly but also to disassemble pre-formed fibrils through electrostatic interactions, leading to the formation of inter polyelectrolyte complexes (IPECs).[18] Similarly, an in silico study has suggested that ssDNA could disassemble Aβ peptides through electrostatic interactions between the DNA phosphate backbone and positively charged residues.[19] Although these preliminary studies are exciting and appear promising to support the design of amyloid modulators based on ssDNA, the toxicity of the formed IPECs has not been evaluated and ssDNAs are known for their poor stability and high promiscuity.[20]
The pancreatic deposition of the islet amyloid polypeptide (IAPP) is a hallmark of T2D and correlates closely with disease progression.[21, 22] IAPP, or amylin, is a 37-residue glucomodulatory hormone that is co-expressed and co-secreted with insulin by pancreatic β-cells.[23] Owing to its primary structure encompassing numerous hydrophobic residues, residues that are prone to H-bonding, and only two charged residues (Figure 1A), IAPP is highly prone to aggregation.[24, 25] IAPP amyloid self-assembly is described as a nucleated polymerization in which the rate-limiting step is the formation of a high energy nucleus[26, 27] and this process gives rise to a diversity of quaternary structures, each being characterized by large conformational ensembles.[28] (Figure 1B). Monomeric IAPP is not cytotoxic, per se, but induces cell death by assembling into transient soluble oligomers and prefibrillar species that damage the plasma membrane and/or trigger oxidative stress and apoptosis.[29, 30] While mature IAPP amyloid fibrils assembled in vitro are usually non-toxic to cells,[23] massive deposition of amyloid plaques in the islets of Langerhans causes inflammatory stress and dysfunction of insulin-secreting cells, contributing to the pathogenesis of T2D.[23] Thus, therapeutic interventions targeting IAPP-associated pathogenicity should not only inhibit the formation of toxic pre-fibrillar species but also promote the elimination/clearance of pre-formed amyloid fibrils. However, in its pre-fibrillar states, IAPP shows a large conformational ensemble that complexifies the design of molecules that could stabilize the peptide in a non-aggregating form[31, 32] Moreover, amyloid deposits are highly stable and resistant to proteolytic and lysosomal degradation, which precludes their elimination from the afflicted tissues.
Inspired by the initial work on the effect of ssDNA on Aβ self-assembly, the present study evaluates the effect of small DNA-based nanostructures on IAPP amyloidogenesis and on the cytotoxicity of the associated proteospecies. Indeed, while short ssDNAs have been shown to interact with Aβ peptides, studies with double-stranded DNA (dsDNA) have been limited to large genomic DNA. Small dsDNA nanostructures have not yet been evaluated in this context. Unlike other polyanionic biopolymers that generally accelerate amyloid self-assembly, 1D-, 2D-, and 3D-DNA nanostructures (Figure 1C) were shown herein to fully inhibit fibril formation. Interestingly, dsDNA nanostructures efficiently disassembled stable amyloid fibrils and trapped highly toxic oligomers, converting both quaternary species into cytocompatible complexes in which the peptide could be digested by proteolysis. In addition, the 1D-, 2D-, and 3D-DNA nanostructures were designed such that IAPP peptide fragments responsible for nucleation could be precisely positioned along each structure to ultimately offer some specificity of interactions. Short IAPP peptides appended to the dsDNA nanostructures did not influence their effect on amyloidogenesis, which is promising for future applications involving targeting. Overall, this study highlights the modulatory effects of DNA nanostructures on amyloid formation and suggests that dsDNA scaffolds could be a source of inspiration for the design of molecular tools to fight protein misfolding disorders.
2 METHODS
2.1 Materials
Fmoc-protected amino acids and O-(1H-6-chlorobenzotriazole-1-yl)−1,1,3,3-tetramethyluronium hexafluorophosphate were purchased from Matrix Innovation. Piperidine was purchased from A&C American Chemicals Ltd. Tris(hydroxymethyl) aminomethane, magnesium chloride, ammonium persulfate, glacial acetic acid, and tetramethylethylenediamine were purchased from Bioshop Canada. Unlabeled DNA strands were purchased from idtDNA. Maleimide-labeled DNA strands were purchased from GeneLink. TAMg buffer was made in-house (40 mM Tris, 12.5 mM MgCl2·6H2O, 20 mM glacial acetic acid, pH 7.8). GelRed stain was purchased from VWR Canada. Precast 4%–15% polyacrylamide gel electrophoresis (PAGE) gels were obtained from BioRad. All solvents were of analytical grade and were purchased from Sigma-Aldrich. Deionized (DI) water was used to prepare buffer solution (pH 7.4). All solvents, reagents, and other chemicals were used as received without further purification.
2.2 Peptide synthesis, purification, and characterization
IAPP was synthesized by means of standard Fmoc solid-phase peptide synthesis and then purified using reverse-phase high-performance liquid chromatography (RP-HPLC), as previously described.[35] Formation of the C2–C7 disulfide bridge was achieved by oxidation in dimethyl sulfoxide at room temperature (RT). The assembled IAPP was purified by RP-HPLC and analyzed by high-resolution mass spectrometry (HRMS) using an Agilent 1200 series HPLC coupled to an electrospray time-of-flight mass spectrometer (ESI-TOF MS) (Figure S1). Peptide fractions with a purity higher than 95% were combined and lyophilized. Subsequently, aliquots of monomerized peptide were prepared by treating the lyophilized powder in 100% hexafluoro-2-propanol (HFIP) at a concentration of 1 mM. The solution was sonicated for 30 min, filtered through a 0.22 µm hydrophilic polypropylene filter, and lyophilized. The lyophilized IAPP powder was solubilized in HFIP, sonicated for 30 min, and lyophilized a second time. Monomerized samples were kept in the frozen dried state at −80°C for a maximum period of 4 weeks.
