Recent advances in AIEgen-based crystalline porous materials for chemical sensing
Abstract
Aggregation-induced emission-based luminogens (AIEgens) have aroused enormous interest due to their unique high fluorescence in a condensed state. To further explore their potential applications, such as chemical monitoring, immobilization of AIE molecules has been widely studied with a variety of supports. Crystalline porous materials, such as metal-organic frameworks, covalent organic frameworks, hydrogen-bonded organic framework, and organic cages, demonstrate well-controlled structures, large surface areas, and promising stabilities, thus providing a perfect platform for AIE agents loading. Outstanding chemical sensing performances are achieved based on these AIE-active crystalline porous materials, such as high sensitivity, short response time, selective identification, and high recyclability, which provide a new alternative to readily detect various hazardous molecules. Furthermore, precise structures of AIEgen-based crystalline porous materials offer an easy way to investigate detection mechanisms. This mini-review will provide a brief overview of AIEgen-based crystalline porous materials for detection and then address how to improve sensing performances remarkably.
Abbreviations
-
- 1D
-
- one-dimensional
-
- 2,4-DNP
-
- 2,4-dinitrophenol
-
- 2D
-
- two-dimensional
-
- 3D
-
- three-dimensional
-
- 5MR-EHCs
-
- five-membered-ring energetic heterocyclic compounds
-
- ACQ
-
- aggregation-caused quenching
-
- ADA
-
- anthracene 9,10-diacrylate acid
-
- AIE
-
- aggregation-induced emission
-
- AIEgens
-
- AIE luminogens
-
- BPy
-
- 4,4′-bypyridine
-
- CB
-
- conduction band
-
- CIL
-
- carbamate ionic liquid
-
- COFs
-
- covalent organic frameworks
-
- CTC
-
- chlortetracycline
-
- DNT
-
- 2,4-dinitrotoluene
-
- DPEB
-
- 4,4′-(2,2-diphenylethene-1,1-diyl)dibenzoic acid
-
- DPMF
-
- 4,4′-((9H-fluoren-9-ylidene)methylene)dipyridine
-
- F-CTFs
-
- fluorescent covalent triazine frameworks
-
- FRET
-
- fluorescence resonance energy transfer
-
- H4TCPE
-
- tetrakis(4-carboxyphenyl)ethylene
-
- H4TCPP
-
- 2,3,5,6-tetrakis(4-carboxyphenyl)pyrazine
-
- H4TCPPE
-
- tetrakis[4-(4-carboxyphenyl)phenyl]-ethene
-
- HOF
-
- hydrogen-bonded organic framework
-
- HTABDC
-
- 4,4′-((Z,Z)-1,4-diphenylbuta-1,3-diene-1,4-diyl)bis(2-hydroxybenzoic acid)
-
- KSV
-
- Stern–Volmer quenching constant
-
- LMCT
-
- ligand-to-metal charge transfer
-
- LMOF
-
- luminescent MOF
-
- LOD
-
- limits of detection
-
- LUMO
-
- lowest unoccupied molecular orbitals
-
- MOFs
-
- metal-organic frameworks
-
- NACs
-
- nitroaromatic compounds
-
- NB
-
- nitrobenzene
-
- NF
-
- nitrofuran
-
- NTO
-
- 5-nitro-2,4-dihydro-3H-1,2,4-triazole-3-one
-
- PET
-
- photoinduced electron transfer
-
- p-NP
-
- p-nitrophenol
-
- POSS
-
- polyhedral oligomeric silsesquioxane
-
- PS
-
- polystyrene
-
- Py3P
-
- tris(2-pyridyl)phosphine
-
- QY
-
- quantum yield
-
- RIM
-
- restriction of intramolecular motion
-
- TABD-COOH
-
- 4,4′-((Z,Z)-1,4-diphenylbuta-1,3-diene-1,4-diyl)dibenzoic acid
-
- TCPE
-
- tetrakis(4-carboxyphenyl)ethylene
-
- TFBE
-
- 1,1,2,2-tetrakis(4-formyl-(1,1′-biphenyl))-ethane
-
- TNP
-
- trinitrophenol
-
- TPE
-
- tetraphenylethylene
-
- tppe
-
- 1,1,2,2-tetrakis(4-(pyridin-4-yl)phenyl)ethane
-
- VB
-
- valence band
INTRODUCTION
Faced with a variety of serious issues associated with the environment, energy, and health, people are attempting to develop diverse sensing devices for efficient detection of analytes. Due to the in situ operability, high sensitivity, excellent selectivity, and satisfactory accuracy, luminescent materials are one of the potential candidates for constructing optical sensors for the fast detection of environmental changes. Despite great emission effect in dilute solutions, most traditional solid luminescent materials demonstrated weak luminescence, which significantly limited their applications. It is known that aggregation of luminescent molecules can cause quenching phenomenon, that is, aggregation-caused quenching (ACQ), which could be originated from the stacking of large planar π-systems in luminophores.
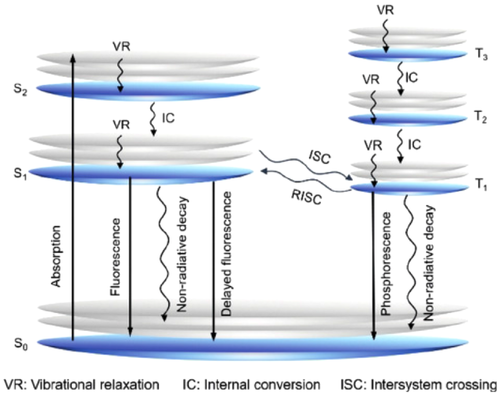
Opposite to conventional ACQ effect, the aggregation-induced emission (AIE) phenomenon was reported by Tang group in 2001.[1] AIE moieties exhibited fluorescence emission behaviors contrary to conventional ACQ molecules, which means that AIE molecules present distinct emissions in aggregated or solid-state, albeit low or insignificant emissions in dilute solutions. Flexible rotated or vibrated molecular moieties are generally necessary for AIE luminogens (AIEgens), such as tetraphenylethylene (TPE), hexaphenylsilol, multiaryl-substituted pyrrole, tetraaryl-substitute 1,3-butadienes, hexaphenylbenzene, and so on.[2] Some mechanisms have been proposed to explain the AIE phenomenon. Among them, restriction of intramolecular motion (RIM), including restriction of intramolecular vibration and restriction of intramolecular rotation, is the most widely accepted.[3, 4] Based on Jablonski diagram, the dissipation of excited state energy could took place via both radiative emission process and nonradiative decay process (Figure 1).[5] In the theory of RIM, AIEgens can deplete energy of the excited state upon nonradiative emission process through intramolecular motions (including free rotation, vibration, movement, etc.) in the unrestricted state, where the interactions between AIEgen molecules have been restricted. When the nonradiative pathways are hindered becuase of RIM in the aggregated state, AIEgens trace the radiative pathway to spread the absorbed energy and present intense fluorescence emissions. Meanwhile, several other plausible mechanisms are also available, such as construction of J-aggregates or excimers, conformational planarization, inhibition of twisted intermolecular charge transfer, E/Z isomerization, excited-state intramolecular proton transfer, and excited-state interlayer proton shift mechanism.[6-11] Furthermore, in some instances, enhanced emissions in aggregation states are attributed to multiple mechanisms with a synergetic effect.[12]
With high surface area, uniform accessible channels and well-modified structures, crystalline porous materials, including metal-organic frameworks (MOFs),[12-14] covalent organic frameworks (COFs),[15, 16] hydrogen-bonded organic framework (HOF),[17, 18] and organic cages,[19, 20] have attracted wide interest in many different areas, such as catalysis, gas separation, electricity, and life science. Recently, these advanced porous materials have attracted a great deal of interest in fluorescent behavior by virtue of their unique designable structures and plentiful organic moieties. In the early age, most of reported luminescent porous materials were constructed with conjugated planar molecules, for example, pyrene, perylene, and other polycyclic aromatic hydrocarbons. However, the possible strong intramolecular π–π interactions in porous materials typically lead to the fluorescence quenching. In other words, the ACQ effect generally happened among those conjugated fluorescent molecules. For this reason, conventional porous materials usually display very low fluorescence emissions, especially two-dimensional (2D) structures, which impedes the development of these porous materials with the fascinating practical applications in fluorescence field. Unlike the ACQ materials, AIEgens show weak fluorescence emissions in dilute solutions but high fluorescence intensities in the solid or aggregated state. Hence, porous materials structured with AIE moieties are potential for high fluorescence emission. What is more, compared to molecular AIEgens with the random arrangement in solid-state, AIEgens could be confined with coordinate or covalent bonds in porous materials, which means the limitation of intramolecular motion and the increasing of the emission efficiency. Besides, definite structures of AIEgen-based crystalline porous materials tender proper platform to explore AIE mechanism. Precise control over photophysical characteristics could also be achieved in porous materials via utilizing predesigned building units. Because of these particular advantages, AIEgen-based porous materials have received increasing concerns in the past few years. Several reviews about AIEgen-based porous materials are available in recent years, for example, Feng and his group reviewed AIEgen-based luminescent MOFs and COFs in 2017,[21] and Roy et al. have reported AIEgens-inspired smart materials. However, no specialized review was available about the chemical sensing of crystalline porous materials.[22] This article summarizes the recent research progress of the fluorescence properties of AIEgen-based crystalline porous materials, discusses the relative AIE mechanism, and highlights their recent applications in molecular recognition.
