A fluorescent film sensor for high-performance detection of Listeria monocytogenes via vapor sampling
Rongrong Huang and Meiqi Li have contributed equally.
Abstract
Foodborne Listeria monocytogenes poses serious threats to public health. Fast and sensitive detection of the pathogen at the point of contamination is thus crucial to halt the spread of bacteria-related diseases. Herein, we report for the first time a fluorescent film sensor to detect the biomarker of L. monocytogenes, 3-hydroxy-2-butanone. The sensor demonstrated unprecedented sensing performance for the analyte with a detection limit lower than 0.05 mg/m3, response time less than 1 s, full reversibility, and excellent selectivity. Further study showed that the sensor can be used to monitor the growth of L. monocytogenes with much-improved sensitivity. The superior performance of the sensor is ascribed to the specific binding, efficient charge transfer emission, and porous adlayer structure of the specially designed sensing fluorophore-based film. Our work paves the way to develop a portable detector to meet the needs for on-site and real-time detection of foodborne pathogens.
1 INTRODUCTION
Listeria monocytogenes, one of the most hazardous bacteria with a mortality rate as high as ∼25%, is widely found in vegetables, fruits, dairy products, poultry, and other foods.[1] They can breed over a wide range of temperature (0-45℃), high-salinity, and low-moisture environments. Patients infected by L. monocytogenes may suffer from bacteremia, complications, and meningitis, especially pregnant women, infants, young children, the elderly, and the sick.[2-4] According to the World Health Organization (WHO), there were 30 incidents worldwide caused by a biological hazard of L. monocytogenes during the first three quarters of 2021.[5] To date, various techniques have been proposed to detect L. monocytogenes in food, such as biochemical tests, chromogenic media, and ionization-time of flight mass spectrometry (MALDI-TOF MS).[6-10] These techniques offer high selectivity and sensitivity but suffer from some drawbacks, such as time-consuming procedures, labor-intensiveness, expensive instruments, and highly trained personnel. Therefore, it is of urgent demand to develop rapid, noninvasive, and easy-to-operate techniques[11-13] for the real-time and on-site detection of L. monocytogenes.
Recently, detection methods based on specific microbial volatile organic compounds (MVOCs) have attracted increasing attention, as they demonstrate promise to meet the requirements for fast, noninvasive, on-site, and real-time pathogen detection and identification.[7, 14, 15] 3-Hydroxy-2-butanone is the main component of the volatile metabolites of L. monocytogenes, and its concentration is highly dependent on breeding time.[16] Therefore, sensitive and selective detection of 3-hydroxy-2-butanone has become an efficient method for detecting L. monocytogenes in food. Gas/liquid chromatography with mass spectrometry is often used to analyze the metabolome of L. monocytogenes, showing high accuracy.[6-9] The method, however, requires sophisticated and bulky equipment, as well as pretrained personnel, which affects its utilization for rapid, cost-effective, and field-deployable surveillance of foodborne pathogens. Chemiresistive “electronic noses” based on different semiconductive materials were also reported for the detection of 3-hydroxy-2-butanone in the vapor phase,[17-25] but only one was used for a real sample test to monitor the growth of L. monocytogenes.[17] Nevertheless, reliable detection of the biomarker at even lower concentrations to enable early finding of L. monocytogenes remains a challenge, not to speak of the dedicated sensors or detectors.
Compared to other techniques, excited-state process-based[26-29] and small molecule-related[30-33] fluorescent techniques, especially those based on fluorescent films,[34-43] demonstrate great sensitivity, improved selectivity, and unprecedented designability. For film-based fluorescent sensors, however, the films must be highly porous and photochemically stable, as they are necessary for sensitive, reversible, and applicable sensing.[44, 45]
Icosahedral o-carborane is a widely employed building block to develop fluorophores possessing aggregation-induced emission (AIE) and screen dense packing in the film state owing to its unique spherical structure.[46-49] Moreover, ever-reported works proved the improved photochemical stability of o-carborane-related fluorophores.[50, 51] Therefore, in the present work, a pyrrole derivative of o-carborane (CbNPy, Figure 1) was purposely designed using o-carborane and pyrrole as the electron acceptor and donor, respectively. In addition, the pyrrole group is expected to function as a binding site for 3-hydroxy-2-butanone. Based on the fabricated CbNPy-film, a centimeter-sized, fluorescent 3-hydroxy-2-butanone sensor was developed. The sensor showed superior sensing performance to the concerned foodborne pathogen than the ever-reported sensors. Moreover, noninvasive, on-site, real-time, and sensitive monitoring of the growth of L. monocytogenes was realized.
