Magnetically accelerated thermal energy storage within Fe3O4-anchored MXene-based phase change materials
Yan Gao and Zhaodi Tang contributed equally to this work.
Abstract
Inherent weak photon-capturing ability is a long-standing bottleneck for pristine phase change materials (PCMs) in photothermal conversion application. To conquer this difficulty, herein, magnetic Fe3O4 nanoparticles were in situ anchored between the layers and the surface of two-dimensional MXene for the infiltration of myristic acid (MA) by an in situ chemical anchoring strategy. Benefiting from the synergistic localized surface plasmon resonance effect of MXene and Fe3O4 nanoparticles, our designed MXene@Fe3O4-MA composite PCMs harvested an ultrahigh photothermal conversion efficiency of 97.7%. During the photothermal conversion process, MXene can capture photons and convert solar energy into heat energy efficiently, and the in situ anchored Fe3O4 nanoparticles further enhanced the photothermal conversion efficiency. Moreover, the introduction of Fe3O4 nanoparticles improved the thermal energy storage density (144.17 J/g) of MXene-MA composite PCMs since Fe3O4 nanoparticles provided more heterogeneous nucleation sites for MA. Simultaneously, MXene@Fe3O4-MA composite PCMs were endowed with excellent paramagnetism, and realized efficient magnetic-thermal conversion. Additionally, MXene@Fe3O4-MA composite PCMs exhibited excellent energy conversion stability, thermal stability, and reliability after undergoing multiple thermal cycles. Therefore, high-performance MXene@Fe3O4-based energy conversion composite PCMs are promising candidates to accelerate efficient utilization of the practical solar energy and magnetic energy
1 INTRODUCTION
With the excessive consumption of traditional fossil energy and increasingly serious environmental problems, improving energy utilization efficiency and developing new energy is imminent, such as solar energy, magnetic energy, biomass energy, etc.[1-5] Emerging thermal energy storage technology-based phase change materials (PCMs) can effectively solve the problem of space and time mismatch between heat supply and demand due to the advantages of high heat storage density, excellent thermophysical stability, convenient accessibility, low cost.[6-8] However, low energy conversion efficiency is a long-term bottleneck in the application of pristine PCMs due to their inherent low light trapping capability. In this regard, many researchers introduced photosensitive materials into PCMs for photothermal conversion and storage, such as carbon-based materials (carbon nanotubes [CNTs], expanded graphite, graphene, etc.[9-11]), metal-based materials (Ag, Ti2O3, CuO, etc.[12-14]), organic materials (polypyrrole, polyaniline, polydopamine, etc.[15-17]), and other Inorganic materials (boron nitride, black phosphorus, Graphitized carbonitride[18-20]). For example, Chen et al.[11] encapsulated paraffin wax into porous CNTs sponge with a photothermal efficiency of more than 40%. Zhang et al.[12] introduced Ag into PEG for the photothermal conversion. The temperature of composite PCMs increased more quickly than that of pristine PEG. Liu et al.[20] used radial ice template to assemble boron nitride (BN)-based composite PCMs for photothermal-electric conversion. Huang et al.[19] designed modified-polymethyl methacrylate (PMMA) to encapsulate eicosane for photothermal conversion.
From inception in 2011, MXene, two-dimensional (2D) transition metal carbides/nitrides, have aroused widespread interest from researchers and engineers in the fields of electricity, optics, magnetism etc., such as battery electrodes,[21] supercapacitor,[22] biosensing[23] and catalysis.[24] Compared with traditional carbon materials, MXene shows two obvious enhanced absorption peaks in the visible and near-infrared regions due to the localized surface plasmon resonance (LSPR) effect, which is similar with metal nanoparticles. It has been reported that the internal photothermal conversion efficiency of pristine Ti3C2Tx is as high as 100%.[25] Therefore, MXene is a very promising candidate to improve the utilization efficiency of solar energy. However, most current reports focus on MXene as a photothermal additive to assist the photothermal conversion of PCMs,[26, 27] and there were few reports of MXene as a supporting material of PCMs for photothermal conversion. Lin et al.[28] prepared MXene@PEG aerogel composite PCMs with a photothermal conversion efficiency of 92.5%. Similarly, Fan et al.[29] prepared MXene@PEG composite PCMs with a photothermal conversion efficiency of 94.5% under actual solar irradiation.