AIEGEN-BASED CRYSTALLINE POROUS MATERIALS
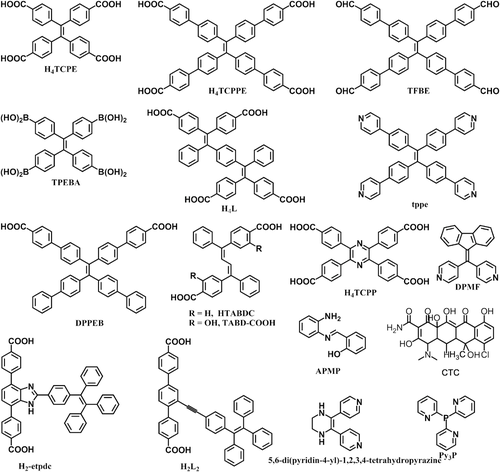
Crystalline porous materials, such as MOFs, COFs, HOFs, and organic cages, are known for their large pore sizes, high specific surface areas, and controllable functionalities.[23, 24] Thanks to the excellent fluorescence properties, AIEgen-based crystalline porous materials have been extensively studied in many areas, such as light-emitting diodes,[16] fluorescence detections,[25] enantioselective sensing,[26, 27] chemical sensing[28, 29], and many others.[2] By virtue of high sensitivity, promising selectivity, short response time, and excellent stability, AIEgen-based crystalline porous material provides a new alternative to readily detect various analytes, including biological molecules, environmental contaminations, explosives, ionic varities, and so on. Based on the mechanism of RIM, many different AIEgen-based building units have been designed for the construction of porous materials, among which TPE derivatives were most widely employed (Figure 2). Supressing of the fast rotation of the phenyl rings and twist vibration of ethylenic C = C bond in TPE-based fragments was considered as the reason of high emission and AIE effect in the rigid frameworks.
Up to now, three primary strategies for introducing AIEgens into porous materials are available. (1) AIE moieties could be designed as coupling units and located in the backbone of the frameworks. The fluorescence efficiency was largely increased as a result of the high comfiement of the phenyl rings, but the exposure of AIE sites was limited.[30] (2) AIE fragments were decorated as the pendant groups of building blocks and were anchored on the channel's walls of the resulted frameworks.[18] Large exposure and easy accessibility of TPE groups were obtained and a sensitive response could be achieved through this strategy. However, the relative research was limited.[31] (3) Small molecular AIE agent could be incorporated into the predesigned frameworks via weak interactions. A greatly expanded selection of AIE agent could be employed in this strategy and the fluorescence properties are easily adjustable, but rare examples are available, possibly due to the insufficient stabilities.
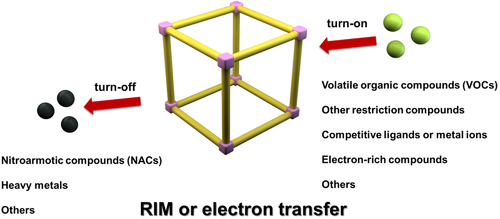
The commonly proposed detection mechanism of TPE-based porous materials is consistent with that of the traditional AIE-based sensors. There are two general kinds of sensors (“turn-off” and “turn-on,” Figure 3), which is subject to the interaction with the guest analytes, including electron and/or energy transfer, suppression of motions, and so on. Electron-rich analyte or restriction of the motions can shift or “turn-on” host emissions, while electron-deficient analyte or relaxation of the motions can “turn-off” host emissions.[3] Furthermore, the specific channels of the porous structures can adsorb certain analytes, which affords a new method for selective detection with high sensitivity. Based on these detection mechanisms, numerous AIEgen-based porous materials have been developed for chemical sensing (Table 1).
| Mode | Sensor | Analyte | KSV (M−1) | LOD | |
|---|---|---|---|---|---|
| Turn off | [Zn2(TCPPE)] | MOF | NB, DNT | ||
| UiO-68-mtpdc/etpdc | MOF | TNP | 2.8 × 104 | ||
| Zn2(H2L)2(Bpy)2(H2O)3·H2O | MOF | TNP | 1.36 × 104 | 110 ppb 0.49 μM | |
| [Zn4O(BCTPE)3] | MOF | TNP | 0.1 ppm | ||
| [Zn4O(BCTPE)3] | MOF | Nitrofurazone | 0.1 ppm | ||
| [Zn4O(BCTPE)3] | MOF | Metronidazole | 0.6 ppm | ||
| {Co(TCPP)0.5(Tipa)}n | MOF | TNP | 5.5 × 104 | 0.326 μM | |
| {[Cu(DPMF)(CN)]·DMAC·MeOH}n | MOF | TNP | 4.03 × 104 | 1.27 × 103 mM−1 | |
| LMOF-263 | MOF | Hg2+ | 459 446 | 3.3 ppb | |
| LMOF-263 | MOF | Pb2+ | 55 017 | 19.7 ppb | |
| JLU-MOF60 | MOF | Cr2O72− | 5.91 × 104 | 0.38 μM | |
| LMOF-2 | MOF | Cu2+ | 550 pM | ||
| {Co(TCPP)0.5(Tipa)}n | MOF | Fe3+ | 1.8 × 105 | 0.098 μM | |
| POSS-T8A | HOF | Cu2+ | 30 305 | ||
| LMOF-241 | MOF | Aflatoxin B1 | 54 227 | 46 ppb | |
| TPE-Ph COF | COF | NH3 | |||
| NUS-30 | COF NS | L-phenylalanine | 13 529 | ||
| NUS-30 | COF NS | L-dopa | 15022 | ||
| F-CTF-2 | COF NS | Nitrofurazone | 1.81 × 105 | 1.75 ppb | |
| Turn on | Zn2(C30H16O8)(H2O)2·4DEF | MOF | VOC | ||
| NUS-1a | MOF | VOC | |||
| [Zn2(TCPPE)] | MOF | VOC | |||
| [(ZnCl2)2Py-TPE] | MOF | VOC | |||
| [Cd2(tppe)(bpdc)2(H2O)] | MOF | VOC | |||
| NUS-13-100% | MOF | VOC | |||
| NUS-13-100% | MOF | Polystyrene | |||
| [Cu4I4(Py3P)2]n | MOF | VOC | |||
| NUS-100 | Organic cage | VOC | |||
| NUS-101 | Organic cage | VOC | |||
| [Cu2I2(tppe)] | MOF | NH3 | |||
| LMOF-2 | MOF | Glutathione or glucose oxidase | |||
| L1-Eu MOF | MOF | Arginine | 15 nM | ||
| TABD-MOF-3 | MOF | 5MR-EHCs |
6.5 ng/cm2 4 × 10−8 M |
||
| Zn(HTABDC)(Bpy)·DMF | MOF | Al3+ | 3.73 ppb | ||
| Zn2(TCPE) | MOF | NH3 | |||
| TMU-40(Co) | MOF | 4-aminophenol | 2.9 × 107 | ||
| ADA−Mn | MOF | CO2 | |||
| Zn-BTEC | MOF | Chlortetracycline | 28 nM |
Sensors based on turn-off mechanism
“Turn-off” sensing based on AIEgen-based crystalline porous materials has been commonly employed for analysis of electron-deficient compounds, metal ions, and so on. Electron and/or energy transfer processes, such as photoinduced electron transfer (PET), fluorescence resonance energy transfer (FRET), and competitive excitation energy absorption, were often suggested to explain the “turn-off” mechanism. Meanwhile, relaxing the RIM of AIEgens might be another possible approach to “turn-off” fluorescence. However, almost no successful examples were available in crystalline porous sensors, which might be an interesting topic in this area.
