Fire-retardant and high-strength polymeric materials enabled by supramolecular aggregates
Special Collection: Distinguished Australian Researchers
Abstract
High-performance polymers have proliferated in modern society across a variety of industries because of their low density, good chemical stability, and superior mechanical properties. However, while polymers are widely applied, frequent fire disasters induced by their intrinsic flammability have caused massive impacts on human beings, the economy, and the environment. Supramolecular chemistry has recently been intensively researched to provide fire retardancy for polymers via the physical barrier and char-catalyzing effects of supramolecular aggregates. In parallel, the noncovalent interactions between supramolecular and polymer chains, such as hydrogen bonding, π–π interactions, metal–ligand coordination, and synergistic interactions, can endow the matrix with enhanced mechanical strength. This makes it possible to integrate physical–chemical properties and noncovalent interactions into one supramolecular aggregate-based high-performance polymeric system on demand. However, fulfilling these promises needs more research. Here, we provide an overview of the latest research advances of fire-retardant and high-strength polymer materials based on supramolecular structures and interactions of aggregates. This work reviews their conceptual design, characterization, modification principles, performances, applications, and mechanisms. Finally, development challenges and perspectives on future research are also discussed.
1 INTRODUCTION
Because of the easy-processing, low-cost, and lightweight advantages, polymeric materials have been the substitutes for traditional ceramic and metallic materials in modern society across a variety of applications, including construction, automotive, aerospace, electronics, and energy. However, the organic and aliphatic structure of most polymers suffers from intrinsic flammability.[1] As a result, their widespread use in the construction field often triggers large fire disasters with immeasurable losses, as happened at the Brazilian National Museum in 2018 and in London in 2017.[2] With the ever-increasing development of energy storage, consumer electronics, and automotive industries, the demand for high-performance polymers with satisfied fire retardancy and advanced mechanical properties has become increasingly urgent.[3, 4] In this regard, mitigation of fire risks of polymers is crucially important. The incorporation of fire retardants is an effective way to endow polymeric materials with high fire safety, easy processing, and low-cost advantages.[5] Nevertheless, a challenge with the use of traditional fire retardants is their adverse effect on the mechanical properties of polymers, due to their poor interfacial compatibility with the polymer matrix.
Mechanical reinforcements of polymers are eternally pursued by researchers.[6] In general, the reinforcing strategies of polymers are mainly divided into two types: (1) external incorporation of rigid nano/micro-additives into a polymer matrix, and (2) intrinsic structure regulation of a polymer network. The fillers used in the first strategy consist of inorganic and organic nanomaterials, such as graphene,[7] MXenes,[8] carbon nanofibers,[9] nanoclays,[10] glass fibers,[11] and cellulose crystals.[12] The second strategy mainly depends on the restraint of polymer chain mobility via the chemical/physical crosslinking of the network, crystallization and alignment of polymer chains, or interpenetration of polymer networks.[13-15] To further enhance the mechanical properties of the polymer composites, these two strategies can also be synergistically combined.[16] For the first strategy, the dispersion state of fillers within the matrix plays an important role in reinforcing performance. Unfortunately, the imperfect compatibility between fillers and matrix often leads to sharp compositional contrast between two phases in the matrix.[17] Meanwhile, these fillers also have an unusually high tendency to self-aggregate with the formation of mechanical defects, due to their high surface energy and large specific surface area.[18, 19] Therefore, to overcome the above shortcomings, it is of great importance to chemically design and modify the structure and morphology of fillers.
Research in supramolecular aggregates in polymers has made triumphant progress since the first proposal of supramolecular chemistry by French scientist Lehn in 1987.[20-22] Supramolecular aggregates with specific structures and functions are generally formed by two or more types of chemical molecules via secondary noncovalent interactions, such as ionic interactions, hydrogen bonding, π–π stacking, metal–ligand coordination, host–guest recognition, hydrophobic interactions, and synergistic interactions of multiple forces (Figure 1).[23-25] Compared to the externally incorporated traditional fillers, the incorporation of supramolecular aggregates into polymers has been proven to be an effective approach to enhance the mechanical strength or toughness of polymer networks, due to the noncovalent interactions formed between multiple associated groups of polymer chains and supramolecular.[26] Furthermore, thanks to the adjustable chemical structure of supramolecular, the fire-retardant elements (such as phosphorus, nitrogen, silicon, or sulfur elements) can also be introduced into as-prepared supramolecular aggregates.[27-29] However, there has not been a comprehensive yet critical review of this topic. In this regard, this review article summarizes the recent achievements from our and other groups on the fire-retardant and reinforcing polymeric materials based on supramolecular aggregates, including their characterizations, modifications, performance, applications, and mechanisms. Furthermore, a discussion of the challenges that need to be solved for their future practical applications and opportunities is also presented.
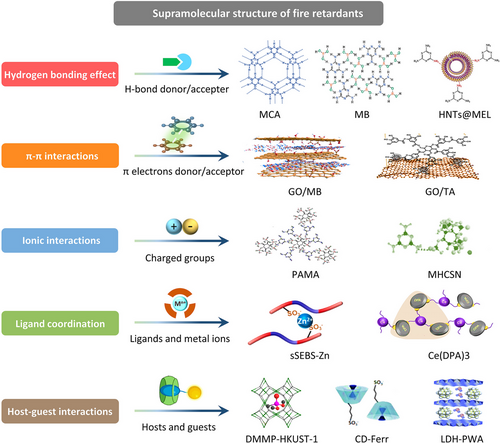
2 FABRICATION OF DIFFERENT FIRE RETARDANT VIA SUPRAMOLECULAR INTERACTIONS
2.1 Supramolecular fire-retardant aggregates based on hydrogen bonding
Hydrogen bonding (H-bonding) is known to be an important noncovalent interaction for polymers owing to their dynamically reversible and highly directional properties, and the bond energy of one single H-bond is around 5–60 kJ/mol.[30, 31] In recent years, various of high-performance polymeric materials based on H-bonding interactions have been successfully prepared and their properties have also been extensively studied.[32] Related studies have confirmed that the mechanical strength/toughness of polymers can be effectively enhanced by adjusting the effective number of H-bonds.[33] Therefore, the supramolecular aggregates containing multiple H-bonds are usually employed to construct supramolecular polymer systems. Upon loading, the interconnected three-dimensional (3D) network formed by multiple H-bond interactions between supramolecular aggregates and polymer chains can restrain the polymer chain movement.[34] As the external load further increases, the shear deformation takes place due to the chain stretching, the initial rupture, and reconstruction of H-bonds, which can induce malleability and stress transfer to dissipate more fracture energy, and thus demonstrating high strength, toughness, and ductility.[35]
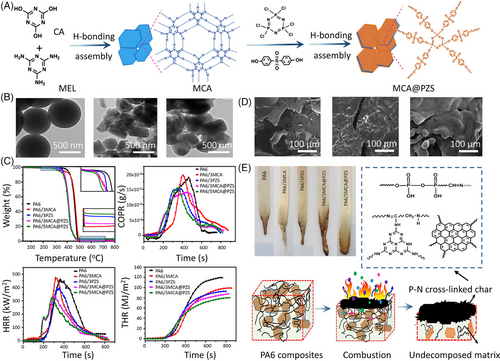
A presentative example of supramolecular aggregate by H-bonding self-assembly is melamine cyanurate (MCA), which is built by melamine (MEL) and cyanuric acid.[36] Araby and coworkers[37] reported that the addition of 5.0 wt% MCA can increase the tensile strength and toughness of thermoplastic polyurethane (TPU) by 8.3% and 2.7%, respectively, owing to the formation of H-bonds between TPU chains and MCA. Furthermore, MCA is also known as a recyclable and environmentally friendly fire retardant due to the high contents of N element within its structure.[38] During combustion, the released inert gas by MCA can not only dilute flammable gases and oxygen in the gaseous phase but also remove heat from the combustible zone. However, to achieve a satisfactory fire-retardant performance, a high content (>15 wt%) of MCA is often required due to its low fire-retardant efficiency, and thus inevitably deteriorating mechanical properties of the host polymer. To counter this challenge, MCA is usually employed to combine with other materials to construct more efficient fire retardant via H-bonding interactions.[39] Araby and coworkers[37] developed MCA@α-ZrP as a novel hybrid fire retardant for TPU. The addition of 5.0 wt% MCA@α-ZrP reduces the peak heat release rate (PHRR) and total heat release (THR) of TPU by 49.7%, and 12.6%, respectively. The combination of the diluting effect of MCA and the catalyzing carbonization effect of α-ZrP is reported to lead to such enhanced fire retardancy.