Similar to MXene, several metal nanoparticles also exhibit LSPR effect.[30] Among them, magnetic Fe3O4 nanoparticles are also considered as photothermal reagents under the action of near-infrared light. Shen et al.[31] reported the photothermal effect of cluster Fe3O4 nanoparticles on tumor photothermal ablation. Nicolas-Boluda et al.[32] found that Fe3O4 nanoparticles can self-assemble into two different conformations. These self-assemblies were assessed for photothermal therapy, an adjuvant strategy for tumor therapy. Additionally, Fe3O4 nanoparticles are capable of integrating magnetic-thermal conversion and photothermal conversion due to the super paramagnetism and superior light absorption ability. Tao et al.[33] designed Fe3O4/polypyrrole/PW composite PCMs for magnetic-thermal conversion under an alternating magnetic field. Tang et al.[34] developed Fe3O4-functionalized graphene-based composite PCMs to simultaneously realize the efficient conversion of electromagnetic energy and light energy to heat energy through the magnetic-thermal effect of Fe3O4 nanoparticles and the light absorption of graphene.
To efficiently integrate the photothermal and magnetic-thermal effects of MXene and Fe3O4 nanoparticles, herein, Fe3O4 nanoparticles were in situ anchored between the layers and the surface of MXene by an in situ chemical synthesis strategy. After MA was infiltrated into MXene@Fe3O4 using a physical vacuum infiltration method, MXene@Fe3O4-MA composite PCMs were prepared to simultaneously integrate the functions of photothermal conversion and magnetic-thermal conversion. During the photothermal conversion process, MXene can capture photons and convert light energy into heat energy efficiently. In addition, the in situ anchored Fe3O4 nanoparticles can further improve the photothermal conversion efficiency of MXene@Fe3O4-MA composite PCMs due to the good photothermal effect of Fe3O4 nanoparticles in the near infrared region. Simultaneously, MXene@Fe3O4-MA composite PCMs were endowed with excellent paramagnetism, and realized efficient magnetic-thermal conversion. Moreover, our prepared MXene@Fe3O4-MA composite PCMs exhibited excellent energy conversion stability, thermal cycle stability and reliability after undergoing multiple thermal cycles. Therefore, our designed novel photothermal and magnetothermal energy storage composite PCMs based on MXene@Fe3O4 will be a promising candidate to improve the practical solar energy and magnetic energy utilization.
2 EXPERIMENTAL SECTION
2.1 Materials
Hydrochloric acid was purchased from Sinopharm Chemical Reagent Co., Ltd. Lithium fluoride. FeCl3⋅6H2O, FeCl2⋅4H2O, Cetyltrimethylammonium bromide (CTAB) and NaOH (10 M) were purchased from Aladdin. Myristic acid(MA, AR, 99%)was purchased from Macklin. Ti3AlC2 (MAX, 98%, 400 mesh) powder was obtained from Macklin.
2.2 Preparation of MXene
Multilayer MXene was prepared by selectively etching Al layer of MAX.1 g LiF and 1 g Ti3AlC2 (MAX) were slowly added to 20 mL HCl (10 M) solution under vigorous stirring for 36 h at 35°C. The obtained product was dissolved in deionized water and centrifuged at 3600 rpm for several times until the supernatant was neutral. The precipitate was freeze-dried to obtain multilayer MXene powder.
2.3 Preparation of MXene@Fe3O4
MXene@Fe3O4 was prepared using an in situ chemical synthesis strategy. Typically, 150 mg MXene was added to a three-necked bottle with 100 mL deionized water. 87.5 mg FeCl3·6H2O and 80 mg surfactant CTAB were added into the reaction system and were vigorously stirred under N2 atmosphere at 55℃ for 0.5 h. Subsequently, 32.2 mg FeCl2·4H2O and NaOH standard solution were added to the mixture to adjust PH to be higher than 10, and then were mechanically stirred for 4.5 h. Finally, the product was washed with deionized water to adjust to neutrality and then freeze-dried to obtain MXene@Fe3O4. In this study, MXene@Fe3O4 with different ratios of MXene and Fe3O4 (4:1, 4:2, 4:3 and 4:4) were prepared by adding different quality of FeCl2 and FeCl3 using the same method.
2.4 Preparation of MXene@Fe3O4-MA composite PCMs and MXene@Fe3O4-MA-PU film
MXene@Fe3O4-MA composite PCMs were prepared using a physical vacuum infiltration method. First, 350 mg MA was added to the ethanol solution at 80°C until MA was completely melted. Then, MXene@Fe3O4 powers were slowly added into the MA-ethanol solution under vigorous stirring for 1 h. Finally, the obtained mixture underwent vacuum drying to obtain MXene@Fe3O4-MA composite PCMs at 80°C for 24 h.
MXene@Fe3O4-MA solution was mixed with waterborne elastic polyurethane, in which the loading content of MXene in PU was 1.5 wt%. The resulting mixture was uniformly dispersed under ultrasound and stirring. Subsequently, flexible MXene@Fe3O4-MA-PU films were prepared by coating the solution onto the glass substrate and drying at 80°C for 5 h.