Nitroaromatic compounds
Because of the increasing applications in the industry, nitroaromatic compounds (NACs) have received extensive attention for the sake of their poisonous nature and threatening explosion. The contamination of groundwater and soil by NACs may bring about many health hazards and environmental problems. As a result, the detection of NACs with fast response, high sensitivity, and selectivity is truly urgent. With strong luminescence and promising stabilities, AIEgens-based sensors have been widely studied in sensing of NACs, especially trinitrophenol (TNP) and other nitrophenols. It should be mentioned that TNP demonstrates higher concentrations in groundwater and soil and poses a more significant health threat than other NACs due to its better solubility in water and commercial availability. Moreover, on account of its higher electron affinities and lower vapor pressure (9.71 × 10−10 atm at 25 °C), it is difficult to achieve the selective identification of TNP from other NACs [32]
Several possible mechanisms have been proposed for the detection of NACs with AIEgens-based sensors, including PET, FRET, and electrostatic and/or hydrogen-bonding. In the PET theory, the lowest unoccupied molecular orbitals (LUMOs) of electron-deficient NACs located between the conduction band (CB) and valence band (VB) of the sensors, therefore, leading to the transfer of the excited electrons from the CB to the LUMO orbitals of NACs upon excitation, which accordingly leads to a quenching phenomenon. In this proposed process, the quenching efficiency increases as the LUMO energy decreases. As for FRET mechanism, effective energy transfer requires the greatly overlapping of the absorption bands of the NACs with the emission bands of the fluorophore. Furthermore, high selectivity was generally obtained for TNP, where electrostatic interaction and/or hydrogen-bonding between the Lewis basic moieties of the porous materials and highly acidic hydroxyl group of TNP may be another important factor other than the low LUMO orbital.
Liu and coworkers presented the first “turn-off” example in 2015.[33] Utilizing the TPE-derivate (tetrakis[4-(4-carboxyphenyl)phenyl]-ethene, H4TCPPE), the Zn-based MOF (Zn2(TCPPE)) with one-dimensional (1D) channels was prepared. Activated Zn2(TCPPE) demonstrated good crystalline and high surface areas (specific surface areas of 1248 m2 g−1) from complementary methods. This material exhibited strong emission at 535 nm with absolute quantum yield (QY) of 11.3%, which could be drastically quenched upon exposure to the vapors of nitrobenzene (NB) and 2,4-dinitrotoluene (DNT). However, sensitivity, selectivity, and quenching mechanism were not reported in this literature. Zang and coworkers elaborated “turn-off” detection of NACs with Tb-MOF.[32] Tb-MOF exhibited higher selectivity for TNP in comparison with other tested nitro compouds. The Stern–Volmer quenching constant (KSV) for TNP was 7.47 × 104 M−1. However, the addition of extra Tb3+ led to irreversible TNP adsorption onto Tb-MOF, which limited the recycle use of this porous material.
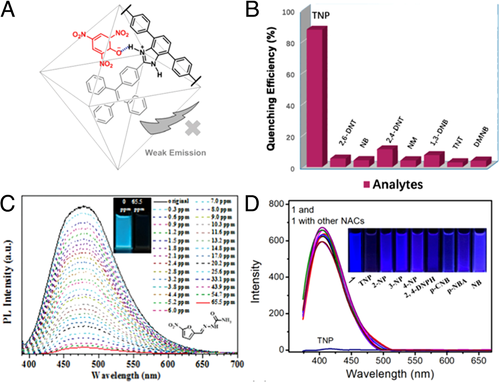
Wang and coworkers lucubrated “turn-off” detection of NACs with UiO-68-mtpdc/etpdc.[29] TPE moiety was decorated as the pendant group and incorporated into the UiO-isoreticular MOF via a mixed strut approach. UiO-68-mtpdc/etpdc emitted notable blue-green light at 490 nm with QY of 48%, which made UiO-68-mtpdc/etpdc a prospective luminescence sensor for NACs. UiO-68-mtpdc/etpdc showed remarkable sensing performance for TNP as over 50% of initial intensity was quenched even though the concentration of TNP was reduced to 40 ppm. The KSV was calculated to be 2.8 × 104 M−1 for TNP, 2.3 × 104 M−1 for 2,4-dinitrophenol (2,4-DNP), and 7.2 × 103 M−1 for p-nitrophenol (p-NP). Meanwhile, high selectivity was observed for TNP, DNP, and p-NP than other nitro aromatic compounds, which could originate from electrostatic and/or hydrogen-bonding interactions between the hydroxyl groups from nitrophenols and N-donor sites of the imidazole in the ligand (Figure 4A). Based on similar mechanism, Zn2(H2L)2(Bpy)2(H2O)3·H2O (MOF-2)[34] was utilized for selective sensing of TNP in aqueous solution with KSV of 1.36 × 104 M−1 and limit of detection (LOD) of 0.49 μM or 110 ppb. Meanwhile, high selectivity of TNP over other nitro explosives was observed, which could be attributed to different electron-withdrawing features (Figure 4B).
Based on the ditopic building block 4,4′-(1,2-diphenylethene-1,2-diyl)dibenzoic acid (H2BCTPE), Liu and coworkers reported another Zn-based MOF ([Zn4O(BCTPE)3], with QY of 64.5%)[35] with LOD of 10, 0.13, and 0.1 ppm for NB, p-NP, and TNP, respectively. Moreover, the NAC analytes were further expanded from explosive to nitro-containing antibiotics, metronidazole, and nitrofurazone (Figure 4C). The emission intensity of [Zn4O(BCTPE)3] was decreased drastically by 93% and 50% upon the presence of nitrofurazone (50 ppm) and metronidazole (50 ppm), respectively, which were discernible by the naked eye. The LODs were estimated to be 0.1 for nitrofurazone and 0.6 ppm for metronidazole, close to that of TNP (0.1 ppm). Additionally, this material can be recovered through centrifugation and subsequent washing with water.
All of the aforementioned materials were prepared based on TPE-based building blocks. Recently, some other ligands without TPE moieties were designed for AIE-MOFs, enriching the diversity of these materials. Utilizing a non-TPE-based ligand 4,4′-((9H-fluoren-9-ylidene)methylene)dipyridine (DPMF), Zhang and coworkers reported the Cu(I) based MOF {[Cu(DPMF)(CN)]·DMAC·MeOH}n[36] for luminescence detection toward TNP with KSV of 4.03 × 104 M−1 and sensitivity of 1.27 × 103 mM−1, much more sensitive than other NACs. With another non-TPE-based AIE ligand 2,3,5,6-tetrakis(4-carboxyphenyl)pyrazine (H4TCPP), {Co(TCPP)0.5(Tipa)}n was synthesized by Huang and coworkers and used for highly selective and sensitive luminescence analysis of TNP in aqueous system.[37] The KSV was calculated as 5.5 × 104 M−1 and LOD was 0.326 μM (Figure 4D). The stability of the sensor was illustrated with pH-dependent assay and recycle test, suggesting high stability with pH values varying from 3.0 to 11.0 after at least five times cycle.