2.3 Design and assembly of DNA nanostructures
Linear DNA nanostructures (DNA-1D) consisted of a 39-base pair (bp) complementary region (Table S1), followed by a four-thymine spacer, and a 15-base addressable overhang. The overhang permits the incorporation of overhang complements (OC, Table S1). The sequences of the pairing region and the overhang were generated using CANADA 2.0,[36] a random DNA sequence generator. Sequences were then cross-analyzed for unwanted binding using idtDNA's Oligoanalyzer tool, based on the work of Allawi.[37] Final sequences were ordered from idtDNA. Unmodified OC strands are used to complete the double-stranded structures bearing no ligands. For the peptide-guided complexing studies, the OC strand was conjugated to a short fragment of IAPP (20–29). Assembly of the desired structure was verified using PAGE (Figure S2, 0.002 nmol of structure per well, pre-annealed from 90 to 23.3°C over the course of 16 h in TAMg buffer; 80 V, 90 min, 4%–15% polyacrylamide). The DNA triangle (DNA-2D) is a novel design created to provide a 2D structure with three overhangs (Figure 1 and Table S1). Built around a central (Tri1) strand, three strands with regions complementary to both the Tri1 and each other were designed using CANADA2.0 to generate unique sequences. Final sequences were chosen based on cross-analyses for undesired binding. The final structure resembles a triangle with three arms, each possessing a structural binding region and an overhang region. Once the complementary sequences were selected, the structure was modeled using GIDEON,[37] a program for DNA nanostructure design. GIDEON was used to determine if extra non-binding bases (thymines) were required at each vertex. Extra bases allow for the continuation of the DNA helix, releasing the strain between the main triangle and each arm. These bases are indicated in black in Table S1. Assembly of the desired structure was based on the predicted design and migration via PAGE. Each strand was added in a stepwise manner, to ensure that changes in mobility were as expected (Figure S2, 0.00435 nmol of structure per well, pre-annealed from 90 to 23.3°C over the course of 16 h in TAMg buffer; 80 V, 90 min, 4%–15% polyacrylamide). The DNA tetrahedron (DNA-3D) was adapted from the design of Dong et al.,[38] with the addition of a four-thymine linker and the addressable overhang at each vertex. To verify assembly, each strand was added in a stepwise manner to ensure that changes in mobility were as expected (Figure S2, 0.00435 nmol of structure per well, pre-annealed from 90 to 23.3°C over the course of 16 h in TAMg buffer; 80 V, 90 min, 4%–15% polyacrylamide.). Each DNA strand was quantified by absorbance at 260 nm prior to use. For each structure, sufficient DNA was added to reach a final concentration of 14 µM in TAMg buffer. The overhang regions were blocked with appropriate amounts of OC, creating fully duplexed structures. The molar ratio of OC per assembly was dependent on the structure. For the linear structure, the ratio was 2:1 (OC: structure). For the triangle, the ratio was 3:1. For the tetrahedron the ratio was 4:1. Due to the prevalence of oligomer formation at high 2D and 3D DNA concentrations, the stock solutions of each structure were diluted 10× with DI water prior to assembly. The stock was mixed and then separated into polymerase chain reaction (PCR) tubes. Using a PCR machine, the tubes were heated to 90°C and then cooled to 23.3°C over the course of 16 h. Samples were recombined and lyophilized to dryness, then resuspended to the initial volume using sterile water. Assembly was verified using PAGE (80 V, 75 min).
2.4 Preparation of peptide-labeled DNA (OC-Pep) structures
Maleimide-labeled DNA strands (OC-mal) arrive as protected maleimido-2,5-dimethylfural cycloadducts. Maleimide deprotection was performed using a microwave deprotection strategy.[39] Briefly, the cycloadduct was deprotected in 1:1 MeOH:H2O solvent mixture with a final DNA concentration of 25 µM in 200 µL. This mix was placed in a CEM microwave reactor. A ‘dynamic’ method was prepared (T = 90°C, Power = 300 W). With this method, the temperature was maintained at T = 90°C by automatic variation of the power. The buildup of pressure kept the sample from boiling, and PowerMax was enabled to provide cooling via compressed air. The reaction was run for 90 min. Once the mixture had cooled, it was transferred to an Eppendorf tube and dried using a SpeedVac. For conjugation to the maleimide, an IAPP peptide fragment containing an additional Cys with free thiol was synthesized (CKGSGSSNNFGAILSS), purified using RP-HPLC, and analyzed by LC-HRMS (Figure S3). The conjugation reaction was then performed in TAMg buffer, with 20 µM of OC-mal and 40 µM of peptide (1:2 ratio). The reaction mixture was placed on a shaker overnight at RT. To ensure conjugation of the peptide to OC-mal, crude reaction product was evaluated by LC-MS, with a gradient of 20%–60% acetonitrile + 0.1% trifluoroacetic acid over 25 min (flow rate 1 mL/min, Agilent Zorbax 300SB-C18, 5 µm, 4.6 × 150 mm column, electrospray ionization, drying gas 12 mL/min, nebulizer pressure of 35 psig, drying gas temperature of 350°C, capillary voltage of 3000 V and fragmentor set to 70). Three peaks were identified through a combination of liquid chromatography controls and mass calculations: OC-mal eluted at 14.5 min, peptide eluted at 4.2 min, and OC-Pep eluted at 15.5 min. Analysis of peak areas revealed no leftover OC-mal, so no further purification was performed. Free peptides may have been present but are minimal in comparison to the large amounts of peptide added for subsequent experiments. Polyacrylamide gel electrophoresis (0.024 nmol DNA-Pep per lane, 90 min at 80 V) confirmed the hybridization of OC-peptide to the OC portions of the linear structure (Figure S3).
2.5 Preparation of IAPP quaternary structures
IAPP quaternary structures were prepared by dissolving the lyophilized and monomerized peptide into 20 mM Tris-HCl buffer (pH 7.4) at a concentration of 75 µM. The freshly prepared solution was used immediately as mainly monomeric species (IAPPM), incubated under fully quiescent conditions at RT for 3 h to form oligomeric and pre-fibrillar species (IAPPO), or incubated for 48 h to generate amyloid fibrils (IAPPF). Solutions were then diluted to reach a final concentration of 25 µM of IAPP to which were added (or not) the DNA nanostructures.