2.5 Simulations
All of the simulated spectra and electromagnetic field distribution were obtained by solving three-dimensional Maxwell equations with the commercial COMSOL Multiphysics software, which is based on Finite Element Method (FEM). The periodic boundary condition was used to simulate the LSPR effect of MXene and MXene@Fe3O4 under incident light. The boundary conditions were set as follows: 1) the incident light illuminated MXene@Fe3O4 in a direction of 45°, 2) the refractive index of Fe3O4 and MXene was 2.34 + 0.11i and -10.75 + 11.28i, respectively, 3) the interlayer spacing of MXene was 150 nm, and the size of Fe3O4 nanoparticle was 100 nm.
2.6 Characterizations
The microscopic morphologies were observed with scanning electron microscope (SEM, SU8010, Japan Hitachi). The functional groups were evaluated by Fourier transform infrared spectrometer (FT-IR, Nicolet 6700, USA). X-ray Diffraction (XRD, M21X, Cu Ka radiation at 40 kV and 150 mA, λ¼0.15406 nm) was used to define the crystal structures. The pore structure was analyzed by nitrogen adsorption and desorption analyzer (Micromeritics ASAP 2420). Thermogravimetric analyzer (TGA, 449F3, Germany) was carried out to explore the thermal stability from 40 to 800℃ at a heating rate of 10℃/min under N2 atmosphere. Thermal properties of pristine MA and MXene@Fe3O4-MA composite PCMs were characterized by differential scanning calorimeter (DSC, DSC3, Mettler Toledo, Switzerland) under 50 mL/min N2 flow at a heating/cooling rate of 10°C/min. The thermal conductivity of sample was measured via a flash method with a laser thermal conductivity analyzer (Netzsch LFA 427, Germany) at room temperature. The samples were pressed into disc-shaped with the diameter of 12.7 mm and the thickness of 1 mm. Then, the pressed standard samples were caught in the disc of analyzer to complete the test. Each sample was tested three times to ensure the accuracy and reliability of the results. The light absorption capacity was evaluated by ultraviolet-visible-near-infrared (UV-Vis-NIR) spectrometer (Agilent, Cary 5000). Vibrating sample magnetometer (VSM) (Quantum Design, USA) was used to test the magnetic properties at room temperature. The thermal response rates were recorded by an infrared thermal imager. The photothermal energy conversion and storage capacity was tested under simulated solar (CEAULIGHT, CEL-S500), and the temperature-time curves were measured by a digital data collector (R2100). An alternating current generator was used to provide an alternating magnetic field for magnetic-thermal conversion and storage, and the temperature-time curves were measured by an optical fiber sensor. The in situ observation of crystal evolution of composite PCMs and MA were performed on a polarizing optical microscopy (POM).
3 RESULTS AND DISCUSSION
3.1 Structural analysis of MXene@Fe3O4
Lithium fluoride and hydrochloric acid solutions were used to etch the Al atomic layer in the MAX phase (Ti3AlC2), thus yielding accordion-like multilayer MXene (Ti3C2) (Figure S1C,D). As seen from XRD patterns, the most peaks of MAX disappeared in MXene, especially the characteristic peak of 38.8° (Figure S1A), indicating the successful preparation of MXene. MXene@Fe3O4 composites were prepared by in situ synthesizing Fe3O4 in MXene (Figure 1A). Free Fe3+ and Fe2+ ions were attached to the surface and interlayer of MXene through electrostatic adsorption due to a large number of F– and OH– ions on the surface of MXene, therefore, Fe3O4 nanoparticles were in situ synthesized under alkaline conditions. Under the assisted action of a large number of negative ions on the surface and the layers of MXene, metal sources of magnetic nanoparticles were firmly anchored on MXene during the in situ synthesis process, and Fe3O4 nanoparticles were uniformly dispersed on the outer surface of MXene and the interlayer space, which would effectively solve the unevenness problems of mechanical, magnetic and heat transfer caused by the agglomeration of magnetic nanoparticles. To further confirm the uniform distribution of Fe3O4 nanoparticles in MXene, the element mapping test of MXene@Fe3O4 was carried out. As shown in Figure S2A–D, C, Ti and Fe elements were uniformly distributed in MXene@Fe3O4, indicating the successful preparation of MXene and the uniform distribution of Fe3O4 nanoparticles in MXene.
