The effect of tailing lipidation on the bioactivity of antimicrobial peptides and their aggregation tendency
Special Issue: Emerging Investigators
Bruce Lin and Andrew Hung are equal co-first authorship.
[Correction added on 20 April 2023, after first online publication: The Figure 1 has been replaced.]
Abstract
Antimicrobial peptides (AMPs) are potentially powerful alternatives to conventional antibiotics in combating multidrug resistance, given their broad spectrum of activity. They mainly interact with cell membranes through surface electrostatic potentials and the formation of secondary structures, resulting in permeability and destruction of target microorganism membranes. Our earlier work showed that two leading AMPs, MSI-78 (4–20) and pardaxin (1–22), had potent antimicrobial activity against a range of bacteria. It is known that the attachment of moderate-length lipid carbon chains to cationic peptides can further improve the functionality of these peptides through enhanced interactions with the membrane lipid bilayer, inducing membrane curvature, destabilization, and potential leakage. Thus, in this work, we aimed to investigate the antimicrobial activity, oligomerization propensity, and lipid-membrane binding interactions of a range of N-terminal lipidated analogs of MSI-78 (4–20) and pardaxin (1–22). Molecular modeling results suggest that aggregation of the N-lipidated AMPs may impart greater structural stability to the peptides in solution and a greater depth of lipid bilayer insertion for the N-lipidated AMPs over the parental peptide. Our experimental and computational findings provide insights into how N-terminal lipidation of AMPs may alter their conformations, with subsequent effects on their functional properties in regard to their self-aggregation behavior, membrane interactions, and antimicrobial activity.
1 INTRODUCTION
Antimicrobial resistance (AMR) has become a looming threat to public health systems worldwide, especially when paired with the stagnant development of novel antimicrobial compounds.[1] Liberal application and misuse of conventional antibiotics have promoted the rise of AMR, notably in the ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.).[2] Infections by ESKAPE pathogens can manifest as sepsis, resulting in $23.7 billion annually in healthcare costs in the United States, which highlights the economic burden these microorganisms pose.[3]
Antimicrobial peptides (AMP), comprising both naturally occurring and synthetic fragments of protein, have demonstrated themselves as an appealing alternative to conventional antibiotics with less tendency to develop resistance.[4, 5] With improved antimicrobial stewardship, better incentive strategies, and economic management, AMPs and other alternative treatments can be optimized as future leading therapeutics to fight AMR.[6, 7] Among many other AMPs, magainin and pardaxin show potent antimicrobial abilities.[8-11] Recently, we further characterized the potential of two leading AMP analogs, pardaxin (1–22) and MSI-78 (4–20), that possess greater antibacterial activity and membrane activity against a range of Gram-positive and Gram-negative organisms,[12] including Escherichia coli and S. aureus as well as bacterial strains including Salmonella typhimurium and Enterococcus faecalis. Mechanistic investigation showed these lead analogs primarily operated through membrane destabilization and disruption mechanisms, as evidenced by membrane permeability and microscopy investigations.[12]
To further optimize AMPs, peptide chemical modifications, such as lipidation involving the attachment of a lipid group onto a peptide,[13, 14] can augment their antimicrobial effects against bacteria and also reduce their toxicity. There is currently a range of lipidated peptide/protein-based therapeutics available for clinical use or undergoing examination in clinical trials, for example, polymyxin B.[15] The attachment of moderate-length lipid carbon chains to cationic bioactive or inactive peptides is a key factor in their self-assembly, micelle formation, and oligomeric format, resulting in various potentials for biomedical applications in vitro and in vivo.[16] Due to their hydrophobicity, the carbon chains can form clusters, inserting into the membrane lipid bilayer, which in turn induce membrane curvature and lead to membrane pore formation, destabilization, depolarization, and leakage.[17, 18] The N-terminal attachment of longer carbon chains, notwithstanding instances of improved AMP function and interaction with bacterial membrane bilayers over acyl chain attachment at other sites.[19, 20] Thus, we undertook to modify pardaxin (1–22) and MSI-78 (4–20) by using various lengths (C6–C12) of carbon chains to develop potent AMPs against bacterial pathogens, followed by toxicity, mechanistic and computational modeling investigations of all lipidated analogs of MSI-78 (4–20) and pardaxin (1–22). Further characterization using aggregation-induced emission (AIE) fluorophores and surface tension for their aggregation properties will provide a better understanding of hydrophobicity and aggregation effects on their activities for future design. The correlation between biological activity and the membrane disruption capability indicates that lipidation of AMPs (either bioactive or inactive) can be an effective strategy to develop more potent peptide-based antibiotic agents.
2 RESULTS AND DISCUSSION
2.1 Peptide synthesis and antimicrobial activity
Synthetic N-lipidated peptides of MSI-78 (4–20) and pardaxin (1–22) (Figure 1) were obtained by using standard automatic Fmoc/tBu solid-phase peptide synthesis (SPPS), followed by a manual coupling of fatty acid with N-terminal amine on the solid support. Then the peptides were cleaved from the solid support with trifluoroacetic acid (TFA) treatment and purified through reversed-phase high-performance liquid chromatography (RP-HPLC). During purification, the lipidated pardaxin (1–22) required high content of acetonitrile to dissolve the crude peptides for chromatography (Figure S1). Such observation indicated the high hydrophobicity of the lipidated pardaxin (1–22) over the lipidated MSI-78 (4–20). To compare the hydrophobicity effect on the activity of pardaxin (1–22), we further designed and synthesized N-acetylated and a Gly1→Ser1 substituted pardaxin (1–22) analogs, which have an extra hydroxyl group over pardaxin (1–22) to increase their hydrophilicity (Figure 1). Each peptide was obtained with a good overall yield (Table S1) and subjected to chemical characterization by analytical RP-HPLC and thermo OrbiTrap Exactive mass spectrometry (Figures S1 and S2; Table S1).