In all, with high emissions and stabilities, varieties of AIE-active MOFs have been developed for sensing NACs via “turn-off” mechanism. With low LUMO energy, large overlap in spectrum as well as electrostatic and/or hydrogen-bonding, high sensitivity and selectivity of TNP was acquired with LOD as low as 0.1 ppm. The detection of other NACs, however, still waited for further exploration, possibly via tuning the CB energy, emission spectrum, or introducing supramolecular interactions. Moreover, the utilizing of other crystalline porous materials in NACs sensing was barely explored.
Heavy metal
Currently, water pollution resulting from hazardous heavy metals (Hg2+, Pb2+, Cd2+, Cr2O72−, and so on) turns out to be one of the imminent and serious environmental problems and gives rise to increasing negative impact on the living conditions and public health. Effective detection of heavy metals in water sources would provide vital protection for global ecology. On the other hand, physiologically relevant metal ions (such as Fe3+) have been involved in various cell biological functions and play critical roles in organisms, but an irregular variation of metal ions levels in biological system may cause some diseases. Thus, the sensing of these metal ions in environment or biological issues is also urgent. AIE-active luminescent MOFs (LMOFs) are considered as potential candidate as chemical sensors due to their high porosity, stability, and emissions. A series of AIEgens-based MOFs have been designed for “turn-off” monitoring a wide range of heavy metals (including Hg2+, Pb2+, Cr2O72−, Fe3+, and Cu2+) with LOD up to ppb or pM level. The “turn-off” effect was commonly contributed to competitive excitation energy absorption, resonance electron, energy transfer, or binding effect between metal ions and the functional groups on the framework.
In 2016, Li and coworkers employed the LMOF-263, which was constructed with TPE derivative of 1,1,2,2-tetrakis(4-(pyridin-4-yl)phenyl)ethane (tppe), for detection of heavy metal for the first time.[38] Demonstrating prominent stability in water, great porosity, and vigorous luminescence, LMOF-263 worked perfectly for fluorescent chemical sensor toward toxic heavy metals with extremely responsive at ppb level (3.3 ppb for Hg2+ and 19.7 ppb for Pb2+) and high selectivity (detection ratios of 167.4 for Hg2+/Ca2+ and 209.5 for Hg2+/Mg2+) (Figure 5A). The Pb2+ KSV value (55,017 M−1) was the highest among LMOFs when reported, followed by the Hg2+ KSV value (459,446 M−1).
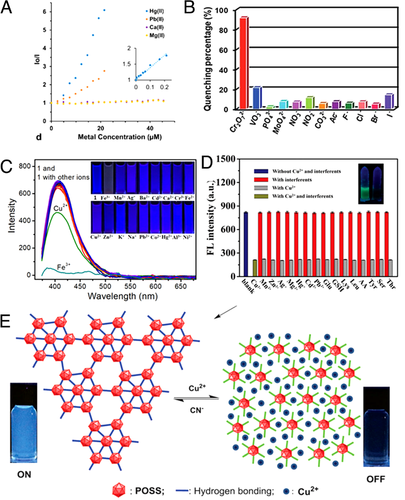
Zr-based MOFs are well known for their promising stabilities. Based on the aforementioned H4TCPP, Zr-based JLU-MOF60 was prepared with outstanding water stability in a broad pH range between 0 and 11 and Zr was connected with hydroxides in the framework.[39] Monitoring of Cr2O72− was then conducted with JLU-MOF60, showing high sensitivity with KSV value of 5.91 × 104 M−1 and LOD of 0.38 μM. Moreover, JLU-MOF60 showed superb anti-interference ability for Cr2O72− over other competing anions (Br−, NO3−, Cl−, CO32−, MoO42−, and PO43−) (Figure 5B). In addition, the quenching efficiency of JLU-MOF60 was maintained even after four cycles, revealing the commendable regeneration ability. FRET and competitive excitation energy absorption could synergistically contribute to the fluorescence quenching of JLU-MOF60 upon Cr2O72−, of competition of excitation and absorption, since of Cr2O72− presented absorption bands from 230 to 500 nm, evidently overlapping the excitation bands (350–420 nm) and the emission bands (420–500 nm) of JLU-MOF60. Moreover, Zr-bonded hydroxides in the MOF easily combined with Cr2O72−, which could promote the energy transfer process.
The aforementioned TNP-sensitive {Co(TCPP)0.5(Tipa)}n37 could also be used for monitoring Fe3+ ion in aqueous solution, with KSV of 1.8 × 105 M−1 and LOD of 0.098 μM. In the anti-interference test, Fe3+ elicited outstanding fluorescence quenching with quenching efficiency of 96%, while equal equivalent of other metal ions induced insignificant fluorescence variation aside from Cu2+ (30% quenching efficiency), indicating the high selectivity for Fe3+ among various metal ions (Figure 5C). PH-dependent emissions and recycle test were also conducted, announcing the high stability with pH ranging from 3.0 to 11.0 and good recyclability (at least five times cycle). A competitively excited energy adsorption mechanism could explain the luminescence quenching phenomenon, since the absorption spectrum of Fe3+ presents an apparent overlap with the excitation bands of MOF, whereas other metal ions do not show markable overlapping of spectral bands.
Liu and coworkers used the novel AIEgens ((E)-2-((2-aminophenylimino)methyl)phenol, APMP) and its derivatives as the guest luminescent species to prepare a series of AIE-active LMOFs.[40] Different from previously discussed structures in which AIEgens were decorated on the framework via strong covalent or coordinate bonds, AIEgens were incorporated in ZIF-8 via weak host–guest interaction. This provided a simple large-scale synthesis method of AIE-active MOF and the fluorescence features of AIE-active MOFs could be tailored through varying the substituents on APMP molecules. More interesting, the acquired LMOFs showed striking fluorescence emission 16-fold higher than original AIEgens, due to the RIM of AIEgens by the ZIF-8 frame. Furthermore, LMOF-2 was used for the detection of Cu2+. The fluorescence intensity could be dramatically quenched by Cu2+ with almost no inhibition from other metal ion or molecules, demonstrating the sensor possessing adequate selectivity for Cu2+ detection and high sensitivity with a low LOD of 550 pM (Figure 5D). Finally, the fluorescence quenching may be caused by the solid binding and fast chelation between Cu2+ and the functional groups on LMOF-2 (e.g., amidogen and oxhydryl) together with the electron or energy transfer from LMOF-2 to Cu2+.
Xu and coworkers put forward a unique “turn-off” pathway based on HOF.[41] Through condensation reaction between AIE-active 4-(1,2,2-triphenylvinyl)benzoic acid and the octa(amino)-substituted polyhedral oligomeric silsesquioxane (POSS) derivative, crystalline HOF POSS-T8A was prepared with high surface area (specific surface area of 101.9 m2 g−1) and remarkable blue-white emission both in the solid state and organic solvents. The fluorescence of POSS-T8A could be quenched with Cu2+ in different organic solvents, among which dimethyl sulfoxide showed the highest quenching efficiency (KSV = 30 305 M−1). Other transition metal ions, such as Mn2+, Co2+, Ni2+, and Fe3+, displayed low quenching effect with 10–26% efficiency, while nearly all alkaline-earth metal ions, such as Mg2+ and Ca2+ ions, did not present fluorescence quenching. Furthermore, the fluorescence could be recovered by the removal of Cu2+ upon addition of cyanide and this process could be repeated several times without considerably sacrificing the sensing activity toward Cu2+. Wide angle X-ray diffractograms revealed that POSS-T8A·Cu2+ showed entirely different patterns from that of POSS-T8A, which reverted after Cu2+ were removed by CN−. The sensitive fluorescence changes of POSS-T8A upon Cu2+ could be attributed to the decline of the RIM of TPE moieties resulting from the disruption of the HOF structure (Figure 5E).