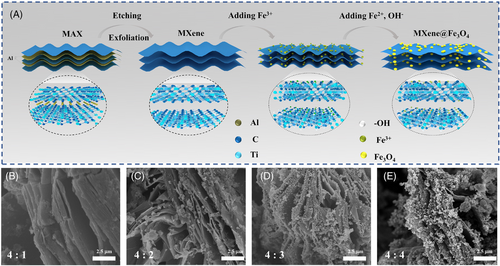
Meanwhile, the average layer spacing of MXene was about 150 nm, and the average size of Fe3O4 nanoparticles was about 100 nm, which provides sufficient space condition for the in situ anchoring of Fe3O4 nanoparticles in the interlayer of MXene (Figure 1B–E and Figure S2E). When the ratio of MXene to Fe3O4 was 4:1, the content of Fe3O4 nanoparticles was too small to observed on the surface and interlayer of MXene. When the ratio increases to 4:2 and 4:3, Fe3O4 nanoparticles can be obviously observed and evenly distributed on the surface and interlayer of MXene. However, with the increase of Fe3O4 content, Fe3O4 nanoparticles tended to agglomerate between MXene layers. Overly agglomerated Fe3O4 nanoparticles in MXene reduce the light absorption ability of MXene, thus influencing the photothermal conversion efficiency of MXene-based composite PCMs.
According to XRD patterns of MXene@Fe3O4 (Figure S1B), the peaks located at 30.0°, 35.3°, 43.0°, 57.0° and 62.7° corresponded to the crystal planes of (220), (311), (400), (511), and (400) of the face-centered cubic Fe3O4, respectively, and the characteristic peak of MXene (002) was also included, indicating the successful preparation of MXene@Fe3O4. In addition, the (002) peak of MXene@Fe3O4 shifted to a low angle (Inset of Figure S1B) compared with MXene, because the in situ formation of Fe3O4 in MXene expanded the interlayer spacing of MXene. To characterize the pore structure of MXene@Fe3O4, N2 adsorption-desorption isotherm was determined (Figure S3). The isotherm of 4:1 MXene@Fe3O4 was type IV with an obvious H3-type hysteresis loop, which was typical for a mesoporous material. The pore sizes of 4:1 MXene@Fe3O4 were mostly distributed between 2–15 nm, which clearly suggested the existence of mesoporous structure. Compared with macropores, mesopores can provide sufficient capillary force to prevent the leakage of liquid PCMs. Mesopores also can provide sufficient space for the free crystallization behavior of PCMs, which can efficiently store and release more heat, and provide mass and heat transfer channels. Therefore, our constructed mesoporous MXene@Fe3O4 provides the possibility to obtain MXene@Fe3O4-based composite PCMs with excellent thermal properties.
3.2 Thermal analysis of MXene@Fe3O4-MA composite PCMs
MXene@Fe3O4-MA composite PCMs were prepared via a vacuum impregnation method. Before loading, vacuum activation of MXene@Fe3O4 was performed to remove the adsorbate in the pores and open the pores. By comparing N2 adsorption and desorption isotherms of MXene@Fe3O4 and composite PCMs (Figure S3), we find the pore volume and specific surface area of composite PCMs are both nearly zero, indicating that abundant MA is fully filled into the pores of MXene@Fe3O4. The XRD and FTIR spectra of MA, MXene@Fe3O4, MXene@Fe3O4-MA pristine were shown in Figure S4. MA has characteristic diffraction absorption peaks at 2θ of 22.5° and 24°. The position of XRD characteristic diffraction peak of MXene@Fe3O4-MA composite PCMs were basically consistent with that of MA and MXene@Fe3O4 (Figure S4B). As seen from FT-IR spectra, the characteristic peaks of -OH at 3415 cm–1 for MXene@Fe3O4, -CH3 at 2920 cm–1 and -CH2 at 2850 cm–1 for MA were well maintained in MXene@Fe3O4-MA, and no new characteristic peaks appeared. These results indicated that only physical interaction occurred without chemical reaction between MA and MXene@Fe3O4 (Figure S4A).
Thermal energy storage efficiency is an important evaluating parameter for composite PCMs. The DSC results exhibited that the addition of MXene and Fe3O4 can effectively reduce the supercooling degree of MA from 13.78℃ to 10.04℃ (Figure 2A,B). The supercooling is mainly caused by the poor self-nucleation ability of the materials during crystallization, which is not conducive to the storage of latent heat. In this experiment, the in situ chemically synthesized Fe3O4 nanoparticles act as nucleating agents in the system, which made MA transform from the original homogeneous nucleation to heterogeneous nucleation, accelerated the crystallization speed, refined the grain structure, and reduced the degree of supercooling.[35]
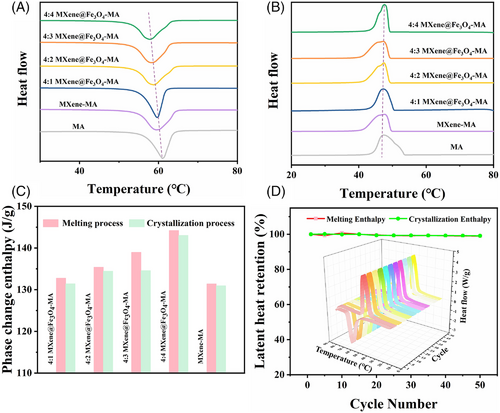
Notably, with the increase of Fe3O4 content, the phase change enthalpies of MXene@Fe3O4-MA composite PCMs loading 70 wt% MA increased from 132.76 to 144.17 J/g (Figure S5), which were increased by 1.04% and 9.60%, respectively, compared with MXene-MA (Figure 2C and Table S1). These results indicated that MXene can be used as a supporting material to encapsulate MA without leakage due to its good adsorption and capillary force. Meanwhile, the addition of Fe3O4 can effectively improve the phase change enthalpies of MXene-based composite PCMs. This may be because the adding magnetic Fe3O4 nanoparticles can provide heterogeneous nucleation sites compared with the homogeneous nucleation of MA, thus further improving the phase change enthalpy of MXene-based composite PCMs. Therefore, it can be concluded that more magnetic Fe3O4 nanoparticles can provide more nucleation sites, thus leading to a higher phase change enthalpy. Furthermore, the phase change temperatures of MXene@Fe3O4-MA composite PCMs were almost consistent with the original samples, and the latent heat retention was nearly 100% after undergoing 50 heating/cooling cycles (Figure 2D), indicating the excellent thermal repeatability and stability.
