Aggregation-induced emission luminogens for organic light-emitting diodes with a single-component emitting layer
Jinhyo Hwang and Peethani Nagaraju have equally contributed to this work.
Abstract
Organic light-emitting diodes (OLEDs) have garnered increasing attention as a promising candidate for application in next-generation displays and solid-state lighting devices. The doping technique commonly used to fabricate light-emitting layers (EMLs) of OLEDs disperses the emitting material in a host matrix to mitigate aggregation-induced quenching (ACQ). Alternatively, owing to their high efficiency and ease of manufacture, nondoped OLEDs have been developed as a substitute for doped OLEDs. When fabricating nondoped OLEDs, it is essential to suppress the ACQ effect in the EML and efficiently utilize the generated excitons for radiative decay. Since the discovery of aggregation-induced emission (AIE) by Tang et al., significant work has been done to improve the stability and efficiency of nondoped OLEDs based on fluorescent AIE luminogens (AIEgens). Fluorescent AIEgens, synthesized using various molecular frameworks, such as silole, tetra/triphenylethylene, tetraphenylpyrazine, and tetraphenylbenzene, exhibit high photoluminescence quantum yields in the solid state. Therefore, many studies have reported that fluorescent AIEgen-based OLEDs have excellent external quantum efficiency and exhibit emission at various wavelengths, including deep-blue/blue/green emission. With the development of fluorescent AIEgens, delayed fluorescent AIEgens with efficient triplet utilization through reverse intersystem crossing and AIE properties have been adopted as ideal emitters and used in nondoped OLEDs. This review systematically discusses and summarizes recent reports on the development of AIEgens for nondoped OLEDs by grouping them into fluorescent and delayed fluorescent AIEgens. The purpose of this review is to provide deep insights into the design of novel AIEgens for high-performance nondoped OLEDs.
1 INTRODUCTION
Research on organic light-emitting diodes (OLEDs) based on organic semiconducting materials has drawn considerable attention from both academia and industry because of the numerous advantages of OLEDs, such as high flexibility, low weight, fast response, high brightness, wide viewing angle, high color quality, and cost effectiveness.
Since the pioneering work of Tang and VanSlyke,1 considerable efforts have been exerted for the development of high-performance materials and fabrication methods for OLEDs. These diodes are thin-film devices in which holes and electrons are injected into an emissive layer, wherein they recombine to generate singlet and triplet excitons at a ratio of 1:3, in accordance with the spin–statistics rule,2 and convert the generated electric current into light. OLEDs can be categorized into fluorescence- and phosphorescence-based devices on the basis of electrogenerated excitons (singlet and triplet excitons).
Initially, the emitting materials employed in fluorescent OLEDs used only singlet excitons for radiative emission, which result in an internal quantum efficiency (IQE) not exceeding 25%. Subsequently, Baldo et al.[3, 4] developed phosphorescent OLEDs (PhOLEDs) with higher efficiencies using a Pt-based complex as an emitter that utilized both singlet and triplet excitons via strong spin–orbit coupling (SOC) and achieved an IQE of 100%. However, the high cost of rare-earth metals (such as Os, Pt, and Ir) due to their low availability and toxicity limits the potential applications of these devices. Several alternative strategies, such as thermally activated delayed fluorescence (TADF),5 triplet–triplet annihilation (TTA),6 and hybridized local and charge transfer (HLCT),7 have been proposed to overcome the disadvantages of fluorescent and phosphorescent OLEDs. In 2012, Adachi et al.8 significantly contributed to the development of TADF materials for efficient OLEDs.