2.6 Ultraviolet-visible spectroscopy
A Mandel UV-1280 spectrophotometer was used to record ultraviolet-visible (UV-vis) absorption spectra from 190 to 390 nm. The light path length was 10 mm, and the internal step was set to 1 nm.
2.7 Fluorescence spectroscopy
Fluorescence measurements were carried out at RT on a PTI Photon Technology International steady-state spectrofluorometer (QuantaMaster 40) and operated with PTI's FelixGX software with a light path length of 3 mm. For thioflavin T (ThT), used at a concentration of 40 µM, the excitation wavelength was set to 440 nm and the emission was recorded from 450 to 550 nm. For 8-anilinonaphthalene-1-sulfonic (ANS), used at a concentration of 100 µM, the excitation wavelength was set to 355 nm and the emission spectra were recorded from 385 to 585 nm. For fluorescein arsenical hairpin (FlAsH), used at a concentration of 0.5 µM and supplemented with 125 µM dithiol British anti-lewisite (BAL) and 500 µM tris(2-carboxyethyl)phosphine (TCEP), the excitation wavelength was set to 508 nm and the emission spectra were recorded from 460–540 nm. For intrinsic fluorescence spectroscopy, the tyrosine fluorescence spectra were recorded from 290–360 nm with an excitation wavelength of 274 nm. The intrinsic fluorescence of IAPP Y37 in the absence of DNA was assigned as F0, and the intrinsic fluorescence of tyrosine in the presence of DNA was assigned as F. The extent of the change in the fluorescence intensity was ascribed to (F0 − F)/F0.
2.8 Kinetics of IAPP fibrillization
2.9 Far-UV circular dichroism spectroscopy
Circular dichroism (CD) spectra were collected on a Jasco J-815 spectrophotometer at RT, in a 2-mm quartz cuvette. Peptide solutions were diluted in 20 mM Tris-HCl, pH 7.4 buffer to reach a concentration of 25 µM, and the CD spectra were collected from 260 to 190 nm. Curves were smoothed using a smoothing moving window of 13 data points through the Savisky-Golay algorithm.
2.10 Dynamic light scattering
Size measurements were performed using the Malvern Zetasizer Nano S90 (Malvern Panalytical) containing a 4 mW helium-neon laser with a wavelength of 633 nm and a scattering angle of 90°. Each measurement corresponded to a triplicate of 10 runs per analysis performed at 25°C. The results were presented as the number distribution (%) of particles versus hydrodynamic radii (nm) calculated from the Malvern software.
2.11 Zeta potential
Measurements were carried out using a zeta potential analyzer (ZetaPlus/Bl-PALS; Brookhaven Instrument Corp.). Each measurement corresponds to a triplicate of 10 runs per analysis.
2.12 Atomic force microscopy
IAPP solutions, with and without DNA nanostructures, were spotted on a freshly peeled mica surface. Excess liquid was removed by wicking, then the surface was rinsed twice with DI water. After being air-dried overnight, atomic force microscopy (AFM) imaging was conducted using a Bruker (MultiMode 8) microscope equipped with a Nanoscope E controller. To characterize the morphology of the resulting complexes, samples were scanned in scanasyst-air mode using a silicon nitride AFM tip (2−12 nm tip radius, k = 0.4 N/m) under ambient conditions. Gwyddion v2.59 was used to analyze images.
2.13 Attenuated total reflection-Fourier transform infrared spectroscopy
IAPP incubated for up to 72 h in 20 mM Tris-HCl, pH 7.4 with, or without 1D-, 2D-, or 3D-DNA were lyophilized and attenuated total reflection-Fourier transform infrared spectroscopy (ATR-FTIR) spectra were recorded from the solid-state sample using a Nicolet Magna 560 spectrometer equipped with a nitrogen-cooled MCT detector.
2.14 Photochemical-induced cross-linking
An IAPPO solution (75 µM) was treated with cross-linking solution (70 µM tris-(bipyridyl)Ru(II) and 1.4 mM ammonium persulfate) and then illuminated with a 150 W incandescent bulb for 5 s. Subsequently, the reaction was quenched with 1 M dithiothreitol, and oligomers were separated by sodium dodecyl sulfate (SDS)-PAGE (10% Tris-tricine gel) and revealed using silver staining.
2.15 Dot blot analysis
IAPPO solution (10 µL at 75 µM) was applied to a nitrocellulose membrane, allowed to air dry, and then blocked in 5% non-fat dried milk in Tris-buffered saline with Tween-20 (TBS-T) for 30 min. The membrane was incubated with an anti-oligomer rabbit polyclonal primary antibody (A11) for 2 h and then washed three times in TBS-T. The membrane was incubated for 1 h with a Horseradish peroxidase-conjugated secondary antibody, diluted at 1:10,000 in 5% non-fat dried milk in TBS-T. Finally, after three washes of the blot in TBS-T, the signal from the secondary antibody was detected by enhanced chemiluminescence.
2.16 Proteolytic digestion by proteinase K
Stability against proteolysis was assessed by subjecting the IAPP fibrils and IAPPF/DNA-1D complexes to 120 U/mL of proteinase K for 1 h and digestion was evaluated by matrix-assisted laser desorption ionization-TOF (MALDI-TOF) MS.