Shape stability and thermal stability are also essential evaluating parameters for the practical application of composite PCMs. Thermal stability of composited PCMs is investigated by TGA (Figure S5). Pristine MA showed totally thermal decomposition from 200 to 300℃. The supporting material (MXene@Fe3O4) presented almost no mass loss from 40 to 800℃. The remaining mass fraction of MXene@Fe3O4-MA was about 30% when the temperature reached 800℃. Obviously, MXene@Fe3O4-MA composite PCMs exhibit high thermal stability below 200℃, which is very important for medium and low temperature practical application. To evaluate the shape stability of pristine MA and MXene@Fe3O4-MA composite PCMs, all the samples were placed in an oven at 80℃. All these samples can maintain their original solid shape at room temperature (25℃). When held at 80°C for 25 s (higher than the melting point of MA), pristine MA began to melt and turns into liquid at 40 s, while MXene@Fe3O4-MA composite PCMs still maintained the solid shape completely without liquid leakage at 80℃ for 60 min (Figure S6). Additionally, the thermal conductivities of MXene@Fe3O4-MA with the ratio of 4:1, 4:2, 4:3 and 4:4 are 0.329, 0.323, 0.321 and 0.315 W/m·K, respectively, which are 49.5%, 46.8%, 45.9% and 43.2% higher than that of pristine MA (0.22 W/m·K) due to the synergistic effect of MXene and Fe3O4 (Figure S7). Firstly, MXene is an excellent thermal conductor due to its intrinsic metal-like conductive structure. Secondly, the homogeneously distributed magnetic Fe3O4 nanoparticles can realize the radial migration of heat from the surface to the interior, shorten the heat diffusion path, thus further improving the thermal diffusion capacity. Thirdly, the magnetic Fe3O4 nanoparticles grown in situ can reduce the interfacial thermal resistance and accelerate the phonon transmission, so as to improve the thermal conductivity (Figure 3F).
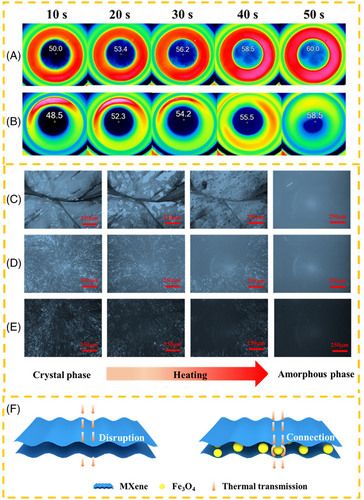
Additionally, polarizing optical microscopy (POM) was applied to the in situ observation of crystal evolution of MA, MXene-MA, and MXene@Fe3O4-MA composite PCMs during the heating process. As seen from Figure 3C–E, MA exhibited a clear cross-extinction mode spherulite morphology at room temperature. This phenomenon can also be observed in MXene-MA and MXene@Fe3O4-MA, but the spherulite size was significantly smaller and the crystal morphology became irregular. This is because MXene and MXene@Fe3O4 limited the fluidity of MA molecules in composite PCMs, and the crystallization process of MA molecules in composite PCMs became heterogeneous nucleation from homogeneous nucleation, which refined the grains. However, the presence of MXene and MXene@Fe3O4 did not change the crystalline structure of MA molecules in composite PCMs. With increasing temperature, the ordered spherical crystals in MXene-MA and MXene@Fe3O4-MA composite PCMs were gradually transformed into disordered amorphous structure until the complete evolution of MA molecules, which were consistent with the evolution of pristine MA, indicating the existence of MXene and Fe3O4 nanoparticles did not affect the crystallization behavior of MA molecules.