Later, the antibacterial assessment of those synthetic peptides (minimum inhibitory concentration [MIC]) showed that the moderate length (C6 and C8) modified MSI-78 (4–20) demonstrated significantly improved activity over the parental peptide and longer fatty acid (C10 and C12), especially against Gram-positive strains (Table 1). However, both the moderate and longer length N-lipidated pardaxin (1–22) were devoid of their antibacterial activity against both Gram-negative and Gram-positive bacteria (Table 1), while the less hydrophobic analogs, acetylated pardaxin (1–22) and Ser1-pardaxin (1–22), retained their activity or slightly improved (Table 1). Given that antibacterial susceptibility testing can be affected by different factors,[21] it has been shown that various ionic compositions and the presence of proteases/proteins in the medium can lead to significant reductions in inhibitory activity against target bacteria. We further evaluated the MIC changes of those AMP analogs against gram-negative bacteria A. baumannii and gram-positive S. aureus in two additional media, including cation-adjusted Mueller–Hinton broth (CMHB) and 10% fetal bovine serum (FBS)-adjusted Mueller–Hinton broth (MHB). The results showed that the lipidated peptides retain their antibacterial activity in the presence of ionic strength and serum with only a two to four-fold increase of the MIC (Tables S2–S6). As shown in Tables S2–S6, the moderate length of fatty acid modification, C8 and C10, was almost consistent with their original values in MHB. Such an observation demonstrated that the lipidation approach, despite increased hydrophobicity, nonetheless had good tolerance for overcoming the decreased antibacterial activity of AMPs in a physiological environment. However, it should be noted that the addition of serum significantly affected the antibacterial activity of the original pardaxin (1–22) and its acetylation and Ser1-substituted analogs (Tables S4 and S5). In contrast, the retained activity of lipidated analogs (Tables S4 and S5) may suggest a potential direction for future optimization.
| Gram-negative pathogens | Gram-positive pathogens | |||||||
|---|---|---|---|---|---|---|---|---|
| Peptide | Acinetobacter baumanii ATCC 19606 | MDR-FADDI-AB156 | Klebsiella pneumoniae ATCC 13883 | MDR-FADDI-KP028 | Staphylococcus aureus ATCC 29213 | MRSA ATCC 43300 | Enterococcus faecalis ATCC 29212 | E. faecalis ATCC 51575 |
| MSI-78 (4–20) | 15.6 | 15.6 | 15.6 | >125 | >125 | 15.6 | >125 | >125 |
| C6-MSI | 7.8 | 15.6 | 15.6 | >125 | 15.6 | 7.8 | 62.5 | >125 |
| C8-MSI | 15.6 | 15.6 | 31.2 | 125 | 15.6 | 15.6 | 31.2 | >125 |
| C10-MSI | 31.2 | 62.5 | 31.2 | 125 | 31.2 | 31.2 | 31.2 | >125 |
| C12-MSI | 62.5 | 125 | 62.5 | >125 | 62.5 | 125 | >125 | >125 |
| Pardaxin (1–22) | 7.8 | 15.6 | 31.2 | >125 | 7.8 | 15.6 | 15.6 | 31.2 |
| Ser-Pard | 7.8 | 15.6 | >125 | >125 | 15.6 | 15.6 | >125 | >125 |
| C2-Pard | 31.2 | 62.5 | >125 | >125 | 62.5 | 62.5 | 31.2 | >125 |
| C6-Pard | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| C8-Pard | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| C10-Pard | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| C12-Pard | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| Gentamicin | 7.8 | >250 | 0.49 | >250 | 0.49 | >250 | 31.2 | >250 |
| Vancomycin | N/A | N/A | N/A | N/A | 0.98 | 0.98 | 1.95 | 250 |
| Colistin | 0.98 | >125 | 15.6 | >250 | N/A | N/A | N/A | N/A |
- Note: The conventional antibiotics, vancomycin, colistin, and gentamicin, were tested in relevant media as the reference values. N/A represents not applicable for the non-tested samples.
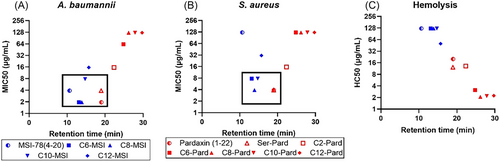
The additional calculated MIC50 and their fold changes in the presence of ions and certain serum further provided clear trends of the fatty acid chain effects on their antibacterial activity (Tables S2, S4, and S6). In combining their antibacterial activities with the hydrophobicity (the retention time generated from RP-HPLC), the moderate-length (C6–C10) modified MSI-78 (4–20) enhanced its antibacterial activity with increased hydrophobicity against both A. baumannii and S. aureus (Figure 2). On the other hand, because the original pardaxin (1–22) is very hydrophobic with an RP-HPLC retention time of 18 min, lipidation increased the hydrophobicity (retention time 25–30 min) and abolished their antibacterial activity (Figure 2 and Table 1). While the Ser1-substitution retained hydrophobicity and antibacterial activity, acetylation significantly decreased the activity. Such interesting activity patterns of the lipidation on either hydrophilic or hydrophobic AMP sequences led us to further investigate the lipidation effects on their bioactivity and modes of action.
2.2 Hemolytic activity and cell viability
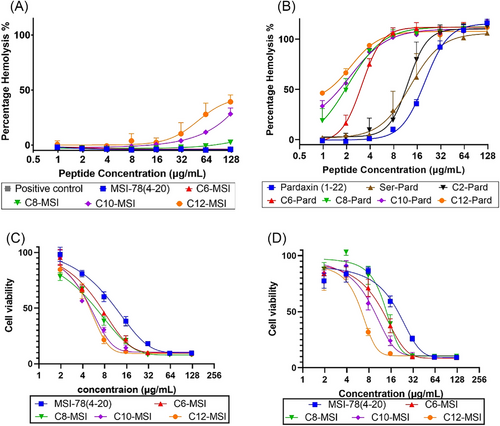
The cytotoxicity of the peptides toward sheep blood cells was determined with HC50, which is the value of 50% hemolysis, a quantity used to predict therapeutic potential. It is generally known that a longer fatty acid chain will increase the cytotoxicity against erythrocytes.[22, 23] As shown in Figure 3, there is increasing toxicity of the peptide with the lipidated modification for both MSI-78 (4–20) and pardaxin (1–22). But the extent of toxicity is specifically sequence-dependent. For instance, although the longer length lipidation increased the toxicity of MSI-78 (4–20), the HC50 was rarely achieved at their highest tested concentration (125 μg/mL). In contrast, the more hydrophobic pardaxin (1–22) analogs showed very strong toxicity toward sheep blood cells as the fatty acid chain increased (Figure 3B). Given the highly hemolytic activity of pardaxin analogs (Figure 3B), we further performed the cell cytotoxicity of MSI-78 (4–20) analogs against epidermal keratinocyte cells via MTT cell viability assay (Figure 3C,D). As the cell viability assay showed, the lipidation on MSI-78 (4–20) slightly increased their toxicity by reducing the epidermal keratinocytes cell viability. A further measurement of the therapeutic index (TI), calculated by the ratio of HC50 over MIC50, displayed their therapeutic potential for future development (Table S7). It is known that a higher TI indicates the AMP's specificity against bacteria with a clinical safety margin as therapeutic agents, while a low TI will restrict their clinical translation into a successful clinical drug. Based on Table S7, the moderate-length chain (C6–C10)-modified MSI-78 (4–20) increased their TI (2–30 times) over the parental peptide and long chain MSI-78, while the lipidation of pardaxin (1–22) significantly reduced the TI values. It should be noted that the extent of TI increases on MSI-78 (4–20) and decrease on pardaxin (1–22) are consistent with both A. baumannii and S. aureus (Table S7). While we incorporate the hydrophobicity to compare their cytotoxicity, it showed a clear correlation between their hydrophobicity and hemolytic activity (HC50) (Figure 2C). Such a co-relationship will guide the design of future AMP analogs with increased TI for their therapeutic application by balancing their hydrophobicity.