Malkappa and Ray[40] prepared MCA@PZS by employing poly(cyclotriphosphazene-co-4,4′-sulfonyl diphenol) (PZS) to functionalize MCA via H-bonding self-assembly (Figure 2A). Transmission electron microscopy (TEM) images illustrate that the surface of MCA became relatively smooth after the surface functionalization of PZS (Figure 2B). Compared to the UL-94 V-2 rating of polyamide 6/3MCA (PA6/3MCA), the sample containing 3.0 wt% MCA@PZS achieves a V-1 rating at the same loading level. In addition, the PHRR and THR values of PA6/3MCA@PZS are 29.1% and 11.2% lower than those of PA6/3MCA, respectively (Figure 2C). The improved fire retardancy can be attributed to the synergistic effect between MCA and PZS in both gaseous and condensed phases. The presence of PZS promotes the carbonaceous process of PA6 to form the thermally insulating P–N cross-linked char to prevent heat and mass transfer between the fire zone and unburned matrix (Figure 2D,E).
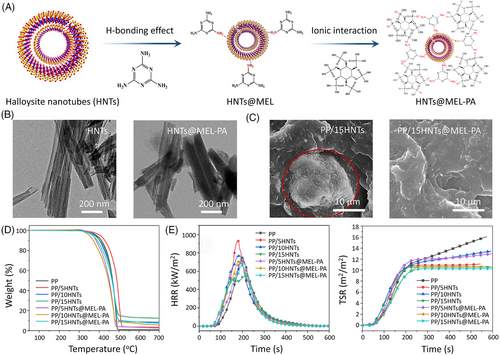
Halloysite nanotube (HNT), as a typical tubular nano-clay, is abundant in nature and possesses a high aspect ratio and good thermal stability.[41] To exploit HNT's potential as a fire retardant, Shang et al.[42] modified HNT with MEL–PA aggregates via H-bonding (Figure 3A). After modification, the surface of HNTs@MEL–PA is decorated with organic nanoflakes while maintaining the hollow tubular structure of HNT (Figure 3B). Compared to the unsatisfactory dispersion state of HNT within polypropylene (PP), the MEL–PA functionalized onto the surface of HNTs@MEL–PA have strong interfacial compatibility with the matrix, which lead to better dispersion (Figure 3C). Under N2 atmosphere, the increased char yields of PP composites at 700°C are attributed to the catalyzing carbonization effect of HNTs@MEL–PA (Figure 3D). The presence of HNTs@MEL–PA also benefits to reduce the total smoke release (TSR) of PP. However, the sole utilization of 5.0 or 10.0 wt% HNTs increases the PHRR and THR values, indicating that the smooth tubular structure of HNTs accelerated the combustion of PP as a result of the wick effect.
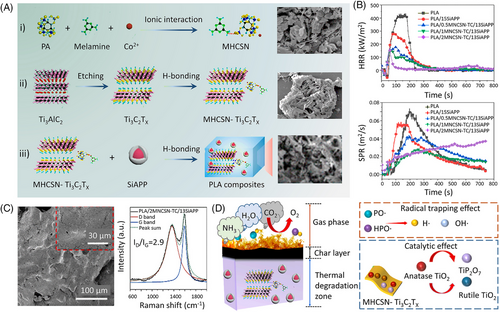
MXenes, a new family of 2D materials, consist of large numbers of hydroxyl groups on its surface, which allow for surface modification through H-bonding. Shi et al.[38] reported that the addition of 3.0 wt.% MXene@MCA can reduce the PHRR of TPU by 40%, while improving the tensile strength, break strain, and toughness by 47.5%, 5.4%, and 56.0%, respectively. In our previous work, MXene modified by phosphorus–nitrogen–transition metal displayed a great fire-retardant and reinforcing effect on TPU and epoxy resin (EP). For instance, 2.0 wt% Zr-MXene enables TPU to show a PHRR reduction up to 63%, a record break strain of 2060%, and great toughness of 316 MJ/m3.[43] For copper–organophosphate–MXene (CuP-MXene), in addition to the good fire retardancy of UL-94 V-0 rating, 5.0 wt% CuP-MXene also increases the tensile strength, elastic modulus, and impact strength of EP by 31.7%, 38.9%, and 25.0%, respectively.[44] Wang et al.[45] further combined P, N, and Co2+ with MXene to construct supramolecular fire retardant (MHCSN-TC) for polylactic acid (PLA). The results show that 1.0 wt% MHCSN-TC and 14.0 wt% SiAPP into PLA reduces the PHRR, THR, and peak smoke production rate (PSPR) by 64.1%, 50.1%, and 61.3%, respectively (Figure 4).
2.2 Supramolecular fire-retardant aggregates based on ligand coordination
Since the first report demonstrating the bonding between metal ions and ligands, a large series of supramolecular aggregates containing various metal ions have been successfully prepared.[46] Interestingly, metal–ligand bonds can serve as reversible cross-linking points in many biomaterials, such as nereids worms, marine mussels, and so on.[47] Metal–ligand coordination bond is the interaction of organic ligands with (transition) metal ions, demonstrating highly thermally stable, saturable, and directional properties.[27, 48] Although this coordination bond is mostly stronger than other supramolecular interactions (e.g., H-bonds and van der Waals bonds), it can be reasonably controlled over a broader time scale due to its rational connecting energy and uncomplicated interaction.[49] For supramolecular aggregates, the typical metal ions are Cu2+, Zn2+, Ca2+, Mg2+, Fe3+, and Al3+, while the commonly used ligands include catechol, triazole, pyridine, and carboxylic acid.[50] To obtain the desired materials with high fire retardancy and reinforcing efficiencies, the structure of resultant aggregates can be controlled by altering the type and quantity of metal ions and ligands.
In this regard, researchers synthesized a series of supramolecular fire retardants based on metal–ligand coordination, which simultaneously improved the mechanical performance of polycarbonate (PC).[51-54] For example, a P–N-containing compound (DPA) is employed as a ligand to synthesize an efficient lamellar fire retardant named Ce(DPA)3 (Figure 5A).[55] Thermo-gravimetric analysis (TGA) results show that both the initial degradation temperature (T–5 wt%) and char yields at 800°C of PC/Ce(DPA)3 are higher than those of PC, indicating the improved thermo-stability due to the Ce(DPA)3 (Figure 5B). The good interfacial compatibility between PC and Ce(DPA)3 benefits to toughen PC while maintaining its tensile strength, owing to the π−π stacking effect (Figure 5C). In addition, the presence of 3.0 wt% Ce(DPA)3 enables PC to reach a limiting oxygen index (LOI) of 26.9% and a UL-94 V-0 rating, with a 55% reduction in PHRR (Figure 5D). The ·OP/R−NH· released by DPA can quench or capture ·OH/H· in the gaseous phase, which can interrupt chain scission reactions during combustion. The generation of phosphonate or P2O5 further functions in the condensed phase by catalyzing carbonization (Figure 5E).