2.17 Cell viability assays
Rat pancreatic β-cell line (INS-1E) were cultured in black wall clear bottom 96-well plates treated for tissue culture at a density of 25 k cells/well in RPMI-1640 media containing 10% of fetal bovine serum (FBS), 100 UI/mL penicillin, 100 mg/mL streptomycin, 10 mM HEPES, 1 mM sodium pyruvate, 50 mM mercaptoethanol, and 2 mM L-glutamine. After 48 h incubation in a humidified atmosphere of 5% CO2 and 95% air at 37°C, cells were treated by the direct addition of IAPPO (25 µM), with or without the co-addition of 1.6 µM of 1D, 2D, or 3D DNA nanostructures, and then incubated for 5 h. For the evaluation of the cytotoxicity of the IAPP-DNA complexes, obtained by preincubation of IAPP (75 µM) with DNAs (4.8 µM) in 20 mM Tris-HCl buffer (pH 7.4) for 72 h, cells were treated with 25 µM of IAPP/DNA complexes and then incubated for 24 h. Cellular viability was evaluated using the resazurin metabolic assay and determined in % from the fluorescence ratio of peptide-treated sample to vehicle-treated control (20 mM Tris-Buffer, pH 7.4).[40] Data of a least three independent assays performed in triplicate were averaged and expressed as the mean ± standard error of mean (S.E.M.). For Live/Dead assays, INS-1E cells were plated in 12-well plates at a density of 150,000 cells/well for 48 h before treatment. Fluorescent microscopy analyses were performed after the incubation with 4 µM of ethidium homodimer-1 and 2 µM of calcein-AM for 45 min incubation.
2.18 Lactate dehydrogenase release assay
3 RESULTS AND DISCUSSION
3.1 DNA nanostructures modulate IAPP self-assembly
Owing to their programmability and addressability, DNA molecules have emerged as versatile nanoscale materials for numerous biomedical applications including vectors for targeted drug delivery as well as sensors for diagnostics and theranostics.[41] Short ssDNA has been shown to be a potent modulator of amyloid formation of the Aβ peptide,[18] however ssDNA is highly flexible and less thermodynamically stable compared to dsDNA, especially when assembled into 2D and 3D architectures.[42] In this context, the present study explored a new paradigm to modulate amyloid formation and its associated toxicity via a DNA-mediated scavenging mechanism. Through the rational design of DNA sequences, almost any desired shape can be constructed with nanometer-scale precision.[43] Small and ordered 1D, 2D, and 3D dsDNA nanostructures were assembled from individual DNA strands (Table S1 and Figure 1). The linear dsDNA strands used in this study were longer relative to the ssDNA previously used,[18] to ensure that all regions remained stably hybridized during the increased temperatures required for in vitro cell studies, especially the overhang regions. The DNA tetrahedron is based on a common motif that has been used in multiple in vitro and in vivo studies,[44] The 2D structure is an original design, created to have approximately the same number of bps (and anionic phosphate groups) as the tetrahedron. DNA stability over 1-week under experimental conditions was confirmed by UV-vis spectroscopy (Figure S4).
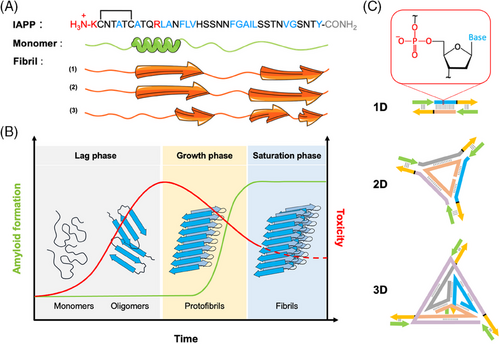
Monomerized IAPP (IAPPM) was incubated in the presence or absence of DNA nanostructures in Tris-buffer, pH 7.4 at RT under fully quiescent conditions. The kinetics of IAPP fibrillization were evaluated over time by means of fluorescence spectroscopy in a microplate reader. Although ThT fluorescence is usually used to probe amyloid formation with good selectivity,[45] ThT exhibits significant emission in the presence of DNA nanostructures[46] that overlaps with amyloid binding (Figure S5), precluding the use of this method. Thus, an alternative strategy based on the fluorogenic FlAsH dye was used to probe the kinetics of IAPP aggregation in the presence of DNA nanostructures. FlAsH is a derivative of fluorescein whose fluorescence quantum yield is dramatically amplified upon its binding to a tetracysteine motif. Accordingly, under reducing conditions that do not affect IAPP fibrillization, a non-contiguous tetracysteine FlAsH-binding site is formed upon the convergence of N-terminal domains that each contain a Cys2-Cys7 motif (Figure 1A), leading to a fluorescent signal.[47] Thus, this method is well-suited to investigate the kinetics of IAPP aggregation by monitoring the formation of quaternary complexes in the presence of dsDNA. As shown in Figure 2A, a prototypical sigmoidal growth curve was obtained for IAPP in the absence of DNA by monitoring FlAsH fluorescence. The extracted lag-time of 3.3 ± 0.1 h and half-time (t1/2) of 5.0 ± 0.2 h were within the range of the kinetics data obtained using ThT fluorescence under these conditions (Figure 2A). In sharp contrast, incubation of 25 µM IAPP in the presence of 1.6 µM of linear (1D), triangular (2D), or tetrahedral (3D) dsDNA nanostructures, induced a sharp increase of FlAsH fluorescence with the absence of any observable lag phase (Figure 2B,C). This observation indicates that the dsDNA nanostructures accelerate the formation of the split-tetracysteine motif through the convergence of the N-terminal domain of IAPP molecules. Different concentrations of dsDNA were evaluated to define the minimal concentration of dsDNA that modulates the kinetics of aggregation. At concentrations of 0.4 µM and above, the accelerating effect of dsDNA is observed with the quasi-absence of a lag phase. In contrast, at a concentration of 0.2 µM dsDNA, a prototypical sigmoidal growth curve of IAPP alone was observed, suggestive of the minimal effect of dsDNA on the kinetics of IAPP aggregation (Figure S6). This observation might be partially explained by the charge ratio between IAPP and DNA. IAPP is a positively charged peptide displaying a net charge of +3 at physiological pH (N-terminus, Lys1, Arg11; Figure 1A), whereas dsDNA is polyanionic with a charge of −2 per bp. The linear structure contains 58 bps and therefore an overall charge of −116. Thus, the opposing charges likely promote their mutual binding through electrostatic interactions and DNA nanostructures likely potentiate IAPP self-assembly by an electrostatic-type mechanism.[48, 49] More specifically, it is likely that the negatively charged DNA interacts with the positively charged N-terminal domain of IAPP, increasing the latter's local concentration, and promoting intermolecular FlAsH binding. Varying the DNA concentration changes the overall negative/positive (N/P) charge ratio. At low DNA concentrations (0.2 µM dsDNA), the N/P ratio was only 0.3, resulting in a loss of templating effects that are expected to facilitate IAPP assembly. In contrast, higher concentrations of DNA increased the ratio of N/P to 0.62, 1.24, and 2.48 for DNA concentrations of 0.4, 0.8, and 1.6 µM, respectively, suggesting a threshold charge ratio for DNA-promoted IAPP assembly exists at a N/P ratio between ∼0.3 and 0.6. No significant differences were observed between the kinetics of IAPP assembly in the presence of the DNA 2D and 3D nanostructures, both of which had relatively high N/P ratios of ∼7.6. Nevertheless, a trend in the maximum intensity of FlAsH was observed as 3D < 2D < 1D, suggesting the influence of the DNA structure on the morphology of the assemblies formed in the presence of IAPP, with greater convergence of the N-termini of IAPP in the presence of 1D and 2D DNA nanostructures.