2.3 Membrane permeability and integrity
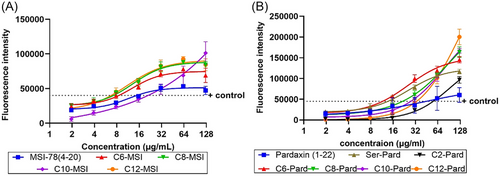
The widely accepted mechanism of AMP activity is through primary interaction with the bacterial membrane, penetration of the membrane, and disruption of membrane integrity. To further understand the effect of lipidation modifications on these modes of action, we applied two specific assays to assess their outer membrane permeability and inner/cytoplasmic membrane integrity, including N-phenyl-1-naphthalymine (NPN) assay and Syto9/propidim iodide (PI) assay, as previously reported.[24] NPN is commonly used as a fluorescent probe to indicate the AMP outer membrane permeability through the specific property of NPN turning fluorescence upon binding to membrane phospholipids.[25] As shown in Figure 4, both the lipidated MSI-78 (4–20) and pardaxin (1–22) displayed an enhanced NPN fluorescence intensity over their parental sequences in a dose-dependent manner. The longer the chain length of the modified AMPs, the stronger the fluorescence intensity, including the Ser1-substituted pardaxin analog (Figure 4). Although the lipidation can increase their outer membrane penetration toward A. baumannii, it does not necessarily reflect their antibacterial activity, nor indicates any correlation between outer membrane permeability and bacterial inhibition (Table 1).
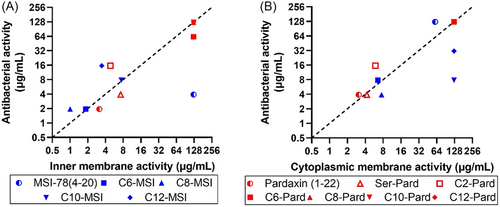
To increase our understanding of their antibacterial activity and membrane integrity, we further performed bacterial inner/cytoplasmic membrane integrity assessment by using PI, a membrane impermeable DNA-binding fluorophore, and Syto9, a membrane permeable DNA-binding dye. When the membrane integrity is destabilized after being challenged with AMPs, the proportion of PI-labeled bacteria increases. Analysis with flow cytometry showed a prominent PI-labeled bacterial population in the presence of the active pardaxin (1–22) and MSI-78 (4–20) analogs in both A. baumannii and S. aureus, which indicated a substantial degree of peptide-induced membrane destabilization (Figure S3). To further demonstrate the co-relationship of membrane integrity and their antibacterial activity, the MIC50 (y axis) was plotted against the peptide concentration causing 50% disruption of the inner membrane or cytoplasmic membrane (x axis) (Figure 5). The graph showed a very clear co-relationship between the peptides’ antibacterial activity and their capability of inner/cytoplasmic membrane destabilization. In comparison with the nonspecific increased NPN outer membrane permeability (Figure 4), the inner/cytoplasmic membrane disintegration analysis (Figure 5) suggested that the disruption of the inner membrane or cytoplasmic membrane as the main mode of action led to bacterial growth inhibition and cell death.
2.4 Structure–activity relationship of lipidated AMPs
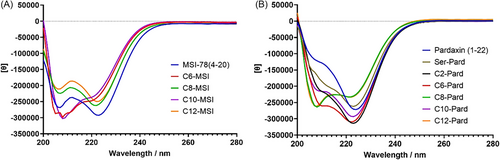
The secondary structure of peptides always plays a critical role for AMPs when they encounter bacterial membranes. Circular dichroism (CD) spectroscopy usually provides the details of α-helices conformational structure, with minima at 208 and 222 nm. The ellipticity at 222 nm indicates the α-helical content, while the excitation band at 208 nm is responsive to the α-helix as a single-stranded α-helices or a two-stranded coiled-coil structure.[26] The intensity ratio of the 222/208 nm bands further provides a gauge of peptides’ conformation with the ratio > 1, indicating a coiled-coil structure and ratio < 0.9 as isolated helices.[27] Based on the intensity at 222 nm, the CD measurement of the lipopeptides indicated that all the pardaxin (1–22) and MSI-78 (4–20) analogs formed α-helical structure in presence of 30% TFE hydrophobic membrane mimic environment (Figure 6), while they mainly displayed nonstructural conformation, except the very hydrophobic analogs, C8-C12-Pard (Figure S4). It should be noted that when we prepared the peptide samples for CD measurement, we observed insoluble precipitates of the C12-MSI and C8-C12-Pard in high concentration at 2 mg/mL. The final concentration of those hydrophobic analogs was reduced to 1 mg/mL for C12-MSI and C8-C10-Pard, and 0.5 mg/mL for C12-Pard. Here, we observed that there is no water solubility issue with shorter or moderate-length modified AMPs, such as the C6-, C8-, and C10-MSI, and C2- and C6-Pard analogs. However, the longer lipid length not only increased their hydrophobicity, but decreased their solubility in both water and aqueous buffer, including for C12-MSI and C8-, C10-, C12-Pard. In comparing the ellipticities of 220 nm in the TFE hydrophobic mimic condition, we observed that the long-length lipopeptide not only increased their hydrophobicity but enhanced their hydrophobic interactions by inducing α-helical structure in benign aqueous buffer condition (Figure S4). In combination with the 222/208 nm ratio (Table S8), the most active analogs, MSI-78 (4–20), C8-MSI, pardaxin (1–22), Ser-Pard, and C2-Pard (Table 1), displayed a high ratio value indicating a preferred coiled-coil stable α-helical structure formation but not isolated helices in hydrophobic membrane mimic condition (Figure 6). The intrachain and interchain interactions of such α-helical coiled-coils structure can stabilize their three-dimensional structure and interaction with bacterial membranes, which may contribute to their improved antibacterial activity.