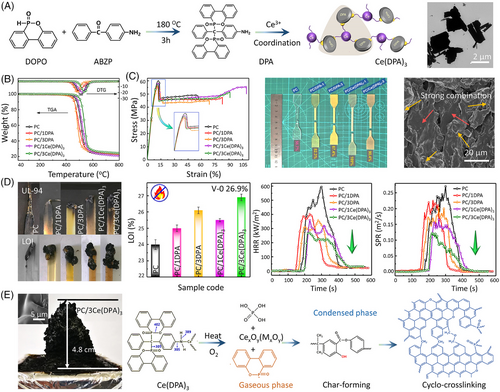
To further achieve higher fire retardancy, sulfonated styrene–ethylene–butylene–styrene (sSEBS) is utilized to coordinate with Na+, Zn2+, and Ce3+ for fabricating thermally stable supramolecular aggregates (sSEBS-M) (Figure 6A,B).[52] Due to π–π conjugation between sSEBS-M and PC chains, sSEBS-M well dispersed within the matrix in the form of submicron phase domains (around 500 nm), and thus PC/sSEBS-M composites demonstrate a comparable transparency and tensile strength to virgin PC. In addition, their impact toughness and ductility are 40% and 116% higher than those of virgin PC. sSEBS-M also shows high fire-retardant efficiency in PC. Incorporating only 1.5 wt% of sSEBS-Ce into PC leads to a desired UL-94 V-0 rating, a high LOI value of 33.5%, and a 53% reduction in PHRR (Figure 6D). The improved fire retardantcy of PC/sSEBS-M is attributed to two reasons: the strong free radical quenching capacity and the catalyzing carbonization effect of (rare earth) sulfonate. The aromatic sulfonate can trap free radicals in the presence of metals in the gaseous phase, while the formation of a char layer in condensed phases can extinguish the flame (Figure 6E).
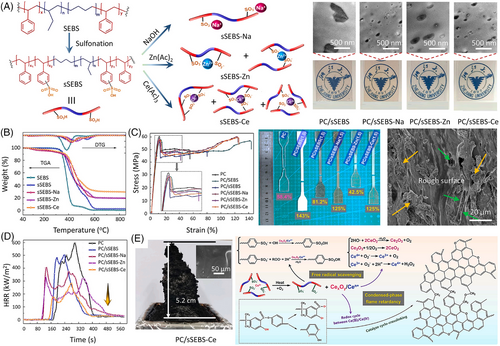
Previous studies have proved the synergistic effect on nano-barrier and char-forming effects of 2D black phosphorus (BP).[56] However, the inorganic BP is incompatible with most polymers, which limits its fire-retardant applications. To counter this problem, Hu and coworkers modified BP by using NH2-BDC as organic ligands to react with the Al3+ of MIL-53 onto the BP surface via a ligand coordination interaction (Figure 7A).[57] As expected, BP@MIL-53 shows high efficiency in improving the thermal stability and fire retardancy of EP. The results show that PC/1.0BP@MIL-53 can achieve a UL-94 V-0 rating and a high LOI value of 30.5% at a low concentration of 1.0 wt%. During combustion, the phosphorus-containing radicals released by BP can trap free radicals, and the char layer formed by MIL-53(Al) and BP serves as physical barriers to protect the unburned matrix. In addition, incorporating the resultant BP@MIL-53 into PC maintains the tensile strength of the matrix, but has some adverse effects on its toughness (Figure 7C).
2.3 Supramolecular fire-retardant aggregates based on host–guest interactions
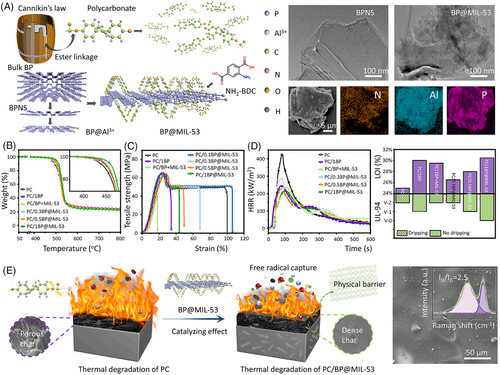
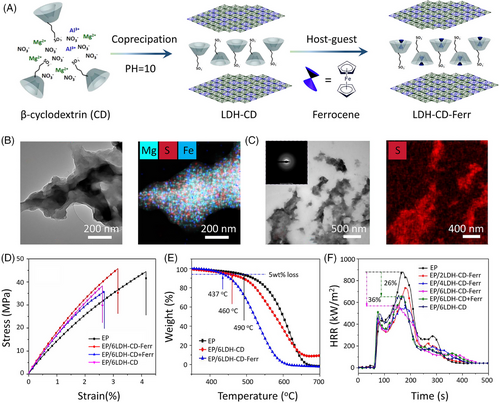
Host–guest interactions are the forces between the guest molecule and the macrocyclic host molecule, which are generally driven by the cumulative effect of various noncovalent interactions, such as ionic bonds, H-bonds, hydrophobic interactions, and van der Waals forces.[58] The unique characteristic of host–guest interaction is the cavity structure of cyclic oligomers, which enables them as hosts to offer spatially oriented binding for size-matched nano/micro-guest compounds. These cyclic oligomers always consist of repeated units, such as cyclodextrins (CDs), cucurbit[n]urils (CBs), crown ethers, pillar[n]arenes, and calixarenes.[59] In addition, layered nanohybrids can serve as host materials by lattice engineering techniques. There are various lattice engineering routes used to prepare functional layered nanohybrids, such as co-precipitation, ion-exchange, exfoliation-reassembling, and calcination-reconstruction techniques.[60] In this regard, plate-like nanohybrids can be rationally assembled with various guest species, such as inorganic nanoparticles, organic molecules, bioactive molecules, and polymers. For instance, Li et al.[61] prepared LDH-CD-Ferr by inserting ferrocene molecules into the nanocavity of sulfonated CDs intercalated layered double hydroxide (LDH-CD) via host–guest interactions (Figure 8A). Figure 8B presents the homogeneous distribution of Mg, S, and Fe elements, revealing that the s-CD and ferrocene are located between the LDH nanosheets. TEM images of EP/6LDH-CD-Ferr show the good dispersion of LDH-CD-Ferr within the EP matrix due to the good compatibility between LDH-CD-Ferr and EP (Figure 8C). Thus, 6.0 wt% LDH-CD-Ferr imparts EP matrix with increased tensile strength and comparable tensile modulus by preventing the propagation of micro-cracks. Compared to untreated EP, EP/6LDH-CD-Ferr shows a higher LOI value of 28.8 vol.% with a V-1 rating in the UL-94 test. In addition, the PHRR value of EP/6LDH-CD-Ferr shows a 36% reduction. However, the presence of LDH-CD-Ferr leads to a lower T–5 wt% (437°C) relative to 490°C of EP/6LDH-CD, as a result of the catalytic degradation effect of ferrocene.