Others
Other than previously discussed NACs and heavy metals, attempts of “turn-off” sensing have also been conducted over some other matters, such as toxins, ammonia, biomolecules, and pharmaceutical molecules, which are important in environmental monitoring, disease diagnosis, and therapy.
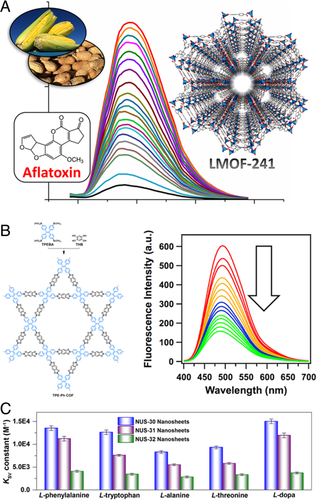
Mycotoxins are toxic substances produced by certain fungi and are capable of inducing health hazards to both humans and other animals. Li and coworkers reported the effective detection of mycotoxins by using Zn2(bpdc)2(tppe) (LMOF-241) (Figure 6A).[30] LMOF-241 exhibited green-emitting with a high QY of 92.7%, due to the RIM of tppe in rigid frameworks. High-speed and supremely sensitive spectral detection of Aflatoxin B1 could be accomplished with KSV of 54,227 M−1 and LOD of 46 ppb, which is much lower than 300 ppb that is set as the tolerant level for corn and peanut feeds for beef cattle by the U.S. Food and Drug Administration. The fluorescence quenching applied to the detection process may be attributed to electron transfer from the MOF to toxin molecules as the bottom of the LUMO (or CB) energy states of LMOF-241 locates above the LUMO energies of aflatoxin B1(AFB1) and aflatoxin B2 (AFB2).
Jiang et al. designed an AIE-COF (TPE-Ph COF) based on TPE-cored boronic acids (tetraphenylethylene-tetraboronic acid, TPEBA).[42] This COF exhibited impressive blue luminescence centered at 500 nm with QY of 32%. Based on density functional based tight binding method (DFTB) calculations, high QY of TPE-Ph COF (two- to three-fold of the model compound) came from suppression of the phenyl rotation via the connection at the monolayer level as well as the π−π stacking of the layered framework. On account of the Lewis acid−base interaction in which boronate served as Lewis acid and ammonia acting as Lewis base, TPE-Ph COF was applied to fluorescence sensing of ammonia (Figure 6B). The corresponding fluorescence quenching rate constant Kq (= KSV/τ) was as low as 6.3 × 1014 M−1 s−1 in toluene solution (2 mL) containing 0.25 mg COF, and the fluorescence intensity had a decrease of 30% in a low concentration of ammonia (1 ppm) in this case. However, no mechanism exploration was carried out in this work, and subsequent works are essential for COF-based AIE sensors.
Based on 1,1,2,2-tetrakis(4-formyl-(1,1′-biphenyl))-ethane (TFBE), Zhao and coworkers constructed a series of extremely thin 2D COF nanosheets (NUS 30−32) through the temperature-swing gas exfoliation of the crystalline COFs and their thicknesses lied in a range of 2−4 nm.[43] These 2D nanosheets with restricted AIE molecular rotors were employed for identifying amino acids and small drug molecules. KSV of L-phenylalanine detection was calculated to be 13,529, 11,225, and 4078 M−1 for NUS-30, NUS-31, and NUS-32 nanosheets, respectively, representing that the binding affinity followed the ascending order ofNUS-30 > NUS-31 > NUS-32 (Figure 6C), which was in line with the overall quantity of azine moieties in the COF networks, suggesting the critical effect of the azine moieties in the affinity with and recognizing of biomolecules. In addition, they further inspected the recognition of L-dopa, a medicine for Parkinson's disease. The KSV for L-dopa was 15,022 M−1 for NUS-30 (11,958 M−1 for NUS-30 and 3713 M−1 for NUS-30), and the apparent quenching constant Kq was estimated to be 2.13 × 1013 M−1 s−1. The biomolecular recognition may be ascribed to effective hydrogen-bonded energy transfer with amino acids. Moreover, the azine units in the COFs (NUS-30 > NUS-31 > NUS-32) could improve specific recognition, revealing that the azine units may make a significant contribution to the formation of hydrogen bonds.
Based on the same AIEgen TFBE, another series of CTF-based nanosheets (F-CTFs) were prepared for luminous detection of antibiotics (nitrofurans, NFs).[44] With F-CTF-2, ultralow LODs were witnessed as 1.75, 2.21, and 3.86 ppb for nitrofurazone, nitrofurantoin, and furazolidone, surpassing all reported fluorescent materials. Several mechanisms were contributed to the high sensitivity synergistically. First, the conspicuous overlap between absorption band of NFs with the excitation band of F-CTFs could lead to energy transfer from the F-CTFs to the NFs. Also, the LUMO level of the NFs was typically below that of the other analytes, which resulted in the more easily electron transfer between F-CTFs and the NFs. Finally, F-CTFs displayed wonderful adsorption ability, high adsorption rate, and great removal performance for NFs and the pre-enrichment from adsorption process can effectively increase the detection capability of F-CTFs toward NFs.
Sensors based on turn-on mechanism
As mentioned above, “turn-off” procedures of AIE-active porous materials have widely been employed in chemical sensing of NACs and heavy metal ions. However, as a result of the intervetion of background noise signal, sensors may encounter an inaccurate fluorescence response based on this mechanism. For “turn-on” sensors, the fluorescence emission was strengthened with a dark background or shifted significantly and the sensing performances, including selectivity, sensitivity, and detection limit, were improved accordingly.[45] “Turn-on” sensors were generally based on those frameworks with low or nonemission, which might raise partially motion of AIE moieties, ligand-to-metal charge transfer (LMCT), PET, and so on. Several approaches have been developed to achieve “turn-on” effect. (1) Incorporating guest molecules (analytes) for further suppression of AIE agents, including volatile organic compounds (VOCs), ammonia, and some other organic compounds (such as polystyrene, glutathione, and glucose oxidase [GOD]). (2) Introducing competitive ligands or metal ions into some frameworks whose emissions were quenched due to some reasons (such as LMCT, PET, or noncomplete restriction of phenyl rotations). (3) Utilizing electron-rich analytes (such as amino and phenols) with LUMO orbitals located over the CB of the sensors, thus excited electrons were dispatched to the CB of sensors, resulting in the enhancement of fluorescence.
Volatile organic compounds
Many VOCs are toxic compounds that have significant vapor pressures at room temperature. It is of particular significance to develop the detection of VOCs in chemical analysis and environmental control. Luminescence detection of VOCs with AIEgen-based porous materials has intriguing application potentials, due to its facile operation, fast response, great sensibility, and visual identification. Most of VOCs, especially electron-rich ones, showed significant “turn-on” effect, which was caused by further restriction of AIEgens and greatly tuned with the conformation, size, and electric density of the analytes.