Given that bacterial inner/cytoplasmic membrane integrity are critical for their antibacterial activity (Figure 5) and helical structure in hydrophobic mimic membrane environments (Figure 6), the interaction of those lipidated peptides was further assessed with phospholipid membranes in both pseudo-membranes model and computational modeling. By using two model membrane systems mimicking Gram-negative and Gram-positive bacterial phospholipid membranes, we performed the calcein dye leakage assay in the presence of lipidated peptides and observed an increased dye leakage with lipidated analog-treated samples over their parental peptides (Figure S5). Both the pardaxin (1–22) and MSI-78 (4–20) analogs demonstrated a significant amount of calcein leakage, indicating their pore formation capability. This is consistent with other reports that lipidation can enhance the peptide interaction with phospholipid membranes.[18] However, in correlation with their antibacterial activity, such increased interaction is dose-dependent and shows no specific interaction to special model phospholipid membranes. Thus, it indicates the interaction with phospholipids, a component of the actual bacterial membrane, only contributes to the binding activity without selectivity.
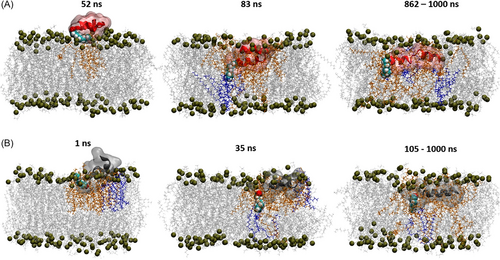
We then applied computational modeling to provide more detailed information on lipidation and their structural effects on membrane interaction. The simulations of monomeric lipidated AMPs and their parental peptides predict the initial adsorption mechanism on a model gram-positive cytoplasmic membrane (POPG:POCL = 9:1). All of the peptides rapidly adsorbed onto their respective membranes within the first 10 ns and retained a close association with the bilayers for the remainder of each simulation trajectory up to 1000 ns. Pardaxin (1–22), C10-Pard, and C8-MSI exhibit the greatest extent of spontaneous insertion into the model membrane, with the longer chained lipidated analogs undergoing a higher degree of interaction with the bilayer. Graphical snapshots are shown for C10-Pard (Figure 7A) and, for comparison, C8-MSI (Figure 7B), illustrating several conformational milestones exhibited by each peptide (similar behavior is observed for C6-MSI). Inspection of the trajectories shows that the N-terminal lipid tails of both C10-Pard and C8-MSI are extended parallel to the membrane surface upon first adsorption (left-hand frames, Figure 7), thus maximizing the initial contact surface area. Subsequently, both lipidated AMPs undergo spontaneous insertion into the bilayer, spearheaded by their respective N-terminal lipid tails, which adopt a perpendicular orientation to the membrane plane in a syringe-like fashion (middle frames, Figure 7). Finally, further insertion of the N-terminal lipid chain promotes partitioning of the entire peptide into the non-polar tail region in sufficient depth to form close interatomic contacts with lipids on the leaflet opposite that of the initial contact leaflet (middle and right-hand frames, Figure 7). Consistent with the aforementioned membrane interaction results, the N-terminal fatty acid chains, therefore, provide a strong initial driving force for membrane insertion of AMPs.
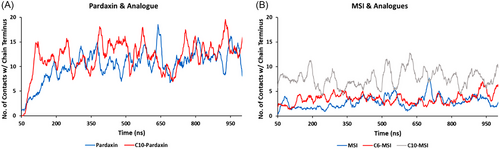
To quantify the extent of membrane insertion for each of the AMPs studied, Figure 8 shows time series plots of the total number of contacts between the parent and lipidated AMPs with the bilayer core lipid terminus carbon atoms. Both pardaxin (1–22) and C10-Pard exhibit substantial contacts with the bilayer terminus carbon atoms, indicative of rapid and deep permeation within the first 100 ns of simulations (Figure 8A). C10-Pard forms a higher number of bilayer core contacts, and at a greater rate, in the initial phase of the simulation, suggesting not only marginally deeper penetration than the parent AMP, but also a more efficient insertion mechanism facilitated by the presence of the C10 chain. Similarly, both MSI-78 (4–20) and C6-MSI exhibit some degree of bilayer penetration (Figure 8B), with C6-MSI forming a marginally higher number of contacts with the bilayer center compared to the parent AMP, particularly in the initial phase of the simulation. However, C8-MSI forms a markedly higher number of contacts compared to both of the other analogs (grey line, Figure 8B), indicative of stronger and more efficient membrane insertion imparted by a longer N-terminal fatty acid chain and their better antibacterial activity over parental sequences (Table 1). This reinforces previous observations that the extent of membrane disruption is dependent on fatty acid chain length.
As for the overall conformational changes induced by the presence of the N-terminus lipid tail upon bilayer adsorption and insertion, we obtained the end-to-end distance time series plots for each of the AMPs (Figure 9). Pardaxin (1–22) initially adopts a compact, bent helical conformation, which begins to transition into an extended ɑ-helix from ∼400 ns after insertion into the membrane (blue line, Figure 9A). C10-Pard adopts a linear ɑ-helical structure much more rapidly and is fully extended by 200 ns (red line, Figure 9A). The influence of N-terminal lipidation is even more pronounced for MSI-78 (4–20). While there is a high degree of conformational diversity for both MSI-78 (4–20) (blue line, Figure 9B, indicating a preference for disordered, extended strands) and C6-MSI (red line, Figure 9B, indicating a preference for compact conformations), C8-MSI stands in stark contrast, with a very rapid transition to a highly rigid extended ɑ-helical structure within the first 10 ns (gray line, Figure 9B). The conformational dynamics of both lipidated MSI-78 (4–20) and pardaxin (1–22) suggest that long N-terminal fatty acid tails promote more efficient coil-to-helix transition kinetics for AMPs in a lipid bilayer environment, which is consistent with our secondary structure measurement in hydrophobic environments (Figure 6).