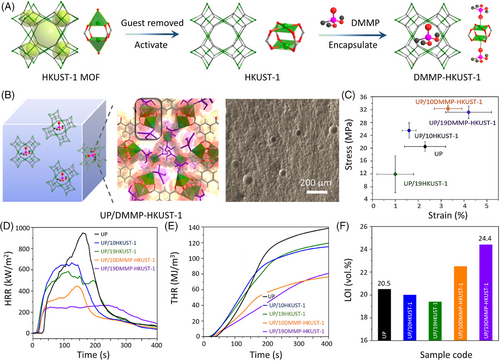
A porous metal−organic framework (MOF) can also be used as the host framework. Recently, Qi et al.[62] prepared a novel fire retardant (DMMP–HKUST-1) by encapsulating dimethyl methylphosphonate (DMMP) into HKUST-1 MOF, and then employed it to improve the flame retardancy of unsaturated polyester (UP) (Figure 9A,B). Because of the hierarchical strategy and the selected components, the affinity and fire-retardant efficiency of DMMP–HKUST-1 are regulated, and thus UP composites show good mechanical reinforcing and fire-retardant performance. For instance, the presence of 10DMMP–HKUST-1 increases the tensile strength of UP by 58%. In addition, UP/10DMMP–HKUST-1 shows a LOI of 22.5 vol.% with 54.2% and 42.9% reductions in PHRR and THR, respectively (Figure 9D-F). Taking advantage of the highly porous inner structure of MOFs, Huang et al.[63] encapsulated P–N containing ionic liquid ([DPP-NC3bim][PMO]) into MOF (NH2‑MIL‑101(Al)) as a novel fire retardant for EP. Incorporating the modified MOF into EP leads to 51.2% and 44.8% reductions in PHRR and COPR, respectively, which also improves the dispersion of ionic liquid within the EP matrix.
2.4 Supramolecular fire-retardant aggregates based on π–π interactions
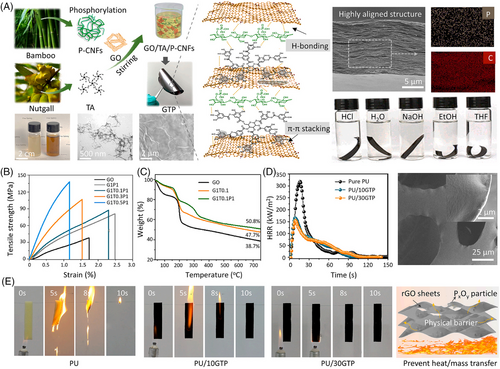
As one of the most important noncovalent forces, π–π stacking interactions exist between relatively electron-poor and electron-rich molecules, which is a special spatial arrangement of aromatic compounds.[64, 65] π–π stacking interactions can be divided into face-to-edge T-type and face-to-face F-type. Currently, some research studies have presented ingenious tactics to develop fire retardants by integrating π–π interaction.[66, 67] Graphene oxide (GO) consists of multiple aromatic regions, which has been one of the representative raw materials for constructing fire retardant via π–π interaction. For instance, Cao et al.[68] fabricate a hybrid fire retardant by combining GO nanosheets, phosphorylated-cellulose nanofibrils (P-CNFs), and tannic acid molecules (TA) based on H-bonding and π–π interactions (Figure 10A). The resultant GO/TA/P-CNFs composite film (GTP) demonstrates an optimized 1D/2D interconnected network with a hierarchical nacre-like structure, leading to good structural stability in various environments and improved mechanical properties. The Young's modulus and tensile strength of GTP film are 7 GPa and 132 MPa, respectively, which are 14 times and 3.6 higher than that of the pure GO (Figure 10B). Then, GTP as a fire-retardant coating is utilized to improve the fire retardancy of polyurethane (PU) foam, leading to a 48% reduction in PHRR at only 10 wt% content (Figure 10D). PU/10GTP can retard fire spread with a low LOI value of 20 vol.%, due to the formation of compact P-rich char in the condensed phase (Figure 10E).
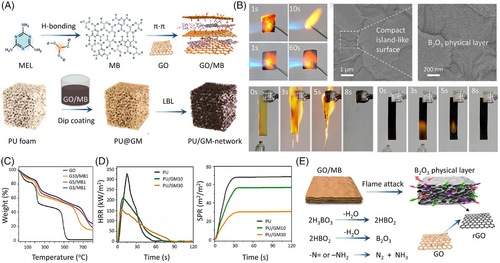
Furthermore, to obtain a GO-based coating with higher fire retardancy, Cao et al.[69] combined supermolecule melamine diborate (MB) with GO to form supramolecular GO/MB via π–π interaction (Figure 11A). The as-prepared GO/MB as a fireproof coating is then used to coat PU foam. Under flame attack, the well-wrapped GO/MB coating (GM) on the foam skeleton displays good structural stability, due to the formation of a B2O3-containing physical char layer. In UL-94 test, a V-0 rating of PU/GM30 is achieved with no obvious spreading flame observed (Figure 11B). The presence of 30 wt% GO/MB coating results in 54% and 56% reductions in PHRR and TSR of PU (Figure 11D). When encountering a flame, the H3BO3, –NH2 groups, and GO sheets in the MB structure can act as an acid source, gas source, and carbon source, respectively. GO can transform into thermally stable reduced GO (rGO), which can restrict the attack of external oxygen and heat (Figure 11E).
2.5 Supramolecular fire-retardant aggregates based on ionic interactions
Ionic interactions exist between positive and negative ions, which can be utilized to synthesize ionic liquids, ionomers, zwitterionic polymers, and polyelectrolyte complexes.[70-72] The ionic interaction intensity is determined by three key factors: electrostatic charge density, dielectric constant, and temperature. This interaction endows the resultant materials with outstanding thermal stability, and mechanical, and electrical properties because of the sufficiently strong binding strength.[73] Incorporating multivalent ions into the system can lead to stronger binding affinity. In addition, ionic interactions between polymer chains and fillers can serve as physical crosslinking points to reinforce the strength or toughness of the matrix.[74] Supramolecular fire retardants based on ionic interactions feature high structural diversity, which can be prepared by various precursors with negatively and positively charged groups.[75, 76] The representative negatively charged groups include phosphonate sulfonate, and carboxylate groups, while the typical positively charged groups are ammonium ions.[77-83]
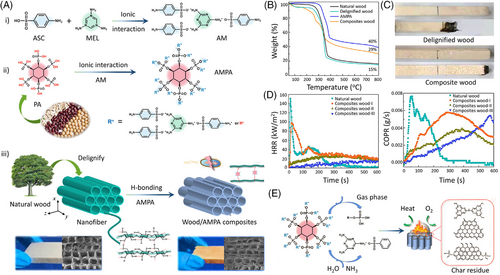
Phytic acid (PA) is mainly extracted from cereals and legumes, which have six phosphorus groups in a single molecule.[84-87] The high phosphorus content and catalyzing effect enable PA to be an environmentally friendly fire retardant.[88-91] Recently, PA has been widely utilized to react with amines and MEL to prepare P–N fire retardants based on ionic interactions.[92-94] For example, Zhang et al.[95] synthesized a phytic acid-based fire-retardant coating (AMPA) based on ionic interactions between p-amino-benzene sulfonic acid and MEL followed by a reaction with PA (Figure 12A). After coating 5.6 wt% AMPA on the surfaces via H-bonding, the thermal stability of sample woods is improved, with high char yields (40 wt%) at 800°C (Figure 12B). In addition, the LOI of the delignified wood obviously increases from 22.5 to 52.5 vol.%, and the PHRR is reduced by 81.0% (Figure 12C,D). Upon exposure to fire, AMPA degrades to release large amounts of inert gases such as NH3 and H2O, which can take the heat away and dilute flammable gases/oxygen concentrations. Meanwhile, PA groups can catalyze the carbonization of wood to form protective char layers (Figure 12E).