The first attempt was carried out by Dincâ and coworkers in 2011.[46] Zn2(C30H16O8)(H2O)2·4DEF based on tetrakis(4-carboxyphenyl)ethylene (TCPE4-) exhibited a blue-shift from 467 to 457 nm upon in the presence of ethylenediamine, and red-shifts of 6 and 10 nm after being exposed to cyclohexanone and acetaldehyde (Figure 7A). Afterward, [Zn2(TCPPE)] with a similar structure but the extended building block tetrakis[4-(4-carboxyphenyl)phenyl]ethene (H4TCPPE) was designed.[29] After exposure to various VOCs (e.g., aromatic and aliphatic hydrocarbons), the maintained framework structure but strongly blue-shifted emissions up to 43 nm were recorded for activated [Zn2(TCPPE)], in which the largest blue shift of 42 nm was observed with mesitylene. Chang and coworkers utilized 1,1,2,2-tetrakis(4-(pyridin-4-yl)phenyl)ethene (TPPE), a TPE-based ligand, to prepare three-dimensional (3D) MOF, [Cd2(tppe)(bpdc)2(H2O)], with a four-fold interpenetrated network. [47] The activated sample exhibited a broad emission band at 526 nm with QY of 63.3%. Blue shift was observed after treatment with different VOCs, in which mesitylene produced the greatest shift of 31 nm. Exposure to VOCs resulted in luminescence enhancement for all the samples. Among the largest enhancement of more than 60% was induced by benzene. In the above examples, TPE moieties were confined in the framework and the sensing performances presumably associated with the conformational changes of phenyl rings of the TPE cores after being exposed to various analytes. Nevertheless, sensitivity and selectivity were not sufficient in these works.
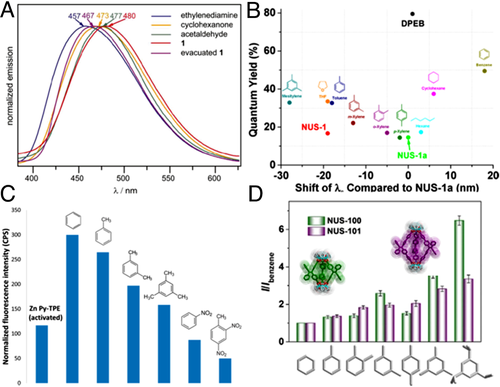
In 2014, Zhao and coworkers reported the innovative work of responsive “turn-on” fluorescence in AIE-active MOFs.[48] Obviously, an “off state” was necessary in “turn on” sensing with promising sensitivity. However, on account of nearly complete RIM of ligands in MOF, intense emissions which affected the turn-on sensing were often obtained. To overcome this problem, a new TPE-based ligand 4,4′-(2,2-diphenylethene-1,1-diyl)dibenzoic acid (DPEB) was designed. DPEB possesses two dangling carboxylate-free phenyl rings that will still be unconstrained in MOFs. As a result, the obtained NUS-1a demonstrated low QY of 15%, which could be greatly enhanced after the restriction of the dangling phenyl rings by VOCs, causing responding of MOF with turn-on fluorescence. In comparison with pristine NUS-1a, NUS-1a⊃benzene displayed the greatest red shift (18 nm), while NUS-1a⊃mesitylene exhibited the most remarkable blue shift (28 nm) (Figure 7B). This phenomenon was due to the conformation latching of surrounding phenyl rings, where a coplanar conformation could strengthen the π electron conjugation resulting in a bathochromic shift, while a perpendicular conformation was apt to lessen the π electron conjugation, inducing a hypsochromic shift. Besides peak shifts, the predicted responsive turn-on fluorescence was also seen (e.g., 49% for NUS-1a⊃benzene), demonstrating validity of the sensing based on turn-on fluorescence. Moreover, it was interesting to find that QY was enhanced along with the increasing of peak shifts (regardless of the type of shift).
Bhosale and coworkers prepared a special infinite 1D ribbon MOF [(ZnCl2)2Py-TPE] with tppe and used it for detecting aromatic compounds containing electron-donating groups (e.g., methyl).[49] The predicted turn-on phenomena of fluorescence were found for each methyl-substituted derivative, with red-shift and increased intensity with declining of methyl-substitution (13.5% for activated sample, 21% for mesitylene, 29% for meta-xylene, 36% for toluene, and 43% for benzene, Figure 7C). The consistent shift and increased intensity suggested that the analytes could restrict the rotation/vibration of the phenyl rings. The peak shift sensitive to each methyl-substituted derivative suggested that they interacted with the framework impelling a coplanar arrangement of the central rings and consequently generating a bathochromic shift.
Recently, a novel mechanism involving guest-lock-induced luminescence enhancement was proposed for realizing the rapid light-up detection of targeted VOCs.[50] Since the molecular vibrations in the synthesized [Cu4I4]-based MOF ([Cu4I4(Py3P)2]n, Py3P = tris(2-pyridyl)phosphine) were locked by the added chlorinated hydrocarbons, its luminescence could be promoted at the ambient temperature. Initial [Cu4I4(Py3P)2]n exhibited a considerably low emission band centered at 580 nm with QY of 0.03%. This emission band was observably increased after being exposed to CH2Cl2 or CHCl3 vapor for 30 min (QY of 23.3% for CH2Cl2, 18-fold as that to air [off state]), which could be explained by the RIM of luminescent linkers with construction of the efficient “binding pocket” generated from the interaction between the iodine atoms in the [Cu4I4] units and the hydrogen atoms in the guest molecules, whereas there was only weak binding from hydrogen bonding between the pyridyl rings and the halogen atoms of the guest molecules(Figure 8A). The short response time of light-up fluorescence upon CH2Cl2 (less than 1 s) could release fast writing/erasing. The luminescent performance maintained even after for 20 transient responding cycles, representing an ultra-rapid naked-eye distinguishable light-up response with reference to other vapor sensors. This sensor exhibited a remarkably stable fluorescence performance, which could be robust for over 2 weeks. Furthermore, light-up detection of chloroaromatics was also conducted with chlorobenzene as a representative sample. After being exposed to chlorobenzene vapor for 455 s, this MOF showed enhanced luminescence with QY of 16.3%.
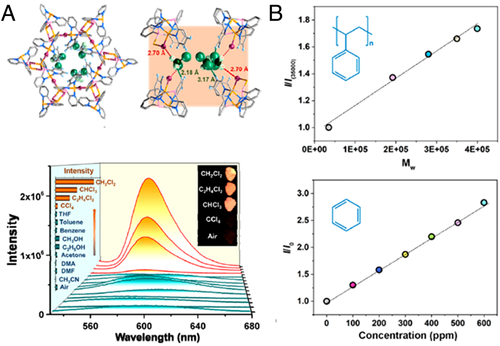
Zhao and coworkers explored the responsive turn-on fluorescence of AIE-MOFs associated with the molecular sizes of a variety of VOCs.[31] TPE moiety was prepared as the pendant groups and a series of MOFs (NUS-13-0% to NUS-13-100%) with different TPE loading were obtained via mixed-linkage strategy. The MOF after being incubated in n-hexane (In‑hexane, 2.8 Å × 8.2 Å) was used as the reference to investigate the relationship between the turn-on fluorescence and molecule sizes of analytes. The relative intensity (IR = I/In‑hexane) of NUS-13-100% correlated positively with the VOC molecular size, such as 2.13 for benzene, 3.05 for toluene, 7.71 for p-xylene, and 19.23 for mesitylene. The molecule sizes of analytes could affect the rotation of phenyl rings and thus restriction degrees that caused the phenomenon of size-dependent turn-on fluorescence. Nevertheless, the VOC with further increasing molecular size (e.g., 1,3,5-triisopropylbenzene) resulted in visible reduction of emission with an IR of 8.20, which might be due to the difficulty in penetrating into the cavities of frameworks. Furthermore, the intensity in hexane/mesitylene mixtures was enhanced with an almost linear relationship with the concentration of mesitylene. Also, alkyl alcohol solutions could trigger the “turn-on” effect of fluorescence of NUS-13-100%, and a distinct linear relationship between emission intensity and molecular length of the analytes was observed (Figure 8B). A “chemical nose” could be developed based on the molecule size-dependent turn-on fluorescence property for quantitative chemical sensing analysis. For example, as expected, the emission intensity of the AIE-MOFs presented a linear relationship with molecular weight of polystyrene, revealing that the fluorescent MOFs could be applied to pratical measurement of molecular weights of polymers based on the variation of fluorescence.