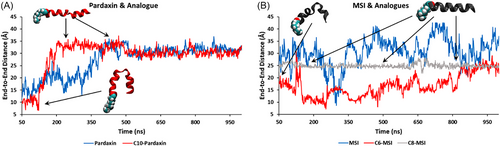
Our present simulations elucidate the impact of N-terminal fatty acid conjugation on the adsorption, penetration, and conformational preferences of pardaxin (1–22) and MSI-78 (4–20). Further simulation work will be performed to explore the possible differences in membrane insertion and disruptive capacities of these lipidated AMPs in oligomeric forms, especially the secondary structure measurement indicating the various lipidations leading to different coiled-coil and isolated helical structure formations (Figure 6 and Table S8).
2.5 LipoAMPs aggregation tendency
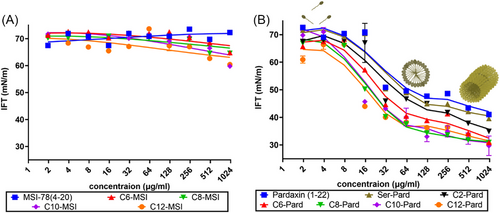
It is known that the more hydrophobic peptides tend to interact with other hydrophobic chains to allow the monomers to form self-association or self-aggregates in an aqueous environment.[28] How their aggregation tendency affects their antibacterial activity will be an interesting aspect to investigate. It is important to identify the physicochemical characteristics, such as the critical micelle concentration (CMC), a breakpoint in the concentration dependence manner, which can lead to a different order of those lipopeptides in solution.[29] Here, we further determined the surface interfacial tension (IFT) of those peptide analogs in water as the standard process, while water was used as a control (IFT ∼ 70 mM/m). As Figure 10 shows, while the lipidated MSI analogs are present as monomers at all concentrations, the incorporation of the alkyl chain into pardaxin sequence and the parental peptide resulted in a surfactant-like behavior and form thermodynamically stable aggregates with an abrupt change of the surface tension. More interestingly, the surface tension values of pardaxin analogs continue to drop beyond the CMC, which suggested the formation of bigger micelles, rods, or disks like the cylindric form.[30]
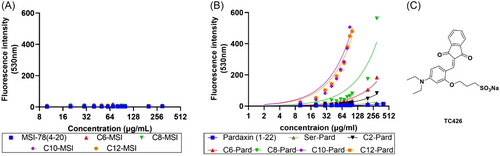
Given the unique properties of AIE fluorescence,[31] further investigations by using an AIE probe will provide more dynamic details of the self-assembly and aggregation process of those lipopeptide analogs. AIE is a unique photo-physical phenomenon that depicts a category of fluorophores showing weak fluorescence in dilute solution but becoming highly emissive in the aggregate state.[32] Taking the advantage of AIE, many fluorescent probes have been developed for the facile and efficient measurement of CMC.[33] After careful observation of the reported CMC probes, one of the previously developed amphiphilic AIE probes, TC-426,[34] was identified as a potential CMC probe to investigate the self-assembly behavior of those lipopeptide analogs. As shown in Figure 11A, this compound contains not only the hydrophobic AIE core but also a hydrophilic sodium sulfonate group (Figure 11C). Therefore, TC-426 exhibits good solubility and thus low fluorescence in most solvents, but is expected to show fluorescence enhancement during the micellization of the peptide analogs. Experiments were carried out using TC-426 as the indicator in AMP water solutions with concentrations ranging from 0 to 300 μg/mL. As shown in Figure 11A, there is no observed fluorescence increase for all the MSI analogs at 530 nm, which indicated no self-assembly or aggregation process, similar to the surface tension measurements (Figure 10A). Unlike the MSI-78 analogs, almost all the fluorescence spectral analyses of pardaxin analogs showed a prominent fluorescence increase at 530 nm (Figure 11B). It is worth noting that the fluorescence emission of probe TC-426 monomer at 530 nm can be ascribed to its electrostatic interaction with proteins[34] once the intramolecular motions of the probe are restricted. According to our observation, the stock solutions (0.5 mg/mL) of C10-Pard and C12-Pard are turbid, suggesting their poor water solubility and high tendency to form aggregates in water. In contrast, all pardaxin (1–22), Ser1-Pard, and C2-Pard showed imperceptible fluorescence at 530 nm (Figure 11B), which suggested their lower aggregation tendency, thus resulting in retained antibacterial activity (Table 1). Among those three pardaxin analogs, C2-Pard exhibited stronger self-assembly capability than pardaxin (1–22) and Ser1-Pard. Additionally, we also observed fluorescence intensity changes of the samples stained by TC-426 at 650 nm (Figure S6), referring to dye aggregates, which can serve as an indication of the typical process of micellization to encapsulate TC-426 probes to form aggregates and trigger the fluorescence emission at 650 nm. These results suggest that the AIE probe is useful for not only measuring the aggregation tendency of lipidated AMPs, which provides consistent results with surface tension measurement, but also provides dynamic details in an efficient way to detect their self-assembly/aggregation process for future hydrophobic peptide design.
2.6 Different peptide format interaction with the bacterial surface
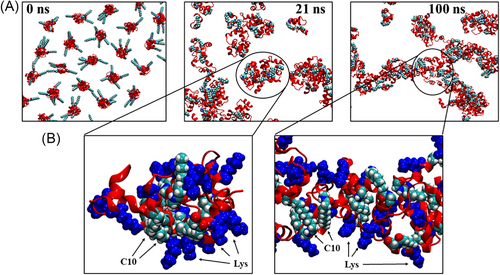
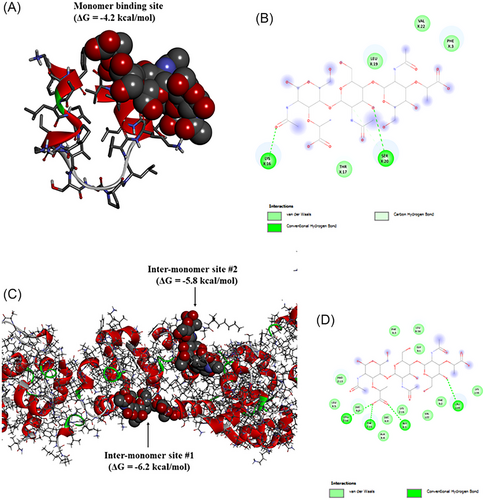
To shed further light on the aggregation propensity and mechanism of C10-Pard, and how oligomerization may impact its interaction with bacterial cell wall oligosaccharides, molecular dynamics (MD) simulations were performed to model the self-assembly of 125 × C10-Pard monomers. This was followed by subsequent molecular docking calculations of the resultant oligomeric complex with a representative trisaccharide fragment (NAM-NAG-NAM) as a vastly simplified model of the outermost regions of bacterial cell wall oligosaccharides such as peptidoglycans (PGN) and lipopolysaccharides (LPS), and the results compared with a similar docking calculation performed on a single pardaxin (1–22) monomer.