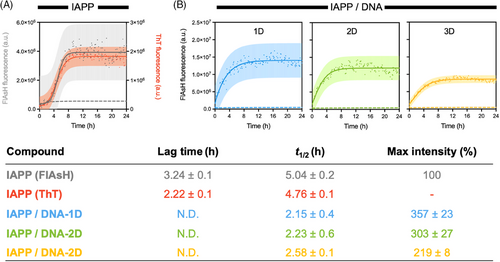
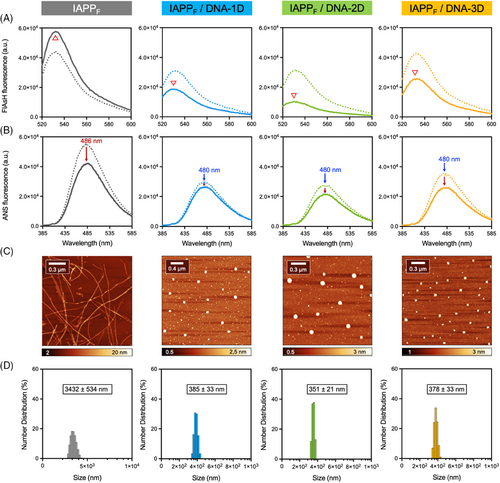
The ability of DNA nanostructures to modulate IAPP self-assembly was further evaluated by performing biophysical analyses and AFM imaging after prolonged incubation of IAPP with DNA. CD spectroscopy revealed that IAPP alone, that is, in the absence of DNA, exhibited a random coil secondary structure with a negative band at 202 nm immediately after its solubilization (0 h), which shifted towards a β-sheet-rich conformation after 72 h of incubation under fully quiescent conditions (Figure S7A). This secondary structure conversion is associated with a sharp increase in ThT fluorescence (Figure S7B), indicative of the formation of the cross-β-sheet quaternary organization.[50] Similarly, FlAsH and ANS fluorescence, respectively, revealed the gathering of adjacent N-terminal regions and the formation of hydrophobic clusters upon 72 h incubation (Figure 3A,B and Figure S7C,D). A modest change to FlAsH fluorescence intensity over incubation with DNA nanostructures was observed, despite changes observed by other techniques (Figure 3A). For IAPP, the formation of amyloid fibrils was confirmed by AFM, with images showing a network of typical fibrils with two major distinctive morphologies: flat ribbons and twisted filaments (Figure 3C and Figure S7E). This morphological heterogeneity is typical for IAPP fibrils assembled in vitro.[51] Because of the strong background signal, CD spectroscopy and ThT fluorescence could not be performed in the presence of DNA nanostructures. Remarkably, incubation of 1D, 2D, or 3D dsDNA nanostructures with monomerized IAPP for 72 h led to the formation of spherical assemblies with the complete absence of fibrillar aggregates (Figure 3C), indicating that these entities inhibit IAPP fibril formation by modulating the mechanism of self-assembly. These spherical aggregates were characterized by the presence of solvent-accessible hydrophobic patches, as observed with the sharp increase in the intensity of ANS fluorescence (Figure 3B). Dynamic light scattering (DLS) analyses revealed that the DNA/peptide complexes had hydrodynamic radii in the 300–400 nm range, independent of the identity of the DNA nanostructure (Figure 3D). These spherical complexes were not observable for the dsDNA nanostructures incubated in the absence of peptides (Figure S8). The formation of spherical assemblies was dependent on DNA concentration, with (predominantly) typical filaments observed at a DNA concentration of 0.2 µM and spherical aggregates at concentrations above 0.4 µM (Figure S9). This observation correlates with the kinetics of aggregation measured at different IAPP:DNA ratios (Figure S6).
Next, this effect, most likely mediated through electrostatic interactions, was tested in solutions of physiologically relevant ionic strength. Thus, monomeric IAPP was incubated in the presence of DNA nanostructures in 20 mM Tris, pH 7.4, supplemented with 150 mM NaCl. Results revealed the formation of similar spherical complexes with a total absence of amyloid fibrils after 3 days of incubation (Figure S10), indicating that IAPP-DNA interactions can occur in the presence of physiologically relevant salt concentrations. Finally, the effect of functionalizing the DNA nanostructures on this inhibitory effect of amyloid formation was tested by appending ligands that could be used for future in vivo targeting strategies. To do so, the nucleating core region of IAPP (segment 20–29 (I10)) was covalently attached to the flanking region of the 1D dsDNA nanostructure by Michael addition and the resulting peptide-labeled DNA structure was incubated in the presence of IAPP. Interestingly, the same effect on IAPP amyloid formation was observed in the presence of 1D-DNA functionalized with the I10 peptide (Figure S11), indicating that dsDNA can be functionalized while maintaining its inhibitory effect on amyloid self-assembly. Overall, these results revealed that linear, triangular, and tetrahedral dsDNA promoted the self-assembly of IAPPM into spherical structures, preventing the formation of amyloid fibrils.