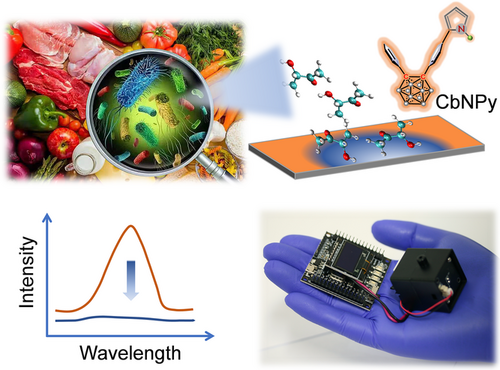
2 RESULTS AND DISCUSSION
2.1 Fluorescence behavior of CbNPy in solution and crystal state
The fluorescence emission spectra of CbNPy depicted in Figure 2A are characterized by two emission bands, where the broad band at longer wavelengths is highly dependent on the polarity of the solvent, an indication of intermolecular charge transfer (ICT) emission. Theoretical calculations using density functional theory (DFT) and time-dependent DFT (TD-DFT) at the CAM-B3LYP/6-31G (d, p) level revealed the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) distributions, as well as their energy levels involved in the transition corresponding to fluorescence emission (Figure 2B). The HOMO is mainly located on the phenyl-pyrrole moiety, while the LUMO partially transfers to the o-carborane and another phenyl unit, which confirms the ICT nature of the electronic transition.
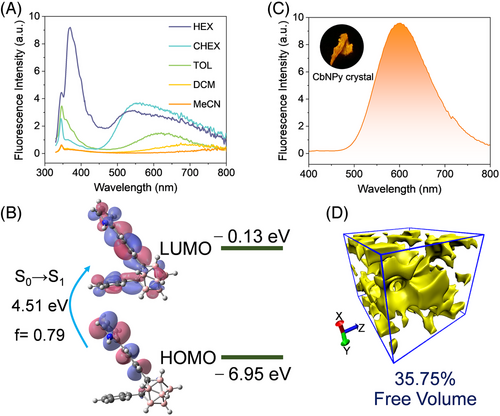
The CbNPy crystal shows strong orange emission at ∼600 nm (Figure 2C, Table S1) under irradiation at 365 nm, which could be a result of ICT owing to the existence of B-H···π (2.57 Å) bonds in the aggregated state (Figure S3). The bathochromic shift of the excitation spectrum of CbNPy in either the crystal or film state further confirms this argument (Figure S4). Regardless, the molecular arrangement in the crystal, not to mention in the film, allows the presence of interstitial space with a free volume up to 35.75%, a result ascribable to the nonplanar o-carborane structure (Figure 2D).[52, 53]
2.2 Fabrication of CbNPy film and validation of its sensitivity to 3-hydroxy-2-butanone
The CbNPy film (Film 1) can be simply fabricated in large quantities by printing a suitable CbNPy solution onto commercially available filter papers (Figure 3A). Compared to crystal emission, the emission of CbNPy in the film state (Figure 3B) is characterized by two emissions, where one is strong and probably derived mainly from the ICT state, and the other is weak and from the locally excited (LE) state. The excitation spectra recorded from the CbNPy film, the crystal and the hexane solution support this argument (Figure S4). The appearance of the LE emission, bathochromic shift of the ICT emission, and especially the comparability between the excitation spectrum of the film and that of the solution must be the result of loose packing of CbNPy compared with that in the crystal state, which favors mass transfer and thus sensing of the analyte.[38, 54] In fact, the fluorescence emission of the film decreased dramatically upon fuming with 3-hydroxy-2-butanone (Figures 3B and 3C), providing potential for detection via vapor sampling.