The initial configuration involved equidistant placement of 125 × C10-Pard peptides in random orientations in solution (Figure 12A). The self-assembly simulation predicts the rapid formation of small oligomeric clusters consisting of approximately 6–10 × peptides (Figure 12A,B, left-hand panel). These oligomers are stabilized by the formation of close contacts between the decanoyl lipid tails within the oligomeric core (cyan spheres in Figure 12B) with the Lys side chains facing outwards in highly solvent-exposed orientations (blue spheres in Figure 12B). Thus, these intermediate oligomers resemble micelles with C10 comprising their non-polar cores and charged Lys residues as the solvent-exposed polar “head groups”. Such micelles may resemble those formed at, or just above, the CMC (Figures 10 and 11). Subsequently, the micelle-like oligomers continue to aggregate, resulting in a single, elongated, fibril-like macro-oligomeric complex (Figures 10 and 11), which remains stable for the rest of the simulation (Figure 12A, right-hand panel). This complex consists of a long linear segment comprising approximately 70 peptides (circled in Figure 12A, RHS) and also contains other substructures, including bends and forks, resembling a complex web-like network, which spans the entire simulation cell. The simulation, therefore, suggests that at high concentrations far above the CMC, C10-Pard may preferentially form elongated 1D rods or even 2D films.
Molecular docking calculations of NAM-NAG-NAM binding to a monomeric pardaxin (1–22) indicate that the trisaccharide preferentially binds along the C-terminal half of the peptide, which constitutes an extended multi-turn helical structure (Figure 13A). Binding is supported by multiple hydrogen bonds, including the side chains of Lys and Ser residues (Figure 13B). The predicted binding affinity with monomeric pardaxin (1–22) is −4.2 kcal/mol. In contrast, docking calculations of NAM-NAG-NAM to the liner segment of the fibril-like oligomeric C10-Pard indicate a markedly higher binding affinity of −6.2 kcal/mol (Figure 13C). The trisaccharide is predicted to preferentially insert deep within crevices between adjacent peptide monomers, with hydrogen bonds and numerous van der Waals’ interactions simultaneously contributed by residues from multiple monomers (Figure 13D). This multi-pronged interaction, supported by multiple neighboring monomers, is facilitated by the oligomeric structure of the C10-Pard aggregate. Thus, while a single pardaxin (1–22) monomer may ephemerally bind to bacterial polysaccharides, with the relatively weak interaction allowing the AMP to readily undergo subsequent permeation through the outer bacterial layer. However, the simulations of macro-oligomeric C10-Pard showed that a stronger interaction with polysaccharides may serve to overstabilize the contact between the peptides and the bacterial cell wall, reducing the ability of this AMP to interact with inner/cytoplasmic membrane for their antibacterial activity.
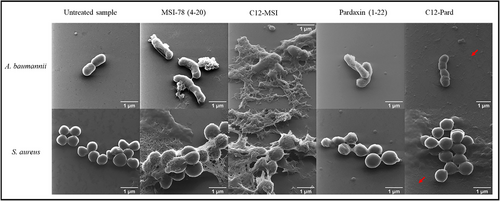
2.7 Microscopic visualization of AMP-treated bacteria
The mode of action, computational modeling, and lipopeptide aggregation capability demonstrated that the inner/cytoplasmic membrane interaction is the key factor for the synthetic lipidated AMP's antibacterial activity, while the highly hydrophobic C12-Pard can form aggregates resulting in the loss of antibacterial activity. Then, helium ion microscopy (HIM) was used to visualize the membrane morphological changes of A. baumannii and S. aureus in the presence of MSI-78 (4–20), C12-MSI, Pardaxin (1–22), and C12-Pard at 1 × MIC. It should be noted that C12-Pard is inactive against A. baumannii and S. aureus (Table 1), of which the HIM samples were applied with the highest tested concentration (125 μg/mL). The tested bacterial samples and controls were fixed onto glass coverslips after incubation for HIM analysis, as previously described.[24, 35] Figure 14 clearly showed morphological changes of A. baumannii and S. aureus with the treatment of MSI-78 (4–20) and C12-MSI, which correlated their antibacterial and membrane destabilization activity (Table 1 and Figure 5), followed by membrane fragmentation and disruption. However, while we can observe the membrane morphological changes of the pardaxin (1–22)-treated samples (Figure 14), the easily aggregated C12-Pard showed no membrane destabilization. Interestingly, we further observed some particles or rods (pointed by the red arrow in Figure 14) in both the C12-Pard-treated A. baumannii and S. aureus samples, but nothing similar was found in other treated samples. Those particles or rods marked by red arrows under microscopy might be the particles or aggregates formed by hydrophobic C12-Pard, consistent with the analysis of surface tension (Figure 10), AIE probes (Figure 11), and computational modeling (Figure 12). Such observations of the morphological charges not only showed the importance of membrane destabilization for antibacterial activity, but indicated the formation of lipopeptide aggregates resulting in activity loss.