3.2 DNA nanostructures convert cytotoxic oligomers into spherical complexes
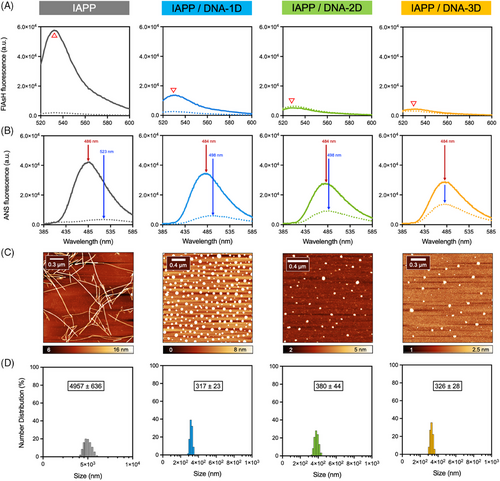
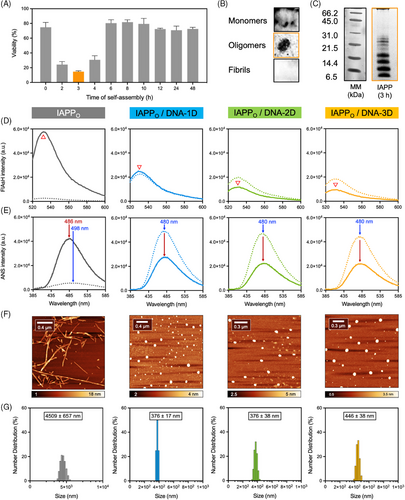
Going beyond previous works and considering that transient prefibrillar assemblies are the most toxic species of the amyloid pathway, the effects of these DNA nanostructures on pre-assembled oligomers were tested. First, the time-resolved toxicity of IAPP proteospecies was evaluated by monitoring the viability of INS-1E pancreatic cells after incubation with proteospecies pre-assembled for different times.[52] This allowed the identification of the mixture of IAPP assemblies having the highest cytotoxicity, and that can subsequently be used for evaluating the effect of DNA nanostructures. As shown in Figure 4A, IAPP cytotoxicity correlated with self-assembly time with the pre-fibrillar proteospecies obtained after 3 h incubation at RT being the most toxic (15% viability of control cells). Accordingly, IAPP oligomers (IAPPO) were prepared by incubating the monomerized peptide for 3 h under fully quiescent conditions (20 mM Tris-HCl, pH 7.4, concentration of 75 µM, RT) before being exposed to DNA nanostructures. The formation of oligomers/pre-fibrillar species under these conditions was confirmed by ThT and ANS fluorescence, CD spectroscopy, AFM, dot blot analysis, and photochemical cross-linking (PICUP). A predominantly random coil conformation was observed in the CD spectra (i.e., sharp band at 202 nm and weak signal at 222 nm) alongside weak ThT and ANS fluorescence (Figure S7A,B). Dot blot analysis conducted with the oligomer-specific A11 antibody and SDS analysis upon PICUP confirmed the formation of oligomeric assemblies (Figure 4B,C).
As expected, when this heterogeneous mixture of prefibrillar aggregates was left to incubate for an additional 72 h in the absence of DNA, a dense network of ANS- and FlAsH-positive fibrils was obtained (Figure 4D,E). Strikingly, incubation of pre-assembled IAPPO with 1D-, 2D-, or 3D-DNA nanostructures led to the formation of spherical aggregates with a complete absence of fibrils (Figure 4F). These assemblies closely resembled those obtained by the incubation of IAPPM with the DNA nanostructures (Figure 3D). Moreover, immediately after mixing the DNA nanostructures with IAPPO solutions, that is, experimental dead time of around 4 min, a strong increase of ANS fluorescence was observed, which likely correlates with the quasi-instantaneous binding of the oligomeric species to DNA thereby generating a high density of hydrophobic patches (Figure 4E). Over 72 h of incubation, these DNA-peptide clusters likely rearrange into core-shell type interpolyelectrolyte complexes in which the hydrophobic IAPP becomes more buried inside the core while the polyanionic DNA sequester to the solvent-exposed shell, as inferred by the decrease of ANS fluorescence. Similarly, FlAsH fluorescence, which reports on the convergence of N-terminal domains of adjacent IAPP molecules, also increased immediately upon the addition of the DNA nanostructures to the IAPPo solutions, before subsequently decreasing over time (Figure 4E). Again, this suggests a rapid clustering of IAPP prefibrillar species onto the DNA nanostructures, leading to the formation of complexes. The height of these clusters observed by AFM imaging and the hydrodynamic radii by DLS were within the range of those observed for the IAPPM-DNA complexes. Overall, these results indicate that dsDNA nanostructures avidly interact with highly cytotoxic IAPP oligomeric species, shifting the amyloid fibril formation pathway towards the formation of spherical complexes.