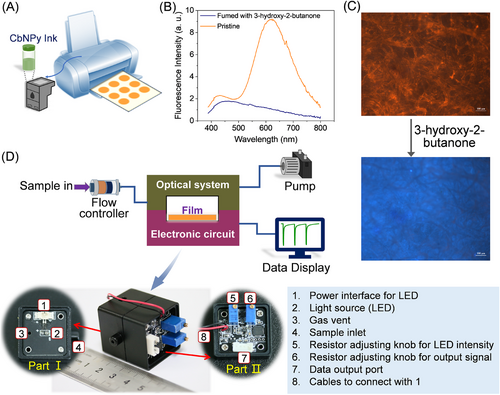
2.3 CbNPy-based fluorescent film sensor
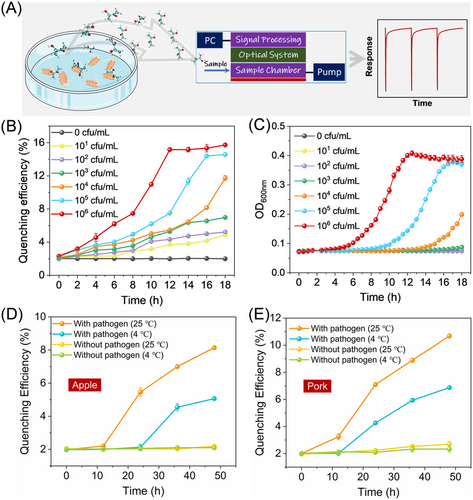
A fluorescent film sensor with CbNPy as the sensing fluorophore was constructed by integrating an electronic system, an optical system, a CbNPy-based sensing film, and a gas sampling unit (Figure 3D). The sensor can be connected to a pump and a display panel to allow surveillance online and in real time. To conduct a sensing process, the gas sample of a known concentration was pulse sampled (∼10 s) into the sample chamber of the sensor, and then the chamber was fully purged with clear air to restore the output of the sensor. Switching between the two operations was realized by using a solenoid valve. In this way, response traces can be generated.
2.4 Sensing performance of the sensor toward 3-hydroxy-2-butanone
As one of the crucial factors affecting the practical applications, the photochemical stability of the CbNPy-based film was first evaluated. As shown in Figure S5, 9-h continuous illumination with 360 nm light showed no observable attenuation in the emission intensity of the film, laying a foundation for the sensing test. Further study showed that the film could maintain the same response behavior over at least 3 months (Figure 4A). Figure 4B shows that saturated 3-hydroxy-2-butanone vapor could induce more than 90% reduction in the fluorescence emission of Film 1, but the quenching could be fully reversed by purging clear air, indicating its usefulness for sensing. Moreover, more than 100-cycle continuous tests showed no noticeable decay of the sensor's performance.
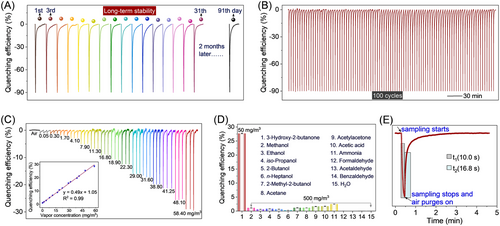
Figure 4C shows that the quenching efficiency increased linearly along with increasing the analyte concentration from 0.05 to 58.41 mg/m3, suggesting that the developed sensor is able to detect the analyte at concentrations greater than 0.05 mg/m3, which is much lower than ever-reported sensors (Table S2).[17-25]
Selectivity tests of the film sensor revealed that the selected interfering gases, which are other main chemicals in the exhausted gases of L. monocytogenes,[16, 17] showed negligible effects upon sensing even though their concentrations (500 mg/m3) were 10 times that of the concerned 3-hydroxy-2-butanone (50 mg/m3) (Figure 4D, Figure S6). Moreover, water vapor also showed little effect on the sensing, which is also crucial for the developed sensor to find real-life applications.