3 CONCLUSION
To combat the uprising AMR, substantial research has been applied to develop novel antibiotic alternatives, including peptide-based antimicrobials. Our previous study on MSI-78 (4–20) and pardaxin (1–22) showed these peptides to possess potent antimicrobial activity with strong membrane activity. Few reports demonstrated that the increased cationic charge and hydrophobicity could improve the binding affinity of AMPs with bacterial membranes, resulting in enhanced antibacterial activity. It is unclear how the lipid carbon chain with increased hydrophobicity affects their antibacterial activity, which also leads to undesired hemolysis.[22, 23] To uncover the details of the carbon chain effect on AMP's bioactivity, in this work, different length lipid carbon chains were added to the N-terminal of MSI-78 (4–20) and pardaxin (1–22), followed by antimicrobial activity investigation. In comparing with their hydrophobicity, we found an enhanced hydrophobicity of hydrophilic sequence (MSI-78 (4–20)) resulted in a stronger antimicrobial activity, while an increase in hydrophobicity of hydrophobic sequences (pardaxin (1–22)) led to the loss of antimicrobial activity. More interestingly, the longer fatty acid chains can lead to higher hemolytic activity toward blood cells (Figure 3), which is consistent with previous reports.[22, 36] Our correlation study between the bioactivity and hydrophobicity of the AMPs, including their antibacterial activity and hemolytic activity (Figure 2), provided insightful information on the balance of the hydrophobicity of AMPs with enhanced antibacterial activity with less hemolytic activity. The mode of action investigation on membrane permeability and integrity demonstrated no specific selectivity for the outer membrane, but a closed correlation between inner/cytoplasmic membrane activity and antibacterial activity (Figure 5). Using CD and computational modeling, the SAR further showed that the N-terminal carbon chain of AMPs assisted the membrane interaction, a greater depth of lipid bilayer insertion, and α-helical conformational adoption. The aggregation analysis of those lipidated peptides using surface tension characterization and AIE fluorescent suggested that increased hydrophobicity contributed to their aggregation process, resulting in less antibacterial activity. Simulation of the aggregation mechanism of C10-Pard suggests that this lipidated AMP may form elongated fibrillar oligomers and extended web-like networks. Simulations suggest that both pardaxin (1–22) monomers and C10-Pard macro-oligomers may bind strongly to bacterial surface oligosaccharides. The stronger binding between C10-Pard macro-oligomers and oligosaccharides may reduce the ability of the (oligomeric) lipidated peptides from reaching the inner membrane. Overall, our experimental and computational findings provide insights into how N-terminal lipidation of AMPs may alter their conformations, with subsequent effects on their functional properties in regard to their self-aggregation behavior, membrane interactions, and antimicrobial activity. This work strongly suggests that a balanced hydrophobicity of AMPs is required to enhance their antibacterial activity with less hemolytic activity for the future design of peptide antibiotics.
4 EXPERIMENTAL SECTION
4.1 Peptide preparation
The peptides pardaxin (1–22), MSI-78 (4–20), and their lipidated variations were synthesized using Rink Amide 4-methylbenzhydrylamine (MBHA) polystyrene resin by Fmoc/tBu solid-phase methods, as previously described.[37] Standard Fmoc-chemistry was used throughout with Rink Amide 4-methylbenzhydrylamine (MBHA) polystyrene resin (0.1 mmol, loading capacity 0.336 mmol/g, GL. Biochem Ltd., swelling in DMF for 10 min), a coupling condition of a four-fold molar (0.4 mmol) excess of the Fmoc-protected amino acids in the presence of four-fold Oxyma Pure (0.4 mmol) and four-fold DIC (0.4 mmol), and a deprotection condition of 20% piperidine on a microwave peptide synthesizer, CEM Liberty Blue 2.0 with a procedure of two times deprotection (2 min, microwave 85°C), double coupling reaction (10 min, microwave 75°C), and double wash by DMF. After the synthesis finish, the peptides were cleaved from the solid support with TFA in the presence of water and TIPS as the scavenger (ratio 95:2:3) for 2 h at room temperature, followed by filtration to remove the resin. The crude peptide products were obtained with nitrogen evaporation and precipitated in ice-cold diethyl ether and washed three times. The crude peptides were then purified with a C18 column (Shimadzu Shim-Pack C18, 5 μm, 20 × 150 mm) by RP-HPLC in water and acetonitrile containing 0.1% TFA with the gradient 0%–100% of buffer B (acetonitrile) at 10 mL/min. The final products were then characterized by both Shimadzu RP-HPLC (HALO 160 Å ES-C18, 5 μm, 4.6 × 250 mm) and Thermo OrbiTrap Exactive Mass Spectrometer.
4.2 Antimicrobial assay and MIC determination
All the synthetic peptides were subjected to antibacterial testing against a panel including Gram-negative A. baumannii ATCC 19606, MDR-FADDI-AB156, K. pneumoniae ATCC 13883, MDR-FADDI-KP028, and Gram-positive S. aureus ATCC 29213, methicillin-resistant S. aureus ATCC 43300, E. faecalis ATCC 29212, and multidrug-resistant E. faecalis ATCC 51575. Vancomycin, colistin, and gentamicin were further used as positive control for Gram-positive species, gram-negative species, and all species, respectively. The resistance and sensitivity of bacterial strains were verified as well at the same time. Briefly, one to two colonies of the corresponding bacterium strain from the agar plate were suspended in 10 mL MHB for overnight incubation at 37°C. On the second day, 1 mL overnight culture was then re-inoculated into 20 mL of fresh MHB and incubated to the late exponential phase at 37°C for bioassays investigation.
MIC determination: In a 96-well flat-bottom microtiter plate, peptides or antibiotics (100 μL) were serially diluted with a dilution factor of two in Mueller–Hinton broth (MHB) OR Cation MHB or 10% FBS in MHB followed by the addition of 100 μL of bacteria in corresponding media (final bacterial concentration as 1 × 106 cells/mL). A microplate reader (PerkinElmer 1420 Multilabel Counter VICTOR3) set at optical density 620 nm (OD620) was then used to analyze the microtiter plates after 24 h of incubation. Any resultant MIC was defined as the lowest concentration of peptide at which there was no bacterial growth upon juxtaposition with media control samples in the 96-well flat-bottom microtiter plate.
4.3 Surface tension
Surface tension measurements were performed on a Dataphysics OCA 20 Optical contact angle measuring and contour analysis system at the Materials Characterization & Fabrication Platform assessed by Bicheng Yao. The tensiometer was calibrated with pure water at 72 mN/m before each experiment. Three independently prepared samples were tested with a pendant drop model and Laplace–Young fitting at 20 degrees.
4.4 AIE for aggregation determination
AIE probe TC-426 was synthesized according to our previously published paper.[34] For the study of AMP aggregates, a stock solution of TC-426 was first prepared in DMSO with a concentration of 10 mM. The stock solution was diluted 100 times using DI water, and a TC-426 solution (100 μM) was finally obtained. Two AMP solutions are needed in this experiment. Stock A was prepared by dissolving a certain quantity of AMP in DI water and the final concentration is 500 μg/mL. Stock B (100 μg/mL) was prepared by diluting stock A five times with DI water. The working solutions containing 10 μM TC-426 and 0, 10, 20, 30, 40, 50, 60, 70, 80, and 90 μg/mL AMP were prepared by adding 10 μL TC-426 solution, 0, 10, 20, 30, 40, 50, 60, 70, 80, or 90 μL stock B, and a certain quantity of DI water to make the total volume 100 μL. The working solutions containing 10 μM TC-426 and 100, 200, and 300 μg/mL AMP were prepared by adding 10 μL TC-426 solution, 20, 40, or 60 μL stock A, and a certain quantity of DI water to make the total volume 100 μL. Finally, after the working solutions were mixed thoroughly with a vortex shaker, they were transferred into a quartz cuvette for fluorescence measurement.