Other than traditional MOFs, organic cages were also utilized for “turn-on” sensing of VOCs. Zhao and coworkers assembled two highly stable Zr-based organic cages (NUS-100 and NUS-101) with TPE groups.[23] Both cages exhibited varying fluorescence emissions after being soaked in diverse VOCs, due to size-dependent effect. A clear positive relationship between the relative intensity and the molecular size of the analytes was found. The size-dependent turn-on fluorescence could be attributed to the different restriction degrees of the phenyl ring rotors (Figure 7D). Different from the previous discussed MOFs in which constrained molecular rotors were inside the network, molecular rotors in organic cages were on the outside surface of the cages, thus they were freely accessible to analytes, even those molecules with giant sizes (such as 1,3,5-triisopropylbenzene).
In summary, extensive efforts have been conducted for monitoring VOCs or other organic compounds. In recent years, several methods have been developed for high sensitivity and selectivity. (1) “Turning-off” the light before exposure to VOCs, which could be obtained via relaxing the motions of AIE fragments through the design of ligand or framework structure. A better background with less noise would be acquired through this strategy, which is beneficial for sensitivity. (2) Designing structures with “binding pockets,” which could form the host–guest complex with specific target agents with relatively strong interactions. This approach could enhance the selectivity and sensitivity at the same time. (3) Taking advantage of the suitable channels as well as exposed AIE sites, which might provide size-dependent sensing and quantitative analysis based on linear response to target compounds. (4) Some other molecules, such as biomolecules, are also suitable analytes based on this mechanism. Nevertheless, relative works are far from enough and there are still many problems to solve.
Other restriction compounds
Other than VOCs, some other compounds could also suppress the partially motion of AIE moieties, therefore, “turn-on” the fluorescence. For example, “turn-on” monitoring of glutathione and GOD was also conducted based on the previous discussed LMOF-2, which was used for “turn-off” sensing of Cu2+.[27] In LMOF-2, AIE-active APMP-2 were attached to the surface of the ZIF-8 network and further restricted motions of APMP-2. The APMP derivates would interact with glutathione or GOD with noncovalent bonds, which could restrain the intramolecular rotations, vibrations, and motions of APMP-2 and therefore enhance the fluorescence emission.
Ammonia was another molecule that could also “turn-on” fluorescence through locking the motion of AIE groups.[4] Protected by network entanglement, the CuI-based MOF [Cu2I2(tppe)] featured high stability, selectivity, and recyclability for “turn-on” sensing toward ammonia. Based on density functional theory (DFT) calculations, multiple weak hydrogen bonds were generated with an overall binding energy of 63.84 kJ mol−1. The rotation of the AIEgen unit could be restricted by the hygdrogen-bonding interaction, leading to inhibition of the nonradiative energy loss of the MOF with AIE groups. Rapid signaling response (< 3 min), at least five stable cycles, and high selectivity were witnessed in the detection of ammonia vapor. While in the detection of aqueous ammonia, two bands below 700 ppm (both Cu2I2 cluster and tppe ligand emitted simultaneously) but one dominated peak above 700 ppm (Cu2I2 cluster) was available, thus the linear range was divided into two regions corresponding to sensitivities of 0.01744 ppm−1 varying from 200 to 700 ppm and 0.04761 ppm−1 for the range between 700 and 1000 ppm, respectively.
In 2019, a strong dual emissive L1-Eu MOF was designed and utilized for ratiometric fluorescence detection.[51] A blue fluorescence emission located at 415 nm was ascribed to the twisted structure of TCPP (L1) in the framework, while a red fluorescence at 620 nm was due to Eu3+ ions in MOF. Upon the addition of arginine, the blue emission (I415) was greatly improved, whereas red fluorescence emission (I620) marginally decreased (Figure 9A). A good linear response observed between the ratio of I415/I620 was linearly correlated with the arginine concentration. An extremely low LOD of 15 nM could be obtained without background interference. In the proposed mechanism, multiple hydrogen bonds were generated between the nitrogen of TCPP ligand and guanidyl of arginine, which restricted the rotation of benzene rings in TCPP, expanded the conjugating region, and therefore enhanced the blue emission. On the contrary, these interactions did not impede the energy transfer from TCPP to Eu3+ ions, which made for steady red emission that served as a reference for quantitative fluorescence detection and visual distinguishment. This paper provided a new pathway for eliminating background noise and increasing sensitivity via introducing a reference signal.
Competitive ligands or metal ions
In some AIE-active frameworks, the emission might be quenched due to various reasons, such as LMCT, PET, or noncomplete restriction of phenyl groups. Introduction of competitive coordination substitution might be one potential pathway for “turn-on” fluorescence.
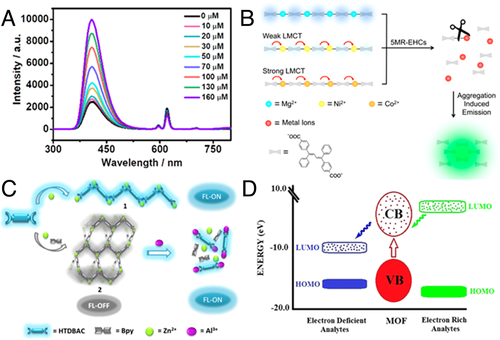
Five-membered-ring energetic heterocyclic compounds (5MR-EHCs) containing high nitrogen content were one example as a competitive ligand.[52] Unlike traditional nitroaromatic explosives, which were electron-deficient and well-studied with “turn-off” phenomenon, sensing of 5MR-EHCs was rarely explored. Using 5-nitro-2,4-dihydro-3H-1,2,4-triazole-3-one (NTO) as the target 5MR-EHCs, 1D MOF TABD-MOF-3 was developed with 4,4′-((Z,Z)-1,4-diphenylbuta-1,3-diene-1,4-diyl)dibenzoic acid (TABD-COOH) as linker and Co2+ as node. Extra-low QY (0.15%) was presented due to the strong LMCT. Upon the addition of NTO, however, the emission was boosted. In spot tests using paper strips, as low as 10 μL of a 1 × 10−6 M solution of NTO (6.5 ng/cm2) was detectable by the naked eye. Interestingly, in bulk solutions, no fluorescence changed in THF but high fluorescence was observed when hexane was added. NTO in THF/hexane could be analyzed in quality in a wide concentration range of 4 × 10−8–10−3 M with LOD as low as 4 × 10−8 M. A possible mechanism was proposed and proved by complementary methods (Figure 9B). TABD-COOH in TABD-MOFs was first released into the solutions as a result of the competitive coordination substitution of NTO. Afterward, THF or hexane arouse aggregation of separated TABD-COOH molecules, which caused a strong AIE effect and enhanced the fluorescence.
Other than competitive ligands, Zhao and coworkers proposed a metal competitive coordination substitution strategy.[53] AIE-active MOFs Zn(HTABDC)(DMF)2 with QY of 50% was constructed with 4,4′-((Z,Z)-1,4-diphenylbuta-1,3-diene-1,4-diyl)bis(2-hydroxybenzoic acid) (HTABDC) and Zn(II). However, the fluorescence could be quenched to an “off” state (Zn(HTABDC)(Bpy)·DMF, QY of 0.99%) utilizing decorative ligand4,4′-bypyridine (Bpy). Three possible fluorescence quenching mechanisms were proposed, including partial vibrational quenching by the guest molecules, noncomplete RIM of the phenyl rings, and the presence of nonradiative deactivation behaviors, such as PET. With quenched emission and abundant –OH groups, Zn(HTABDC)(Bpy)·DMF was employed for sensitively detecting oxyphilic metal ions, especially Al3+. Upon addition of Al3+, highly effective fluorescence enhancement along with an apparent red shift of 26 nm could be readily distinguished by the naked eye and a superior sensitivity with LOD of 3.73 ppb was obtained. However, all other metal ions demonstrated only minor intensity changes and hardly interfered in the detection of Al3+ in competition measurements. The turn-on mode could be explained with eliminated PET process from HTABDC to Bpy due to the coordination of HTABDC with Al3+ and resulted in dissociation of the framework (Figure 9C), which could be testified by the fluorescence red shift, 1H NMR, and electrospray ionization mass spectrometry.