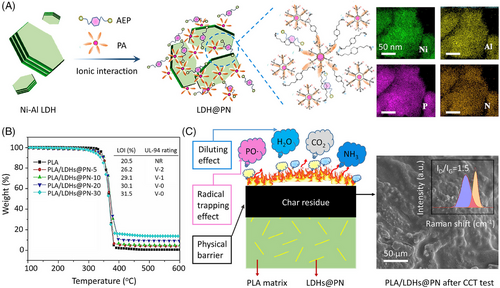
Wang et al.[96] synthesized a P–N supramolecular aggregate (PN) by reacting PA with 1,4-bis(3-aminoethyl) piperazine (AEP), and then decorated it onto NiAl-LDHs nanosheets (Figure 13A). Scanning electron microscopy mapping results show that Ni, Al, P, and N are homogeneously distributed on the surface of LDHs@PN. Incorporating 20 wt% LDHs@PN into PLA can result in a UL-94 V-0 rating and achieve a LOI of 30.1 vol.%. The T–5 wt% and char yields at 600°C of composites are higher than those of untreated PLA, indicating that LDHs@PN improves the thermal stability of PLA (Figure 13B). The synergistic effect between LDHs and PN can explain the enhanced fire retardancy of PLA. On the one hand, LDHs@PN promotes the char formation for PLA to form compact char layers. On the other hand, NiAl-LDHs and AEP decompose to release H2O and NH3, which dilute the oxygen and combustible gases; PA produces phosphorus-based radicals to capture hydrogen and oxygen radicals (Figure 13C).
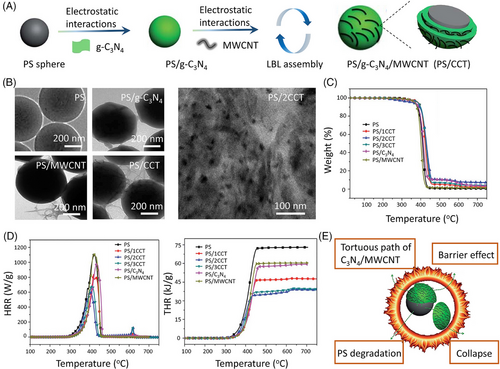
Shi et al.[97] developed an multi-wall carbon nanotube (MWCNT) and g-C3N4-coated polystyrene (PS) systems via electrostatic interactions (Figure 14A). The MWCNT/g-C3N4 bilayers exhibit the good distribution on PS spheres (Figure 14B). In contrast to the uncompact network structure formed by thermolabile MWCNTs, the bilayers can induce the construction of the “tortuous path” to endow PS with better thermal stability and fire retardancy (Figure 14E). For example, compared to untreated PS, the initial degradation temperature is increased by 15.5°C (Figure 14C). Meanwhile, the PHRR and THR values are reduced by 45% and 47%, respectively (Figure 14D).
2.6 Supramolecular fire-retardant aggregates based on synergistic interactions
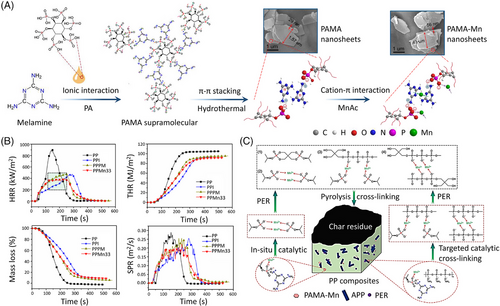
For practical use, the supramolecular fire-retardant aggregates with higher fire retardancy are always constructed by multiple interactions due to the potential synergistic effect between various fire-retardant elements or groups. In addition, multiple interactions are beneficial for the construction and maintenance of highly ordered assembly structures, which play an important role in the reinforcing effects of fillers. Recently, many studies have focused on integrating multiple interactions into a single system to pursue simultaneous improvements of polymeric materials with ordered structures. For example, Li et al.[84] prepared a melamine phytate supramolecular nanosheet flame retardant containing manganese ion (PAMA-Mn) via multiple interactions, including ionic interactions between PA and MEL, π–π stacking interactions, and cation–π stacking interactions (Figure 15A). The results show that polypropylene composites (PPMn33) filled with 4.5 wt% PAMA-Mn, 9.0 wt% ammonium polyphosphate (APP), and 4.5 wt% pentaerythritol can reach a UL-94 V-0 rating with a LOI value of 31.9%. Compared to untreated PP, PHRR and PSPR values of PPMn33 are reduced by 56% and 23%, respectively (Figure 15B). The synergistic effects of PAMA-Mn, APP, and PER account for the improved fire retardancy of PP, including the catalytic crosslinking and carbonization effects (Figure 15C).
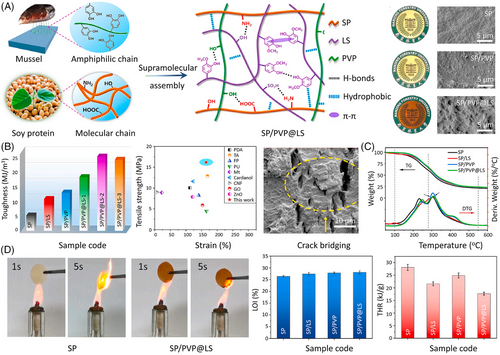
Furthermore, host–guest interactions based on hydrophobic effects also occupy a pivotal position. The hydrophobic interaction is a reversible effect formed by two or more nonpolar molecules or groups. As the main body in host–guest interactions, hydrophobic molecules in an aqueous solution tend to aggregate to minimize their contact with water in dynamic equilibrium. For example, inspired by the supramolecular chemistry of mussels, Li et al.[98] fabricated a high-performance soyprotein (SP)-based film by introducing polyvinylpyrrolidone (PVP)-modified lignosulfonate (LS) into the system (Figure 16A). This interconnected cross-linking SP/PVP@LS network is formed based on hydrophobic interactions, π–π interactions, and hydrogen bonding. Because of the synergistic effect of multiple interactions, the tensile toughness and strength of the resultant composite are improved up to 23.5 MJ/m3 and 16.2 MPa, corresponding to increases of 386.5% and 111.4% relative to the untreated SP (Figure 16B). The presence of unique aromatic rings and phenolic hydroxyl groups within the PVP@LS structure endow SP with improved thermal stability and fire retardancy (a LOI of 28.1%). The PHRR and THR of the SP/PVP@LS film are 31.1% and 37.0% lower than those of untreated SP, respectively (Figure 16D).