4.5 MD simulation with membranes
All-atom MD simulations were performed using GROMACS 2019[38] and the CHARMM36m[39] forcefield. Gram-positive cytoplasmic membranes were modeled using a bilayer composed of POPG and palmitoyloleoyl cardiolipin (POCL) in a 9:1 ratio. The membrane patch employed had a surface area of 7.15 × 7.15 nm2 and was built using the CHARMM-GUI Membrane Builder webserver.[40] The initial structures of pardaxin (1–22) and MSI-78 (4–20) were predicted using the PEPFOLD3 server as described previously.[12] Forcefield topologies for the N-terminal lipidated peptides were created by defining hexanoic (C6), octanoic (C8), and decanoic acid (C10) as new residues, which were connected to the N-terminus of MSI-78 (4–20) (for C6-MSI and C8-MSI) and pardaxin (1–22) (for C10-Pard) via peptide bonds. Bonded and non-bonded parameters for the lipid chains were adapted from linear alkane parameters in the CHARMM forcefield. Five peptides were simulated: pardaxin (1–22), C10-Pard, C6-MSI, and C8-MSI. Each peptide was simulated in a water solution for 700 ns using the simulation parameters described below, and the highest populated conformation of each peptide was selected as the starting structure for subsequent bilayer adsorption simulations.
For each bilayer adsorption simulation, the peptides were initially placed in an orientation such that the N-terminus faces the water–membrane interface, approximately 0.4 nm above the mixed bilayer. Periodic boundary conditions (PBC) were applied, and a simulation box with dimensions of 71.5 × 71.5 × 97.9 Å3 was defined around the peptide-membrane system and solvated with TIP3P[41] water molecules, potassium (K+), and chloride (Cl−) ions to achieve neutrality and an approximate salt concentration of 150 mM. The integration time step was set to 2 fs. Van der Waals interactions were switched to zero between 0.8 and 1.2 nm. Electrostatic interactions were evaluated using the fast smooth particle-mesh Ewald (PME) method[42] with a Coulombic potential cutoff of 1.2 nm. Covalent bonds involving hydrogen atoms were constrained using the LINCS algorithm.[43] For the production simulations, the velocity rescale thermostat of Bussi et al.,[44] with a coupling time constant of 0.1 ps, was used to maintain the temperature of all simulations at 310 K. The pressure was maintained at 1 bar using semi-isotropic coupling with the Parrinello–Rahman barostat algorithm and a coupling constant of 5 ps. The systems were energy minimized using the steepest descent algorithm for a maximum of 10,000 steps. The systems were equilibrated using a series of equilibration simulations according to the protocol specified in the CHARMM-GUI Membrane Builder webserver,[40] in which non-hydrogen atoms were restrained to their initial positions using force constant values of progressively lower values at each equilibration simulation step. Subsequently, 1000 ns (1 μs) of production equilibrium simulations were performed for each of the five peptide-bilayer systems, resulting in a total of 5 μs worth of trajectories. Molecular structures were visualized using Visual Molecular Dynamics version 1.9.3 (VMD).
4.5.1 MD simulation of C10-Pard aggregation
Simulated self-assembly and aggregation of C10-Pard was performed by initially placing 125 × C10-Pard peptides separated from each other by approximately 40 Å within a dodecahedron box of dimensions 235.035 × 235.035 × 235.035 Å3. The relative orientation of each peptide was set randomly. The simulation box was solvated with 282,757 water molecules and sufficient K+ and Cl− to neutralize the system and produce a salt concentration of approximately 150 mM. The system was geometry-optimized according to the procedure outlined above and subsequently subjected to 100 ns of MD simulation.
4.5.2 Molecular docking of trisaccharide to pardaxin (1–22) and oligomeric C10-Pard
To investigate the interaction between a simplified representation of bacterial polysaccharides, such as peptidoglycan (PGN) and lipopolysaccharide (LPS), with monomeric pardaxin (1–22) or aggregated C10-Pard, Autodock Vina was used to perform molecular docking calculations.[45] An exhaustiveness parameter of eight was used for both docking calculations. Bacterial PGN or LPS was represented by the bacterial cell wall trisaccharide (N-acetyl-beta-muramic acid-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-N-acetyl-beta-muramic acid, NAM-NAG-NAM). Binding studies of NAM-NAG-NAM to monomeric pardaxin (1–22) were performed by defining the former as the “ligand”, with all rotatable torsional angles set to be flexible, while the latter was defined as the rigid “receptor”. A docking grid box with dimensions of 60 × 61 × 54 Å3 was used. Binding studies of NAM-NAG-NAM to the fully aggregated (fibril-like) C10-Pard was performed by setting the latter as the rigid “receptor”. Docking was performed by defining a grid box around a linear segment of the fibril-like aggregate, of dimensions 76 × 62 × 68 Å3. Subsequently, both sets of docking results were visualized using Discover Studio Visualizer.
ACKNOWLEDGMENTS
The National Health and Medical Research Council (NHMRC) of Australia and Australian Research Council (ARC) are thanked for financial support over many years for the peptide chemistry and chemical biology studies reported in the authors’ laboratories. WL is the recipient of NHMRC Investigator Grant EL1 (APP2018256) and Australian Dental Research Foundation Grant. NMOS is the recipient of NHMRC funding (APP1142472, APP1158841, APP1185426), ARC funding (DP210102781, DP160101312, LE200100163), Cancer Council Victoria funding (APP1163284), and Australian Dental Research Funding in antimicrobial materials and research is supported by the Division of Basic and Clinical Oral Sciences at The Melbourne Dental School. This study was funded in part by grants from Australian Research Council (FT210100271 to YH) and Australia-China Science and Research Fund-Joint Research Centre on Personal Health Technologies (ACSRF65777 to YH). WL, RM, and YH are the recipients of the Melbourne Dental School Collaborative Research Grant 2022 round. Computational resources were provided by the National Computational Infrastructure (NCI), which is supported by the Australian Government, and the Pawsey Supercomputing Centre with funding from the Australian Government and the Government of Western Australia. This work was performed in part at the Materials Characterisation and Fabrication Platform (MCFP) at the University of Melbourne and the Victorian Node of the Australian National Fabrication Facility (ANFF).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.