Electron-rich compounds
PET process could also be one important approach of “turn-on” sensing. The electrons from the electron-donor are excitated to jump from the HOMO to LUMO. Subsequently, the excited electrons are transferred to the LUMO of the electron-acceptor, resulting in the variation of fluorescence emission. Different from electron-deficient analytes, such as NACs, the LUMO orbitals of electron-rich analytes were located over the CB of the sensors, thus excited electrons were transferred to the CB of sensors, bringing about fluorescence enhancement.
Dincâ and coworkers reported Zn2(TCPE) for selective sensing for ammonia at 100°C.[54] Different from traditional fluorescent organic crystals and polymers, Zn2(TCPE) maintained fluorescence even being heated to 350°C (near by the decomposition temperature of 400°C) and exhibited selective sensing of ammonia with emission shift at 100°C, while no selectivity was observed at room temperature. Other than the previously discussed effect of RIM, the “turn-on” effect was due to binding between ammonia and Zn2+, which affected the electronic structure of metal and the HOMO and LUMO energies of linkers via a charge-transfer mechanism. And the stronger binding energy of ammonia (e.g., NH3 binding was 38 kJ mol-1 higher than H2O with Zn2(O2C−)4 unit) contributed to the temperature-dependent selectivity.
Then, Morsali and coworkers reported a convenient one-pot synthesis method for fast-response AIE fluorescent MOFs[55] utilizing 5,6-di(pyridin-4-yl)-1,2,3,4-tetrahydropyrazine (a TPE-free AIE fluorophore), which was in situ generated through the C = C aldimine-coupling reaction and cyclization of N,N’’-Bis(pyridin-4-yl-methylene)ethane-1,2-diamine. The resulted TMU-40 (Zn), TMU-40 (Cd), and TMU-40 (Co) demonstrated great selectivity and sensitivity for detection of phenol derivatives, by virtue of the interaction of the free nitrogen on the ligand with the phenolic compounds. Significantly, TMU-40 (Co) could promptly and efficiently detect 4-aminophenol (KSV = 2.9 × 107 M−1, LOD less than 65 nM). Restriction of AIE agents via supramolecular interactions was one possible explanation, but PET process from LUMO orbitals of phenols to CB of TMU-40 was believed more importantly. In this case, the LUMO orbitals of electron-rich phenols were located over CB of the MOF, and the excited electrons in LUMO orbitals were transferred to the CB of MOF, rendering fluorescence enhancement (Figure 9D). Besides, improved adsorption property of TMU-40(Co) might also contribute to the more significant effect on sensing in comparison with TMU-40.
Others
Developing fluorescence-based CO2 sensors was difficult due to the nonluminescent nature and chemical inertness of this compound. However, quantitatively produced carbamate ionic liquid (CIL) via reaction of CO2 with amines may offer a promising strategy to develop innovative CO2 sensors. Xie and coworkers reported a special dynamic AIE-active MOF (ADA-Mn, ADA = anthracene 9,10-diacrylate acid), which promoted the ACQ ligands to be AIE-active upon framework construction.[56] ADA-Mn exhibited nonfluorescent due to the twisted acene rings (with a dihedral angle of about 3°) and twisted J-aggregates between neighboring ligands. However, the emission enhanced along with decreasing of particle size and the fluorescence was linearly related to viscosity in the low viscous region. The tests involving viscosity confirmed the rare dynamic AIE activity of ADA−Mn. On the basis of the viscous CIL generated from the interaction between CO2 and amines, CO2 fluorescence quantification was carried out with dipropylamine. In mixed gas of CO2/N2, a linear relationship between the fluorescence intensity and CO2 concentration was observed in the range of 2.5−100%. Moreover, nearly identical data could be acquired when 1−10 μL of H2O was added, illustrating the excellent anti-interference ability.
Another instance was based on AIE-active analytes rather than AIE-active sensors. Selective and sensitive sensing of chlortetracycline (CTC) was achieved with AIE-free Zn-based MOF (Zn-BTEC).[57] It was proposed that the fluorescence enhancement was attributed to AIE effect originated from the assembly of CTC in the MOF. The fluorescence intensity displayed a good linear correlation with the CTC concentration in the range of 0−8 μM with LOD of 28 nM. Moreover, CTC exhibited excellent anti-interfere performance over other TC derivatives, other biological substances, or metal ions.
CHALLENGES AND OUTLOOKS
In comparison to conventional luminescent porous materials that exhibit weak emission (or nearly nonemissive) in aggregation, the AIEgen-based crystalline porous materials exhibit a high emission due to the rigid architecture coordinate or covalent bonds that limit intramolecular motion in the aggregated or solid state. Considering that the AIEgen-based crystalline porous materials have high emission, outstanding stability, and high surface area, they offer a new platform for monitoring NACs, VOCs, heavy metals, and so on. Albeit many relative exciting results have been reported, future prospects for this thriving research area are worth looking into. Some key aspects may need to be underlined for upcoming investigation. (1) AIEgens-based crystalline porous materials still lack sensitivity and selectivity to some extent. There are still some interferences, such as background signal, false response, or error in “turn-off” mode as well as off-state emission in “turn-on” mode. Several strategies have been developed for molecular recognition and specific detection for target analytes, including structural design of building blocks, postmodification, and construction of supramolecular “pocket” for specific recognition. However, relevant works are still nascent and further investigations are necessary. (2) Structure–function correlations are not clear in this system, and further investigation is needed to shed light onto the sensing mechanism that is imperative for guiding rational design and synthesis of high-efficient sensors. (3) Although AIE-MOFs have been well studied as sensors, the explorations of other sensitive crystalline porous materials are limited. For instance, with well-defined structures, outstanding stabilities, and the absence of LMCT or MLCT, COFs might be ideal candidates for sensing, which were rarely studied. (4) Most of the sensors available were based on TPE or derivate. Exploring some TPE-free frameworks might give out some interesting results. (5) Biosensing and bioimaging are intriguing prospects for the further development of AIE-based crystalline porous materials. The use of these materials in biological field will raise concerns over their biocompatibility and toxicity. Meanwhile, aqueous dispersity is another major parameter for biological applications of AIEgens-based crystalline porous materials.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (21571079, 21621001, 21390394, 21571076, and 21571078), ″111″ project (B07016 and B17020), and the program for JLU Science and Technology Innovative Research Team.
Biographies

Yaozu Liu obtained his BS from Xinzhou Normal University in 2017 and his MS from Jilin University in 2020. Currently, he is finishing his PhD under the supervision of Prof. Qianrong Fang in the State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, Jilin University, China. His current research interests focus on the design synthesis of covalent organic frameworks.

Xinyu Guan obtained his BS from Zhejiang University in 2016. Currently, he is finishing his PhD under the supervision of Prof. Qianrong Fang in the State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, Jilin University, China. His current research interests focus on the synthesis and application of covalent organic frameworks.

Qianrong Fang received his BS (2001) and PhD (2007) from Jilin University in China. From 2007 to 2014, he completed his postdoctoctoral study in University of California at Los Angeles, Texas A&M University, University of California at Riverside as well as University of Delaware. In 2015, he went back to the State Key Laboratory of Inorganic Synthesis & Preparative Chemistry at Jilin University, as a full professor. His current research focuses on the design and synthesis of covalent organic frameworks (COFs) for applications in adsorption, separation, catalysis, and a number of others.