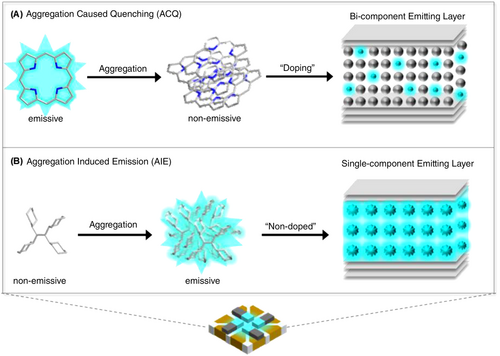
In 2001, Tang et al.9 reported that the AIEgens of tetraphenylsilole (TPS) can overcome the drawback of aggregation-caused quenching (ACQ). They showed low or no emission in the solution phase but become highly emissive in the aggregated state as they continuously undergo intramolecular vibrational and rotational motions in dilute solutions, which result in rapid nonradiative decay. By contrast, such motions are inhibited in the aggregated state, leading to highly suppressed nonradiative decay. Additionally, the twisted structures of AIEgens can weaken the intermolecular π–π stacking interactions in the aggregated state. Generally, AIEgens exhibit restricted intramolecular motion (RIMs) and twisted intramolecular charge transfer (TICT).
Since the discovery of the AIE phenomenon, fluorescent AIEgens have received considerable attention from researchers owing to their high photoluminescence quantum yield (PLQY) in the aggregated state. Within a few years, the application scope of AIEgen materials has gradually expanded to various fields and devices, such as organic optoelectronic devices,10 circular polarized luminescence (CPL),11 chemo- and bio-sensors,12 bioassays, biometric imaging,13 bioimaging and healthcare applications, and14 ion and explosive detection.15
Additionally, excellent device performance has also been reported by using fluorescent AIEgens in nondoped OLEDs containing a single-component EML. The nondoped fluorescent OLEDs manufactured by vacuum deposition and a solution process achieved the maximum efficiency exhibited by a fluorescent device. Notably, upon controlling the structure of the AIEgens, the OLEDs showed various emission wavelengths ranging from deep- blue to green. Significant research has been conducted by various researchers on fluorescent AIEgens based on different AIE cores, such as silole, tetra/triphenylethylene (TPE/TriPE), tetraphenylpyrazine (TPP), tetraphenylbenzene (TPB), and chiral binaphthyl.
Meanwhile, owing to the small energy gap (ΔEST) between their first excited singlet and triplet states, TADF materials harvest electrically generated singlet and triplet excitons through an efficient reverse intersystem crossing (RISC) process, similar to phosphorescent materials. Consequently, they exhibit an IQE of 100%.[5, 16] Furthermore, TADF emitters for OLEDs have been recognized as useful materials, in addition to conventional fluorescent and phosphorescent materials.17 Since the discovery of aggregation-induced delayed fluorescence (AIDF) by Chi et al.,18 many researchers have focused on the integration of delayed fluorescence (DF) into AIEgens to produce high-performance nondoped OLEDs.19 Such an AIDF emitter showed excellent performance when applied to nondoped OLEDs and is recognized as a promising material along with fluorescent AIEgens.[10, 20]
The emitting materials in high-efficiency OLEDs comprising AIEgens can be primarily categorized into fluorescent and TADF materials. Well-known fluorescent and newly developed TADF materials have a low molar mass. Therefore, EMLs for OLEDs are typically manufactured by a vacuum process employing the sublimation properties or solution process because of their high solubility in organic solvents owing to their low molar mass. The thin-film-forming ability of the emitter material is essential for the single-component fabrication of EMLs for nondoped OLEDs containing AIEgens. In addition to low-molar-mass materials, medium- and high-molar-mass materials are considered to be good candidates for robust EML fabrication. Nevertheless, only a few studies on AIEgens with a large molar mass have been conducted thus far. Most of the related research has been conducted recently because of the possibility of manufacturing thin-film devices by solution processes.
Several review articles have been published recently on the use of AIEgens in optoelectronic devices, chemosensors, and biosensors. Further, their potential application as a stimulus-responsive material has been investigated.21 In addition, the development of high-performance OLEDs based on AIEgens can stimulate the future utilization of AIEgens in nondoped OLEDs. A recent review article by Ma[10] summarizes the latest developments showing the use of AIEgens in blue and hybrid white OLEDs and as hosts for PhOLEDs. Tang et al.[10] have analyzed the research trends for high-performance nondoped OLEDs based on organic delayed fluorescent materials up to the year 2018. In this context, it is necessary to summarize the research data related to the application of AIEgens in OLEDs in the last 5 years. This article provides a reliable basis for the research and development of new AIE materials with superior properties for specific OLEDs.