3.3 Photothermal energy conversion
Inherently, MXene exhibits an excellent electromagnetic wave absorption ability. The absorbed electromagnetic waves can across the MXene lattice structure and undergo internal reflections between the layers, and eventually are absorbed within the structures, which is an essential prerequisite for the photothermal conversion behavior.[36] The UV-Vis-NIR absorbance spectra of MXene and MXene@Fe3O4 were shown in Figure 4B. Compared with pristine MA (Figure S8), MXene and MXene@Fe3O4 exhibited almost complete absorption ability in the entire UV-Vis-NIR range. Notably, two enhanced absorption peaks were observed at 680 and 1150 nm due to the LSPR effect of MXene and Fe3O4 nanoparticles. Moreover, compared with MXene, the absorption peak of MXene@Fe3O4 at 1150 nm was significantly enhanced due to the excellent light absorption of Fe3O4 nanoparticles in the near infrared range, which is conducive to the rapid solar harvesting for photothermal conversion and storage of MXene@Fe3O4-MA composite PCMs. Benefiting from its metal-like and semi metallic properties, MXene is endowed with significant LSPR effect. Meanwhile, Fe3O4 nanoparticles, as photothermal reagents, also have LSPR effect. The synergistic LSPR effect of MXene and Fe3O4 nanoparticles can further improve the photothermal conversion performance of MXene@Fe3O4-MA composite PCMs (Figure 5A).
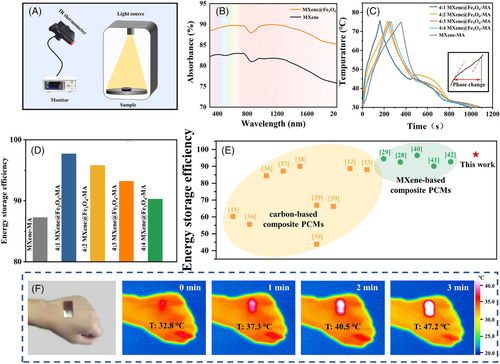
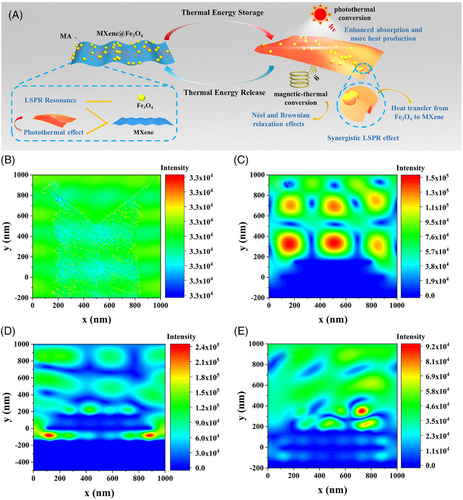
Figure 4C presented the photothermal energy conversion curves of MXene@Fe3O4-MA composite PCMs under the simulated solar of 0.15 W/cm2. When the solar source was switched, the temperature of the prepared composite PCMs rapidly increased from 30℃ to 52℃. When exceeding 52℃, the temperature began to rise slowly between 52℃ to 60℃, corresponding to the photothermal conversion process of MXene@Fe3O4-MA composite PCMs, which was consistent with the phase change temperature. The absorbed solar energy was stored in the form of latent heat. Besides, compared with other ratios of MXene@Fe3O4 (4:2, 4:3, 4:4), MXene@Fe3O4 (4:1)-MA composite PCMs showed a fastest heating rate and reached the phase change point first, indicating that its photothermal conversion rate was the highest. The reason may be that excessive Fe3O4 content would agglomerate on the surface of MXene, affecting the photo absorption performance of MXene, thereby weakening the photothermal conversion performance of the composite PCMs. Too little Fe3O4 content cannot form a heat transfer channel between the MXene layers, slowed down the thermal diffusion rate, which in turn affects the photothermal conversion performance. Meanwhile, it took only 180 s for MXene@Fe3O4 (4:1)-MA composite PCMs to rise temperature from 30℃ to 75℃, which shortened the illumination time by 55% compared with MXene-MA composite PCMs (400 s) under the same light radiation, indicating its potential application value in the field of rapid heating.
As shown in Figure 4D, the photothermal storage efficiencies of MXene@Fe3O4 (4:1, 4:2, 4:3, 4:4)-MA composite PCMs were 97.7%, 95.8%, 93.2% and 90.2%, respectively, which were higher than MXene-MA (87.26%). Interestingly, as the content of Fe3O4 nanoparticles increases, the photothermal storage efficiency decreases, which was consistent with the results of Figure 4C. This phenomenon can be explained as follows: 1) The synergistic LSPR effect of MXene and Fe3O4 nanoparticles enhanced the light absorption ability of MXene@Fe3O4-MA composite PCMs; 2) A small amount of Fe3O4 nanoparticles in situ anchored in the MXene layers could provide a fast transfer channel for phononic diffusion, and prolong the mean free paths of phonons, thus, improving the heat transfer rate; 3) Excessive Fe3O4 nanoparticles tend to agglomerate on the surface of MXene, which conversely reduce the solar-trapping properties of MXene@Fe3O4.