3.3 dsDNA nanostructures disassemble stable amyloid fibrils into spherical complexes
Amyloid fibrils are highly stable structures that are resistant to thermal denaturation, chemical treatment with mild denaturants, and proteolytic degradation.[53] This stability results in the life-long and massive accumulation of amyloid deposits in vivo, which ultimately cause tissue dysfunction despite the low cytotoxicity of individually organized fibrils.[54] Considering that it has been astonishingly reported that ssDNA could disassemble Aβ fibrils,[18] and in light of the results above, a comparable test of the effect of dsDNA nanostructures on mature IAPP fibrils (IAPPF) was warranted. Fibrils were prepared by incubating the monomerized peptide for 48 h in 20 mM Tris-HCl pH 7.4 under quiescent conditions at a concentration of 75 µM, a procedure known to reach the saturation phase.[55] The formation of amyloid fibrils was confirmed by ThT fluorescence, CD spectroscopy, and AFM imaging (Figure S7). The resulting fibrils were characterized by intense FlAsH and ANS fluorescence signals, respectively resulting from the convergence of N-terminal domains and the presence of hydrophobic domains (Figure 5A,B). These amyloid fibrils also resisted thermal denaturation and proteolytic degradation by proteinase K, as confirmed by AFM imaging, CD spectroscopy, and MALDI-TOF MS (Figures S12 and S13). As expected, incubation of these pre-assembled fibrils in the absence of DNA for an additional 72 h did not significantly alter FlAsH or ANS fluorescence, nor the morphology of the fibrils (Figure 5). In sharp contrast, the presence of 1.6 µM of either 1D-, 2D-, or 3D- DNA nanostructures in the IAPPF mixture led to the total disappearance of the fibrillar aggregates and the formation of spherical nanostructures (Figure 5C,D), with similar shapes and sizes to those reported for IAPPM (Figure 3) and IAPPO (Figure 4). This mesoscopic morphological conversion was accompanied by a slight decrease in FlAsH and ANS fluorescence (Figure 5A,B). The mixtures obtained after incubation with DNA were subsequently exposed to proteinase K to evaluate whether IAPP could be digested within these spherical complexes. As observed by MALDI-TOF MS, after incubation with the protease, IAPP originating from stable amyloid fibrils within the DNA-peptide complexes were digested to an extent similar to IAPPM, in contrast to IAPPF that were fully resistant to enzymatic hydrolysis (Figure S13). These observations indicate that DNA nanostructures not only have the capacity to interact with monomeric and oligomeric IAPP proteospecies but can also disassemble thermodynamically stable and proteolytically resistant amyloid fibrils into spherical aggregates, in which the polypeptide can be degraded by proteolysis.
3.4 DNA binding promotes the random coil conformation of IAPP
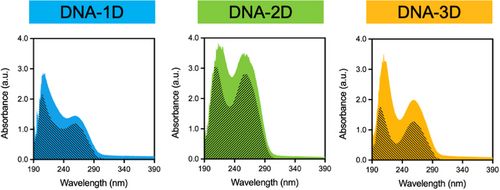
Results obtained thus far revealed that the interaction of IAPP with DNA nanostructures induced the formation of spherical complexes, regardless of the peptide's initial secondary or quaternary structure (i.e., randomly coiled monomers, heterogeneous oligomers/prefibrillar species, or organized β-sheet-rich amyloid fibrils). In fact, for all IAPP assembly states, the resulting spherical structures obtained upon incubation with DNA nanostructures showed similar characteristics in terms of morphology, hydrophobicity, size, and peptide clustering. IAPP-dsDNA interactions were further investigated by monitoring changes to the spectroscopic properties of IAPP and DNA molecules. As described above, monomers, oligomers, and fibrils were obtained by dispersing the lyophilized and monomerized IAPP in 20 mM Tris-HCl buffer (pH 7.4) at 75 µM. Each solution was then diluted to reach a final concentration of 25 µM of IAPP in the absence or in the presence of 1.6 µM of DNA nanostructure. As observed in Figure 6, in the absence of peptide, the UV-Vis absorption spectrum of dsDNA showed a broad band in the UV region with maximum absorption at 260 nm that does not overlap with the absorption maximum of IAPP species (Figure S14). To assess the direct interaction between DNA and the proteospecies, the UV-Vis absorption spectra of IAPP/DNA mixtures were scrutinized for shifts in the position of the maximum bands.[56] The spectra of the 1D-, 2D-, and 3D-DNA nanostructures in the presence of IAPPM, IAPPO, and IAPPF species were characterized by hyperchromism and slight bathochromism (redshift) (Figure 6 and Figure S15). Combined with zeta potential measurements (Figure S16), these observations indicate direct binding of dsDNA to the different peptide proteospecies.
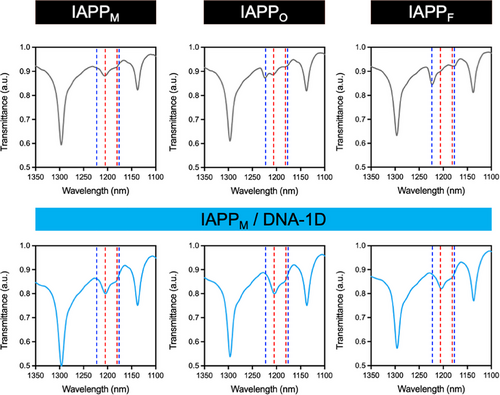
Since CD spectroscopy could not be used due to the strong signal of DNA, ATR-FTIR spectroscopy was employed to gain insight into the secondary structure of IAPP species and to monitor conformational changes associated with the interaction of the peptide with DNA nanostructures. The amide III region of the spectrum (∼1175–1310 cm–1) is mainly associated with C-N stretching and NH bending vibrations of the amide group and is highly sensitive to the secondary structure of the polypeptide chain (i.e., α-helix, β-sheet, β-turn, and random coil).[57, 58] In particular, this region does not overlap with the spectrum of DNA, contrary to the amide I region, nor does it overlap with the absorption of water.[58-60] Figure 7 shows the FTIR spectra of IAPP species obtained during the different phases of the fibrillization process. The spectrum of IAPPM was characterized by a random-coil secondary structure with a band at 1209 cm−1 and a shoulder at ∼1186 cm−1. The FTIR spectrum of IAPPO was characterized by the same band at 1209 cm−1 with a shoulder at ∼1186 cm−1 as well as a new band at 1226 cm−1 corresponding to the oligomeric transition state from random-coil to β-sheet structure. In contrast, the FTIR spectrum of IAPPF displayed a clear signature of a β-sheet secondary conformation with a characteristic band at 1226 cm−1 and a shoulder at ∼1181 cm−1. In the presence of DNA nanostructures, no band was observed at 1226 cm−1 for any of the IAPP-DNA mixtures, and all FTIR spectra were indicative of a random coil peptide secondary structure (Figure 7 and Figure S17). Next, we harnessed the intrinsic fluorescence of IAPP to further investigate the interaction of DNA nanostructures with IAPP species. As shown in Figure S18, the fluorescence intensity associated with the emission of Tyr37 was significantly quenched when DNA nanostructures were added to the solutions, further confirming the binding of IAPP to DNA. Overall, these results suggest a templating-based mechanism, in which the negatively charged phosphate backbone of DNA avidly interacts with the positive charges of IAPP, forming nanoscale IPECs in which the peptide is locked in a disordered non-amyloidogenic conformation.