3 COMPARISON OF FIRE RETARDANCY AND MECHANICAL PERFORMANCES
Tables 1 and 2 compare the contribution of supramolecular fire retardants to fire retardancy and mechanical properties of typical thermoplastic polymer composites, including PC and TPU. Overall, supramolecular fire retardants based on ligand coordination, hydrogen bonding, and ionic interactions present improvements in terms of both the PHRR/THR/TSR reductions and the increase in tensile strength (σt). The high processing temperature (around 220°C) of PC requires the high thermal stability of supramolecular fire retardants. Recently, various supramolecular fire retardants based on ligand coordination are developed for PC systems, such as sSEBS-Na, sSEBS-Zn, sSEBS-Ce,[52] Zr-BDC, Ce-HPP,[51] Ce-(DOPO)3,[99] and Ce-(DPA)3.[55]
| Supramolecular interaction | Modifier | Matrix | Contents (wt%) | ∆PHRR (%) | ∆THR (%) | ∆TSR (%) | ∆T–5 wt% (°C) | ∆Char-TG | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Ligand coordination interaction | sSEBS-Na | PC | 1.5 | −31.7 | −6.2 | −7.4 | −49.0 | +2.3% | [52] |
| sSEBS-Zn | PC | 1.5 | −43.5 | −14.2 | −17.2 | −37.0 | −12.0% | [52] | |
| sSEBS-Ce | PC | 1.5 | −52.5 | −44.8 | −20.3 | −14.0 | −2.8% | [52] | |
| Zr-BDC | PC | 2.0 | −36.2 | −17.8 | −17.1 | +10.0 | −2.5% | [51] | |
| Ce-HPP | PC | 2.0 | −35.7 | −5.1 | −4.9 | −1.0 | +11.7% | [51] | |
| Ce-(DOPO)3 | PC | 2.0 | −43.1 | −20.6 | −16.1 | +30.0 | +14.0% | [99] | |
| Ce-(DPA)3 | PC | 3.0 | −54.9 | −30.3 | −23.9 | −21.0 | +15.0% | [55] | |
| Ionic interaction | α-ZrP | TPU | 5.0 | −35.3 | −7.0 | −12.9 | −1.6 | 1.1 times | [37] |
| f-MoS2 | TPU | 2.0 | −25.3 | −45.4 | – | −9.8 | +62% | [100] | |
| ZnO-TA | TPU | 4.0 | −22.7 | −8.9 | −16.1 | −3.0 | −3 | [101] | |
| Fe2O3-TA | TPU | 4.0 | −14.1 | −11.8 | −8.7 | −3.5 | 1.1 times | [100] | |
| Co3O4-TA | TPU | 4.0 | 19.3 | −9.4 | −22.2 | −21.0 | +44.5 | [100] | |
| PDDA/MXene | TPU | 3.0 | −50.0 | −15.8 | −47.0 | −22.0 | 4.1 times | [102] | |
| CTAB/MXene | TPU | 2.0 | −51.2 | −2.9 | −6.1 | – | – | [103] | |
| TBPC/MXene | TPU | 2.0 | −52.2 | −1.5 | −0 | – | – | [103] | |
| Hydrogen bonding interaction | rGO/MXene | TPU | 2.0 | −18.3 | −29.1 | −54.0 | – | 21 times | [104] |
| PPPA/MXene | TPU | 1.0 | −24.5 | −32.6 | −54.4 | – | – | [105] | |
| MCA | TPU | 5.0 | −15.8 | −3.6 | −4.0 | +3.3 | +28% | [37] | |
| MCA/MXene | TPU | 3.0 | −40.0 | – | – | −15.0 | 3 times | [38] | |
| APP@CS/MXene | TPU | 9.5 | −77.8 | −73.0 | −77.3 | +15.3 | 4.1 times | [107] | |
| ZrAMP/MXene | TPU | 2.0 | −63.2 | −59.3 | −52.0 | −12.0 | 4.3 times | [43] | |
| DOPO-HQ/MXene | TPU | 2.0 | −27.3 | −17.3 | −43.8 | +2.5 | 1.4 times | [108] | |
| P-HBPSi@GO | TPU | 2.0 | −63.5 | −20.9 | −36.4 | −43.3 | 1.1 times | [106] |
- Abbreviations: APP, ammonium polyphosphate; GO, graphene oxide; MCA, melamine cyanurate; PC, polycarbonate; PHRR, peak heat release rate; rGO, reduced graphene oxide; sSEBS, sulfonated styrene–ethylene–butylene–styrene; THR, total heat release; TSR, total smoke release; ∆T–5 wt%, change of initial degradation temperature; ∆Char-TG, change of char residue in thermo-gravimetric test; Ce-HPP, cerium phenylphosphonate; DOPO, 9, 10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide; CTAB, cetyltrimethyl ammonium bromide; TBPC, tetrabutyl phosphine chloride.
| Supramolecular interaction | Modifier | Matrix | Contents (wt%) | Δσt (%) | Δεb (%) | ΔE (%) | Δτ (%) | Δδ (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Ligand coordination interaction | sSEBS-Na | PC | 1.5 | +0.2 | +115.6 | – | +141.1 | +32.1 | [52] |
| sSEBS-Zn | PC | 1.5 | −1.8 | −25.8 | – | −27.7 | −21.1 | [52] | |
| sSEBS-Ce | PC | 1.5 | −1.0 | +115.4 | – | +135.8 | +39.7 | [52] | |
| Zr-BDC | PC | 2.0 | +2.7 | −7.1 | – | −4.3 | – | [51] | |
| Ce-HPP | PC | 2.0 | −5.5 | −54.0 | – | −56.5 | – | [51] | |
| Ce-(DOPO)3 | PC | 2.0 | −1.7 | −50.9 | – | −51.8 | – | [99] | |
| Ce-(DPA)3 | PC | 3.0 | −5.5 | +59.4 | −1.1 | +50.7 | +19.7 | [55] | |
| Ionic interaction | α-ZrP | TPU | 5.0 | +31 | +3.0 | – | +29.4 | – | [37] |
| f-MoS2 | TPU | 2.0 | +40.9 | −58.9 | – | −42.3 | – | [101] | |
| ZnO-TA | TPU | 4.0 | +77.4 | +8.4 | – | +94.0 | – | [100] | |
| Fe2O3-TA | TPU | 4.0 | +53.8 | −1.1 | – | +53.0 | – | [100] | |
| Co3O4-TA | TPU | 4.0 | +80.5 | +1.6 | – | +75.0 | – | [100] | |
| PDDA/MXene | TPU | 3.0 | +31.2 | −30.3 | – | −8.5 | – | [102] | |
| CTAB/MXene | TPU | 2.0 | +5.5 | +3.7 | – | +7.4 | – | [103] | |
| TBPC/MXene | TPU | 2.0 | +12.2 | +6.8 | – | +19.8 | – | [103] | |
| Hydrogen bonding interaction | rGO/MXene | TPU | 2.0 | – | – | – | – | – | [104] |
| PPPA/MXene | TPU | 1.0 | – | – | – | – | – | [105] | |
| MCA | TPU | 5.0 | +8.3 | −3.2 | – | +2.8 | – | [37] | |
| MCA/MXene | TPU | 3.0 | +47.5 | +5.4 | – | +56.0 | – | [38] | |
| APP@CS/MXene | TPU | 9.5 | – | – | – | – | – | [107] | |
| ZrAMP/MXene | TPU | 2.0 | +23.0 | +37.3 | +41.6 | +88.1 | – | [43] | |
| DOPO-HQ/MXene | TPU | 2.0 | +32.8 | +10.4 | – | +56.8 | – | [108] | |
| P-HBPSi@GO | TPU | 2.0 | −21.6 | −6.6 | – | −22.0 | – | [106] |
- Note: Δσt, Δεb, ΔE, Δτ, and Δδ refer to the change of tensile strength, the change of break strain, the change of tensile modulus, the change of tensile toughness, and the change of impact strength, respectively.
- Abbreviations: APP, ammonium polyphosphate; GO, graphene oxide; MCA, melamine cyanurate; PC, polycarbonate; rGO, reduced graphene oxide; sSEBS, sulfonated styrene–ethylene–butylene–styrene; TPU, thermoplastic polyurethane.