To further confirm the synergistic photothermal enhancement effect of MXene and Fe3O4 prepared by an in situ synthesis strategy, MXene/Fe3O4 composite was prepared by a physical mixing method (Figure S9B). After comparing the photothermal conversion curves, the photothermal conversion efficiency of MXene@Fe3O4 (4:1)-MA composite PCMs was better than that of MXene/Fe3O4 (4:1)-MA (η = 86.2%). Generally, carbon-based composite PCMs were widely reported for photothermal conversion with a photothermal efficiency from 55.5% to 92.0%.[11, 12, 37-40] Recently, MXene-based composite PCMs gradually were reported for photothermal conversion with a photothermal efficiency from 90.1% to 96.5%.[28, 29, 41-43] Comparatively, our prepared MXene@Fe3O4-MA composite PCMs in this study showed a higher photothermal conversion efficiency (97%) under the synergistic effect of MXene and Fe3O4 nanoparticles (Figure 4E and Table S2).
Additionally, we also measured the photothermal conversion curves of MXene@Fe3O4 (4:3)-MA under different light intensities (Figure S9A). The photothermal conversion ability of composite PCMs were correspondingly improved with the increased light intensity from 0.1 W/cm2 to 0.2 W/cm2. This is because higher light intensity can induce more photothermal electrons due to LSPR effect, meanwhile, MXene@Fe3O4-MA can absorb more light waves per unit time.
Considering the excellent photothermal conversion efficiency of MXene@Fe3O4 (4:3)-MA, we mixed it with waterborne elastic polyurethane (PU) to prepare wearable thermal management MXene@Fe3O4-MA-PU film. As shown in Figure 4F, the prepared MXene@Fe3O4-MA-PU composite film was attached on the skin of the hand, and infrared thermal imaging was used to record the surface temperature evolution of the hand. After being exposed to the sun for about 3 min, the temperature of the composite film increased from 32.8°C to 47.2°C, while the temperature of other parts of the hand had very little change. These results indicated that MXene@Fe3O4-MA-PU composite film exhibited excellent photothermal conversion performance in practical applications. In the cold environment, it can be a wearable flexible heating material driven by solar.
To further reveal the synergistic LSPR effect between MXene and Fe3O4 nanoparticles and provide a deep understanding of the photothermal transformation phenomenon in MXene@Fe3O4-based composite PCMs, the numerical models of Finite Element Method (FEM) based on Maxwell formula were established (Figure 5B–E). In particular, the optical properties of materials are dominated by their LSPR, defined as the collective motions of the conduction electrons induced by light irradiation, which is associated with a considerable enhancement of the electric field. The surrounding electric field intensity of MXene@Fe3O4 (4:1) was about 2 times and 8 times higher than that of MXene and pure air when the incident light irradiated. In addition, the electric field intensity of MXene@Fe3O4 (4:4) decreased instead, indicating that the agglomeration of magnetic Fe3O4 nanoparticles reduced the synergistic LSPR effect between MXene and Fe3O4 nanoparticles (Figure 5B–E), which were consistent with the experimental results of photothermal conversion. Therefore, the synergistic LSPR effect between MXene and Fe3O4 nanoparticles can be proved by comparing the simulated induced electric field intensity.
3.4 Magnetic-thermal energy conversion
VSM was conducted to evaluate the magnetic property of MXene@Fe3O4 (Figure 6B). Obviously, MXene@Fe3O4 exhibited an extremely low magnetic retentivity and coercivity, indicating its superparamagnetic nature. These superparamagnetic characteristics mean that an applied magnetic field can attract MXene@Fe3O4 while there is no residual magnetism when the magnetic field is removed. Therefore, the magnetic Fe3O4 nanoparticles did not tend to aggregate together after removing the magnetic field. Figure 6C showed the temperature-time curves of MXene@Fe3O4-MA composite PCMs with different contents of Fe3O4 nanoparticles under an alternating magnetic field (the frequency of 1.05 MHz, the alternating current of 10 A). When the alternating magnetic field was applied, the temperature of MXene@Fe3O4-MA increased rapidly from room temperature to 50℃ in the form of sensible heat, then the temperature platform appeared on the curves and the generated thermal energy was stored in the form of latent heat. After the phase change process of MA was completed, the temperature further increased rapidly. In addition, the intensity of alternating magnetic field could be enhanced by increasing the intensity of alternating current. It can be seen that the magnetic-thermal conversion capacity increased when the intensity of the alternating magnetic field increased (Figure 6D). These results indicated that MXene@Fe3O4-MA exhibited excellent magnetic-thermal conversion and storage capacity under an alternating magnetic field.