3.5 DNA nanostructures prevent IAPP cytotoxicity by generating non-toxic complexes
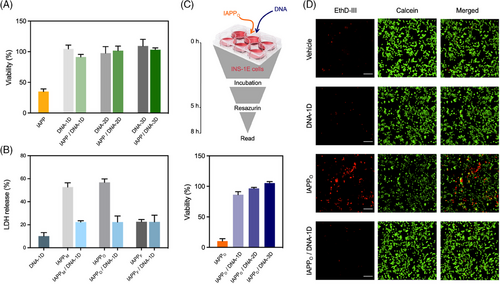
The cytocompatibility of the spherical IAPP-DNA complexes was evaluated using INS-1E pancreatic cells, a model commonly used to study IAPP-associated toxicity. While the incubation of INS-1E cells with 25 µM IAPPM for 24 h reduced their viability by 60%, the pre-formed IAPP-DNA complexes (i.e., 25 µM IAPP and 1.6 µM DNA pre-incubated for 72 h), did not affect cellular viability, nor did the DNA nanostructures alone affect the viability (Figure 8A). For instance, exposure to pre-formed spherical complexes assembled from IAPPM and DNA-1D maintained over 95% viability of the treated control cells. Next, the activity of the cytoplasmic enzyme LDH, a common indicator of plasma membrane permeabilization, was measured in the presence of DNA-1D, IAPP-DNA-1D complexes, and IAPP alone. The LDH activity measured in the cell media after 6 h treatment indicated that both IAPPM and IAPPO avidly disrupted the plasma membrane (Figure 8B). In sharp contrast, DNA alone as well as pre-assembled IAPP-DNA complexes induced no significant increase of LDH activity in the cell media, indicating a lack of perturbation of the plasma membrane. It is known that pre-fibrillar oligomers are the most toxic IAPP species and contribute to pancreatic β-cell death in T2D.[61, 62] Accordingly, it would be interesting to evaluate whether the DNA nanostructures could trap pre-assembled toxic oligomers and convert them into cytocompatible complexes. As described above, IAPP was pre-incubated at 75 µM in 20 mM Tris-HCl for 3 h to obtain IAPPO, and these proteospecies reduced cellular viability by almost 90% after 5 h of incubation with the cells (Figure 8C). Strikingly, the co-addition of 1.6 µM DNAs in the cell culture, that is, without pre-incubation, fully abrogated the cytotoxicity of 25 µM IAPPO. This result was confirmed by fluorescence microscopy analysis using the LIVE/DEAD assay, with the IAPPO/DNA-1D showing quasi-absence of red-labelled dead cells (Figure 8D). These observations indicated that DNA nanostructures promptly interact with toxic oligomers in complex biological media containing high concentrations of a diversity of biomolecules, including those from FBS, and convert them into non-toxic proteospecies.
4 CONCLUSION
The present work demonstrates the ability of linear (1D), triangular (2D), and tetrahedral (3D) dsDNA nanostructures to fully inhibit IAPP amyloid fibril formation as well as their unique capacity to efficiently disassemble fibrillar IAPP quaternary species. Importantly, these DNA nanostructures readily trapped highly toxic oligomers, even in complex biological media, and fully abrogated their cytotoxicity by converting them into cytocompatible and enzymatically biodegradable spherical complexes. The effects observed on IAPP's amyloidogenic pathway and associated toxicity were independent of the architecture of the DNA nanostructure and were unaffected by peptide ligands appended to the dsDNA nanostructures, which could ultimately be exploited for designing a targeted therapeutic based on the observed phenomenon. The results obtained herein suggest that the dsDNA nanostructures rapidly interact with IAPP proteospecies via, most likely, electrostatic interactions, leading to a conformational shift from a cross-β-sheet organization to a random coil structure. The lack of a distinctive difference between the 1D/2D/3D nanostructures seems to suggest that penetration of the nanostructure into the peptide assembly is unlikely. Nevertheless, adsorption of the nanostructures to the surface of the aggregate could impose stress on the assembly, altering it sufficiently to increase its dynamics and promote a change in quaternary morphology. Indeed, the FlAsH experiments suggest that the convergence of the N-termini of the peptides is similar in the fibrillar (cross-β-sheet) and spherical (random-coil) forms of the aggregates. While the precise molecular mechanisms underlying these observations remain to be elucidated, the efficient capacity to sequester highly toxic and metastable amyloid oligomer/pre-fibrillar species in complex media containing FBS and high concentrations of salts and biomolecules is remarkable and points to the need to further probe interactions of amyloid peptides and circulating DNA in relation to amyloid-associated diseases. Particularly, structure-activity relationship studies using a diversity of DNA sequences and structures will need to be conducted to elucidate the molecular basis of this phenomenon. Overall, this study provides novel insights into the design of DNA-based entities that could potentially modulate the aggregation of amyloid peptides in vivo, either for therapeutic purposes or for fundamental studies on these diseases. However, while DNA-based nanostructures have been evaluated for several biological applications, including biosensing,[63, 64] cell modulation,[65] bioimaging,[66] and drug delivery,[67, 68] challenges associated with specificity, metabolic stability, and distribution of DNA molecules will need to be addressed before their use as therapeutics.
ACKNOWLEDGMENTS
This research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) grants RGPIN-2018-06209 to Steve Bourgault, RGPIN-2021-03301 to Marc A. Gauthier, RGPIN-2018-05799 to Ali Nazemi, and by a new initiative grant from the Regroupement Intersectoriel de Recherche en Santé de l'Université du Québec (RISUQ) to Marc A. Gauthier and Steve Bourgault. Nadjib Kihal acknowledges a scholarship from the Center of Excellence in Research on Orphan Diseases-Fondation Courtois (CERMO-FC). Marc A. Gauthier is a Research Scholar of the Fonds de Recherche du Québec Santé (FRQS) and Steve Bourgault holds the Canada Research Chair in Chemistry of Biological Nanoassemblies.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.