As shown in Table 1, a relatively low content (≤3.0 wt.%) of supramolecular fire retardants enables PC to show an obvious PHRR reduction, ranging from 31.7% to 54.9%. In addition, these fire retardants seem to be effective in reducing the TSR of PC. For instance, only 1.5 wt.% sSEBS-Ce decreases the TSR of PC by 20.3%.[52] It is worth noting that the effects of supramolecular fire retardants on the thermal stability and mechanical properties of PC depend on their structure and groups. For example, the sSEBS-containing fire retardants show negligible effect on the tensile strength of PC (Table 2), and adverse effect on the ∆T–5 wt%, due to the pyrolysis of ionomers before PC and the potential catalytic degradation effect of transition metals on PC.[52] Furthermore, the ∆Char-TG of PC/sSEBS-Ce is much lower than samples containing Ce-HPP, Ce-(DOPO)3, and Ce-(DPA)3, indicating that the structure of ligand plays an important role in the char-forming performance of PC composites.
In general, the main chains of TPU are mostly formed by reacting diisocyanates with hydroxyls or amino-terminated polyols. The H-bonding interactions between TPU chains can serve as cross-linkers to increase the cross-link density of the hard domain, which plays an important role in enhancing the mechanical strength of TPU.[43] Therefore, to improve the tensile strength of TPU, various supramolecular fire retardants with hydroxyls or amino groups are prepared via hydrogen bonding or ionic interactions.[100-105] In addition, the resultant H-bonding network endows TPU composites with good flame retardancy. For instance, 4.0 wt.% ZnO-TA is reported to decrease the PHRR of TPU by 22.7%, accompanied by a 16.1% reduction in TSR and a 77.4% increase in tensile strength.[100] In addition, the addition of 2.0 wt.% ZrAMP/MXene reduces the PHRR and TSR of TPU by 63.2% and 59.3%, respectively.[43] However, compared to untreated TPU, the presence of an H-bonding network cannot efficiently increase the ∆T–5 wt% of TPU composites due to the sensitivity of H-bonds to heat. Furthermore, the catalytic degradation effect of transition metals or functional groups (e.g., P-OH) of fire retardant on TPU also brings thermal disadvantages.[37, 100, 106] Therefore, to simultaneously improve the fire retardancy and thermal stability of polymeric materials, regulation of the noncovalent interactions between polymer chains and fire retardants is of great importance.
By comparing different types of supramolecular flame retardants, their advantages and disadvantages differ. For example, supramolecular flame retardants based on H-bonding interaction feature two merits. The first advantage is their good compatibility with certain polymers containing H-bond donors or acceptors (e.g., TPU), which can simultaneously improve the flame retardancy and mechanical properties (tensile strength or toughness) of the matrix. The second advantage is ease of processing for large-scale production. However, due to its dynamic and reversible properties, H-bonds are generally sensitive to humidity and heat, leading to the structural damage of supramolecular flame retardants.
Supramolecular flame retardants based on ligand–coordination interaction feature three major advantages. The first advantage is their high flame retardancy, due to the synergistic flame-retardant effects between fire-retardant ligands (e.g., DOPO) and transition metal catalysts. The second advantage is that there are abundant sources of ligands containing different fire-retardant elements (e.g., P, N, Si, S) and transition metal compounds (e.g., Zr4+, Fe3+, Cu2+). The last merit is their good thermal stability, which is suitable for the polymers with high processing temperature. Despite this, the major disadvantage is that the synthetic routes of most fire-retardant ligands are complicated and of high cost.
Supramolecular flame retardants based on host–guest interaction involve two advantages. One merit is that the guest flame-retardant molecules do not leach out or migrate to the sample surface during their particle use. Another one is that it solves the problem of the low compatibility of the guest molecular with the matrix. However, there are very few types of the host species with high flame retardancy currently being developed. Another disadvantage is that the synthetic route is complex and not easy to control.
Based on π–π interactions, supramolecular flame retardants are generally rich in aromatic structures, which contributes to the char-forming effect of polymers during combustion. The compact char residue can retard heat and mass transfer between the fire zone and the unburned matrix. Another advantage is their good compatibility with certain polymers containing large amounts of aromatic rings (e.g., PC), which can improve the mechanical properties of the matrix. Nevertheless, this type of supramolecular flame retardant is often ineffective in increasing the LOI values and UL-94 ratings of polymers due to their relatively lower contents of flame-retardant elements than that of other aggregates.
Based on ionic interactions interaction, the synthetic routes of most supramolecular flame retardants are facile and highly effective. In addition, various negatively charged 2D materials (e.g., MXene and GO) can be employed as the source of raw materials to develop flame retardants with heterostructure. This type of supramolecular flame retardants can combine the barrier effect of 2D materials and the catalytic effect of the flame-retardant surface modifiers. However, these aggregates usually exhibit unsatisfactory compatibility with the matrix than those of supramolecular flame retardants based on H-bonding and π–π interactions.
4 FLAME RETARDANT MECHANISMS
Supramolecular interactions play an important part in the construction of the different categories of flame retardants, which combine two or more types of flame-retardant chemical species. Generally, the flame-retardant mechanisms of these supramolecular flame retardants vary in a wide range mainly depending on three factors: (1) the content and the type of flame-retardant elements/groups in their chemical structure, (2) the reactivity (radical quenching, catalytic crosslinking, or catalytic carbonization effects), and (3) the thermal degradation behavior. In this regard, supramolecular interactions may affect the thermal degradation behavior of flame retardants to some degree. However, polymeric materials usually burn vigorously upon ignition, leading to the fast thermal degradation of the flame-retardant groups. The combustion process of polymeric materials involves very complicated physical and chemical reactions, which mainly take place in the gas phase, the condensed phase, and both phases. Therefore, the effects of supramolecular interactions on the flame-retardant mechanism are insignificant, and always being neglected by researchers.
Figure 17 illustrates the possible modes of action for the supramolecular flame retardants reported so far. The radical quenching effects and diluting effects are involved in the gas phase. For example, phosphorus-containing compounds can release phosphate radicals (e.g., PO·, PO2·, HPO·) to exert a radical quenching effect or a flame inhibition effect.[109] In addition, the inert gases (e.g., NH3, C1N4, C2N3, C3N2, C3N3, and C3N4) generated by the degradation of MEL-based supramolecular flame retardants (e.g., MCA@ZrP) can dilute oxygen and flammable gases, thus reducing the intensity of fire.[37]
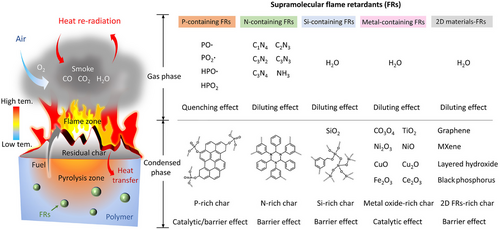
The condensed-phase mechanism mainly relates to the catalyzing cross-linking catalyzing carbonization, and physical barrier effects. For example, the phosphoric derivatives formed during thermal degradation can catalyze the cross-linking and the carbonization of the polymer matrix via dehydration and cyclization reactions.[92] For Si-containing flame retardants, the SiO2 in situ formed by Si-containing synergists can enhance the structural compactness of the char residue.[110] In addition, metal oxides (e.g., TiO2, Fe2O3, Ce2O3) have been reported to show an ability to catalyze carbonization of the polymer matrix, thus improving the char yields.[100] Furthermore, 2D materials (e.g., graphene, layered hydroxide, MXene) can serve as physical barriers and form a tortuous path within the polymer matrix, thus retarding heat and mass transfer between the combustion zone and the unburned matrix.[111]
5 CONCLUSIONS, KEY CHALLENGES, AND FUTURE PERSPECTIVES
5.1 Conclusions
Supramolecular aggregates have been intensively employed to create fire retardants and reinforcing agents for various polymers. Compared to conventional fire retardants, supramolecular aggregates can simultaneously reduce the flammability (i.e., reducing heat/smoke release), and improve the mechanical properties of polymeric materials. Herein, we discuss the recent remarkable advances in fire-retardant and high-strength/toughness polymers based on typical supramolecular interactions, including hydrogen bonding, ligand coordination, host–guest interactions, π–π interactions, ionic interactions, and synergistic interactions of multiple forces. In addition, the related mechanisms, design strategies, properties, structure–property relationships, potential applications, and challenges have been reviewed.