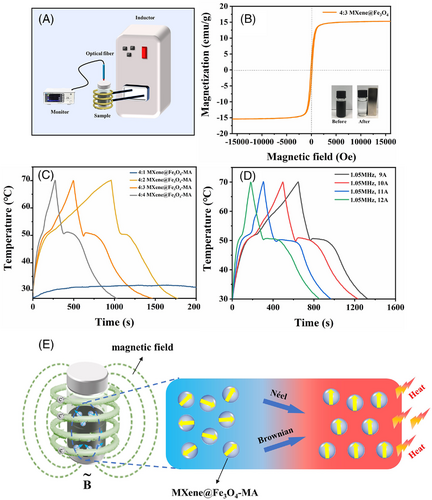
The principle of magnetic-thermal effect can be explained as follows: the orientation of the magnetic moment is disordered in the substance without an applied magnetic field according to the Néel and Brownian relaxation effects (Figure 5A). At this time, the magnetic entropy is larger and the adiabatic temperature of the system is lower. After a magnetic field is applied, the magnetic Fe3O4 nanoparticles are magnetized, and the molecular magnetic moment tends to be aligned. Resultantly, the magnetic entropy decreases and the adiabatic temperature increases, which in turn releases heat to the environment through heat exchange (Figure 6E).
3.5 Cyclic stability
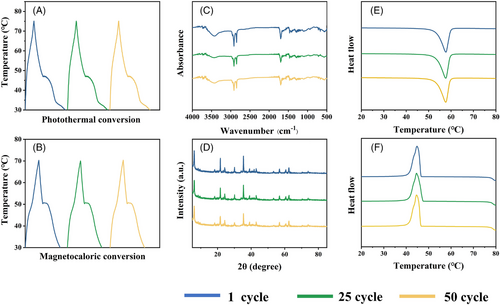
Cyclic stability of thermal energy conversion and storage is very important for composite PCMs. In this study, our prepared MXene@Fe3O4-MA composite PCMs were subjected to 50 photothermal and magnetic-thermal cycles (Figure 7A,B). Resultantly, the photothermal and magnetic-thermal conversion curves after 50 cycles are almost consistent with the original curves, indicating excellent cyclic stability of thermal energy conversion. Additionally, the XRD, FT-IR and DSC results were analyzed after 50 cycles (Figure 7A–F). The phase structures and functional groups types of MXene@Fe3O4-MA composite PCMs are almost unchanged. Importantly, the phase change temperature and latent heat of MXene@Fe3O4-MA composite PCMs after cycling are almost similar to that before cycling (Figure 7E, F and Table S3). The XRD, FT-IR and DSC results indicate that MXene@Fe3O4-MA composite PCMs show superior thermal cycle reliability, which provide a reliable guarantee for pratical application in the heat storage field.
4 CONCLUSION
In this study, we designed MXene@Fe3O4-MA composite PCMs for the simultaneous realization of excellent photothermal and magnetic-thermal conversion and storage via simple in situ chemical synthesis and vacuum infiltration methods. Compared with pristine MXene-based composite PCMs, MXene@Fe3O4-MA composite PCMs exhibited an ultrahight photothermal conversion efficiency of 97.7% due to the synergistic LSPR effect of Fe3O4 and MXene. Furthermore, a small amount of Fe3O4 nanoparticles in situ anchored in the MXene layers could provide fast transfer channels for photonic diffusion, and prolong the mean free paths of phonons, thus improving the heat transfer rates. Meanwhile, the superparamagnetism of Fe3O4 also endowed the composite PCMs with magnetic-thermal conversion capability. These results showed that MXene@Fe3O4-MA composite PCMs have broad application prospects in the field of multifunctional thermal storage. Additionally, our prepared MXene@Fe3O4-MA composite PCMs exhibited excellent photothermal and magnetic-thermal cycle stability, thermal cycle stability and reliability, showing a great possibility of renewable thermal energy storage applications.
ACKNOWLEDGMENTS
This work was financially supported by the National Natural Science Foundation of China (grant number: 51902025), Fundamental Research Funds for the Central Universities (grant number: 2019NTST29 and FRF-BD-20-07A), China Postdoctoral Science Foundation (grant number: 2020T130060 and 2019M660520), and Scientific and Technological Innovation Foundation of Shunde Graduate School, University of Science and Technology Beijing (grant number: BK20AE003). The authors congratulate the 70th anniversary of University of Science and Technology Beijing.
CONFLICT OF INTEREST
The authors declare no competing financial interest.
ETHICS STATEMENT
There are no ethics issues in this work.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