As shown in Figure 4E, the sensor showed an immediate response (<1 s) toward the biomarker vapor. The signal reached a maximum at the end of the corresponding pulse sampling, which is 10 s, as mentioned above. The signal began to return upon finishing the sampling. The recovery time (the time taken by the sensor output from the maximum to 90% recovery of the initial intensity) is less than 17 s. To illustrate the advantages of the developed sensor, a full comparison with recently reported 3-hydroxy-2-butanone sensors was made (Table S2). Clearly, our sensor would be applicable for the on-site and real-time detection of L. monocytogenes in contaminated foods owing to its fast, sensitive, selective, and reversible sensing performance.
2.5 Listeria monocytogenes detection
The sensing performance of the developed film sensor toward the incubation of L. monocytogenes solutions (Figure 5A) with concentrations from 101 to 106 cfu/mL was further examined. The results are depicted in Figure 5B. Compared to the widely adopted turbidity method and the only reported sensor ever used for monitoring the growth of L. monocytogenes,[17] our film sensor showed much improved sensing performance, as the presence of L. monocytogenes can be found at a much earlier stage (Figure 5C), demonstrating great potential for the sensor to be developed into a portable dedicated fluorescent L. monocytogenes detector. Additionally, we investigated the sensing performances of the developed sensor toward foods infected with L. monocytogenes. As shown in Figures 5D and 5E, the sensor shows a significant response toward the infected foods, especially those stored at room temperature (25℃), probably due to the higher propagation ability at 25℃. The food stored in the refrigerator could also induce a response of the sensor at later time. According to the Codex Alimentarius Commission (CAC), for ready-to-eat foods in which growth of L. monocytogenes will occur, the criteria for L. monocytogenes are less than 100 cfu/g.[55, 56] Therefore, our developed sensor is promising for fast and on-site surveillance of L. monocytogenes in foods.
2.6 Sensing mechanism
TD-DFT calculations revealed the absorption properties of CbNPy and the possible associating complex formed between CbNPy and 3-hydroxy-2-butanone. As shown in Figure 6A, complexation with the analyte leads to a large perturbation to the orbital distribution and energy gap of CbNPy. The HOMOs of CbNPy-3-hydroxy-2-butanone and CbNPy are mainly located on their phenyl and pyrrole units, whereas the LUMO of the former partially transfers from the pyrrole unit to 3-hydroxy-2-butanone, resulting in a remarkable decrease in the oscillator strength f for the S0→S1 transition from 0.79 (pure CbNPy) to 0. This means forbidden of the corresponding transition, which explains why the presence of the analyte will quench the fluorescence emission of the CbNPy-based film.
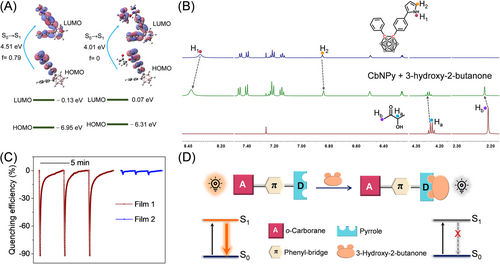
Specific binding of the analyte by the synthesized fluorophore is confirmed by the results from 1H NMR studies. Figure 6B depicts the 1H NMR traces of CbNPy upon addition of 3-hydroxy-2-butanone. Obviously, the proton signals of H1 on pyrrole and Ha and Hb on 3-hydroxy-2-butanone exhibited some downfield shifts, but H2 on pyrrole showed an upfield shift, strongly indicating complexation between them. Further temperature-dependent 1H NMR measurements revealed that the binding constant of the sensing fluorophore CbNPy to the analyte in DMSO-d6 is 3.57 × 103.