These multiple interactions enable the supramolecular aggregates to form abundant adjustable chemical structures. For instance, based on ligand–coordination interaction, ligands containing fire-retardant elements (such as phosphorus, nitrogen, silicon, or sulfur elements) such as PA can be easily employed to react with various transition metal (such as Zr4+, Fe3+, and Cu2+) to synthesize supramolecular fire retardants. In addition, based on the hydrogen-bonding effect, fire-retardant molecular or polymers rich in hydroxyl/amino groups (such as MEL, cyanuric acid, and TA) are always used to construct supramolecular aggregates. Furthermore, based on ionic interactions, the combination of fire retardants with other synergists (e.g., 2D nanomaterials) also represents a promising approach for creating highly effective fire retardants. The 2D nanomaterials (such as MXene, graphene, and LDH) mainly work in the condensed phase by exerting the physical barrier, catalytic carbonization, “tortuous path”, and volatile absorption effects. Particularly, graphene consists of multiple aromatic regions and has been one of the representative raw materials for constructing fire retardant via π–π interaction. In summary, supramolecular fire-retardant aggregates have demonstrated enormous potential for fire retardancy and reinforcing applications so far, despite some challenges given below for its industrial applications in the future.
5.2 Key challenges
Due to the strong demand for high-performance polymers in many fields with fire-retardant requirements, studies on developing fire retardants based on supramolecular interactions play an important role in the progress of our society. However, the development of high-performance polymers with good fire-retardancy and mechanical properties via supramolecular chemistry is still in the developing stage (Figure 18).
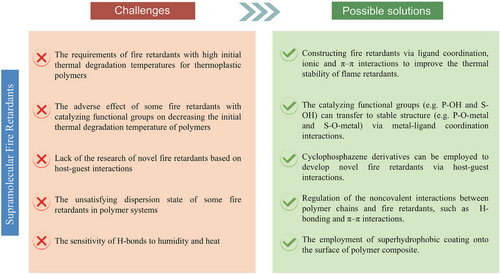
First, fire retardants with high initial thermal degradation temperatures are always required for thermoplastic polymers (such as PP, PC, and nylon 66), due to their high processing temperature. However, the different bond energies among various noncovalent interactions lead to the various thermal stability of fire retardants. For example, as the metal–ligand coordination bonds are generally stronger than H-bonds, fire retardants based on ligand coordination interactions show higher initial thermal degradation temperature and char yields than other types. Therefore, to meet the different demands for versatile materials, there is an urgent need to develop more fire retardants with suitable thermal features.
Second, fire retardants with catalyzing functional groups (such as APP) always decrease the initial thermal degradation temperature of polymers, demonstrating the adverse effect on the thermal stability of the matrix. For practical applications, the fire-retardant groups within the structure of supramolecular aggregates need to be regulated
Third, except for CD-based derivatives, there are few examples available in the literature associated with the research of novel fire retardants based on host–guest interactions, yet it still has a non-negligible value in some studies of preparing strong and tough polymers. Currently, the supramolecular assembly of cyclophosphazene derivatives has attracted particular attention in the inclusion material field. The phosphorus nitrogen backbone of the phosphazene ring is also suitable for constructing fire retardants. Therefore, for developing novel fire retardants based on the host–guest interaction, there remains a huge progress to be made.
Fourth, supramolecular interactions between fire retardants and polymer chains play an important role in enhancing the mechanical properties of the matrix. Based on the energy dissipative mechanism, the polymer networks with better uniformity or higher crosslinking degree are beneficial to dissipative energies upon mechanical loading. Therefore, selecting suitable chemical structures and groups for synthesizing fire retardants is important for improving their dispersion state in polymer systems.
Fifth, the reversibility of noncovalent bonds like H-bonds is favorable for the healability and recyclability of polymer composites. However, due to its dynamic and reversible properties, H-bonds are generally sensitive to humidity and heat. As a result, enhancement of the stability via the H-bonding effect is not thermally stable. The negative effect of the noncovalent bonds on the stability of polymer composites is also a critical aspect to be addressed.
5.3 Future perspectives
In response to the above-mentioned key challenges associated with supramolecular fire retardants, some possible solutions are proposed as follows (Figure 18).
To obtain fire retardants with satisfying thermal stability, a promising method is constructing fire retardants via ligand coordination and ionic and π–π interactions. For instance, thermally stable inorganic compounds can be immobilized onto GO surfaces to increase the thermal stability of GO based on π–π interactions. During combustion, these immobilized inorganic materials can prevent GO from thermal degradation by retarding heat and oxygen transfer.
To mitigate the adverse effect of fire retardant on the initial thermal degradation temperature of the matrix, the catalyzing functional groups (e.g., P–OH and S–OH) can transfer to stable structure (e.g., P–O–metal and S–O–metal) via metal–ligand coordination interactions. Alternatively, silicone or boron-containing groups can be selected to construct fire retardant due to its good thermal stability.
With respect to the limited options of host molecules, cyclophosphazene derivatives can be employed to develop novel fire retardants via host–guest interactions. The high contents of phosphorus and nitrogen in its backbone enable it as a potential effective fire retardant for various polymeric materials, due to the synergistic effect between phosphorus and nitrogen.
Regarding the compatibility and dispersion state of supramolecular fire retardants within the matrix, regulation of the noncovalent interactions between polymer chains and fire retardants is of great importance. For TPU, H-bonding plays a decisive role in improving the compatibility between fire retardant and matrix. By contrast, π–π interaction plays a decisive role in fire-retardant PC composites.
For the sensitivity of H-bonds to humidity and heat, the H-bond crosslinking polymer composited can be surface coated by the superhydrophobic coating, such as polydimethylsiloxane and silica nanoparticles. These silica-containing coatings can endow the polymer with humidity and thermal resistance properties.
ACKNOWLEDGMENTS
This work was financially supported by the National Natural Science Foundation of China (grant no. 52303088), the Natural Science Foundation of Shandong Province (grant no. ZR2023QB108), the Natural Science Foundation of Qingdao Municipality (grant no. 23-2-1-84-zyyd-jch), and the Australian Research Council (nos. DP190102992, FT190100188, DP240102628, and DP240102728).
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest.
Biographies

Lei Liu received his PhD degree from Tongji University, China, in 2018 under the supervision of Prof. Zhengzhou Wang. He worked with Zhejiang A&F University as a postdoctoral research fellow from 2019 to 2021, and has been co-supervised by Prof. Pingan Song. After completion of a 2-year postdoctoral position, he joined Qingdao University of Science and Technology in 2021 as an assistant professor. His research interest is in the design and preparation of advanced fire-retardant polymers.

Pingan Song received his PhD from Zhejiang University in 2009. He is currently a full professor and ARC Future Fellow at the School of Agriculture and Environmental Science and Centre for Future Materials of University of Southern Queensland, Australia. His research interests involve fire retardants and fire-retardant coatings, fire-warning sensors, and polymers and polymer composites as well as their structure–property correlations. In addition to one book and two book chapters, to date he has published over 250 peer-reviewed journal articles, which have received over 16,000 times of citations, leading to an H-index of 77 (Google Scholar). He is a Clarivate's Highly Cited Researcher (2023).