To specify the interaction site of CbNPy with 3-hydroxy-2-butanone, a compound of CbMPy was designed, where the H atom of pyrrole in CbNPy was replaced by a methyl group (Scheme S1), and used to fabricate a reference film (Film 2). As shown in Figure 6C, Film 2 exhibited a 22-fold lower response toward the saturated vapor of 3-hydroxy-2-butanone (∼4%), again confirming that the specific association is crucial for the selective and sensitive sensing of the biomarker. Accordingly, the sensing process can be reasonably described by the cartoon shown in Figure 6D.
3 CONCLUSION
A new fluorescent film sensor was developed using a specially designed pyrrole derivative of o-carborane as a sensing fluorophore. The sensor displays multiple advantages toward the sensing of 3-hydroxy-2-butanone, a biomarker of L. monocytogenes, including fast response, full reversibility, good selectivity, and excellent stability. In addition, the experimental detection limit to the analyte is lower than 0.05 mg/m3 (<0.02 ppm). Further studies demonstrated its applicability for the early discovery of L. monocytogenes. The superior sensing performance of the sensor as developed is ascribed to the preferential affinity of the sensing fluorophore to 3-hydroxy-2-butanone, porous adlayer structure, efficient charge transfer emission of CbNPy in the aggregated state, and the great photochemical stability of the film. Our work is the first example to detect a 3-hydroxy-2-butanone biomarker via a fluorescent film technique. We believe that the sensor has great potential to be developed into a portable and dedicated L. monocytogenes detector for meeting the needs to monitor food safety on-site and in real time.
4 EXPERIMENTAL SECTION
4.1 Chemicals and synthesis
Chemical reagents were obtained commercially and used without further purification. All solvents used were at least analytical grade and obtained from China Sinopharm Chemical Reagent Co. Ltd. Solvents used in fluorescence measurements and chemical reactions were distilled in advance. Detailed synthesis methods and characterization data are provided in the Supporting Information.
4.2 Instruments and characterizations
1H NMR, 13C NMR, and 11B NMR spectra were obtained on a Bruker AV 600 NMR spectrometer. Mass spectra (MS) were obtained from Bruker Microflex using an APCI source. UV–vis absorption spectra were obtained on a U-3900 (Hitachi) spectrophotometer. Fluorescence measurements were carried out on an FLS 980 (Edinburgh). Absolute fluorescence quantum yields were recorded on a quantum efficiency measurement system (Hamamatsu, Quantaurus-QY). Single X-ray diffraction (SXRD) measurements were made by Bruker Smart Apex 2. The fluorescent images were taken by a Canon E70D digital camera. Fluorescence images of the film morphology were taken on a laser confocal fluorescence microscope (Olympus FV1200). The bacteria were cultured in a constant-temperature oscillator and incubator at 37℃. The optical densities (ODs) of the systems were recorded on a SpectraMax M5 at the wavelength of 600 nm.
4.3 Film fabrication and sensing measurements
4.4 Bacterial culture
The sample of L. monocytogenes (ATCC 19111) was first spread onto the PALCAM medium slant for activation for 24 h at 37℃. Then, a single colony from the slants was incubated in tryptic soy broth and grown overnight at 37℃ in a gyratory shaker at 180 r/min. After that, the obtained bacterial culture was diluted to 101, 102, 103, 104, 105, and 106 cfu/mL. The diluted samples were incubated for 18 h at 37℃, and the sensing tests were performed every 2.0 h. The variability of the bacterial growth was also investigated by measuring the optical densities of the samples every 0.5 h on a microplate reader.
4.5 Crystal information
CCDC 2145443 (CbNPy) and 2145444 (CbMPy) contain the supplementary crystallographic data for this study. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
CONFLICT OF INTEREST
There are no conflicts of interest to declare.
ACKNOWLEDGMENT
This work is supported by funding from the National Natural Science Foundation of China (21820102005, 22132002), 111 Project (B14041), and Fundamental Research Funds for the Central Universities (2019CBLY001). Binbin Zhai, Jiaqi Tang, and Qingwei Jiang are thanked for their help in taking the photo images. We also appreciate Taihong Liu and Zhongshan Liu for their suggestions and discussions on this work. Rongrong Huang and Meiqi Li contributed equally to this work.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