This article provides a description of various AIEgens and their applications in nondoped OLEDs. We discuss the molecular design strategies as well as photophysical and electroluminescent properties of AIEgens used in nondoped OLEDs. The results of studies on nondoped OLEDs based on fluorescent and delayed fluorescent AIEgens are summarized in two sections: fluorescent AIEgens (Section 1) and delayed fluorescent AIEgens (Section 2). Section 1 describes fluorescent AIEgens according to the core structure and provides the characteristics of nondoped OLEDs based on such AIEgens. In Section 2, delayed fluorescent AIEgens are described based on their emission color. Blue, green, yellow, orange, and red AIDF emitters as well as macromolecules (dendrimers and polymers) with AIDF characteristics are elucidated. The ACQ- and AIE-based emitters for OLEDs and their corresponding doped and nondoped EMLs are compared in Figure 1. The relevant data from the studies reviewed in Sections 1 and 2 are summarized in Tables 1–9, and the related molecular structures are shown in Figures 2-11.
| Emitter | ΦPLa (%) (sol.) | ΦPLa (%) (film) | Device configuration | Von (V)b | CEc (cd A−1) | PEd (lm W−1) | EQEe (%) | Lf (cd m−2) | λmaxg (nm) | CIE(x,y)h | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TPBS-H | 1 | 28.2 | ITO/PEDOT:PSS/TFB/TPBS-H/TmPyPB/LiF/Al | 2.8 | 3.15 | 3.3 | 3.5 | 2258 | 438 | (0.15,0.10) | [24] |
| TPBS-F | 1.1 | 32.7 | ITO/PEDOT:PSS/TFB/TPBS-F/TmPyPB/LiF/Al | 2.8 | 3.28 | 3.4 | 3.6 | 2139 | 438 | (0.15,0.10) | [24] |
| TPBS-B | 1.5 | 55.2 | ITO/PEDOT:PSS/TFB/TPBS-B/TmPyPB/LiF/Al | 2.8 | 2.89 | 3.0 | 3.1 | 2281 | 438 | (0.15,0.10) | [24] |
| TPBS-M | 1.7 | 70.3 | ITO/PEDOT:PSS/TFB/TPBS-M/TmPyPB/LiF/Al | 2.8 | 3.13 | 3.3 | 3.4 | 2390 | 438 | (0.15,0.10) | [24] |
| (PBI)2DMTPS | 3.4 | 62.1 | ITO/NPB/(PBI)2DMTPS/LiF/Al | 2.5 | 13.3 | 14.51 | 4.25 | 14,155 | 538 | (0.36,0.56) | [25] |
| (PBI)2MPPS | 3.2 | 58.7 | ITO/NPB/(PBI)2MPPS/TPBi/LiF/Al | 2.6 | 15.06 | 16.24 | 4.84 | 46,689 | 560 | (0.42,0.55) | [25] |
| (PPI)2DMTPS | 13.8 | 49.5 | ITO/NPB/(PPI)2DMTPS/TPBi/LiF/Al | 2.9 | 10.49 | 10.83 | 3.36 | 16,614 | 542 | (0.37,0.56) | [25] |
| (PPI)2MPPS | 14.5 | 50.2 | ITO/NPB/(PPI)2MPPS/TPBi/LiF/Al | 2.7 | 14.03 | 12.69 | 4.51 | 43,117 | 560 | (0.44,0.54) | [25] |
- a ΦPL: total fluorescence quantum yield after nitrogen purging.
- b Voltage at 1 cd m−2.
- c Maximum current efficiency.
- d Maximum power efficiency.
- e Maximum external quantum efficiency.
- f Maximum luminance.
- g Maximum electroluminescence wavelength.
- h Commission internationale de l'eclairage.
| Emitter | ΦPLa (%) (sol.) | ΦPLa (%) (film) | Device configuration | Von (V)b | CEc (cd A−1) | PEd (lm W−1) | EQEe (%) | Lf (cd m−2) | λmaxg (nm) | CIE(x,y)h | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TPE-F | 3.5 | 50 | ITO/NPB/TPE-F/TPBi/LiF/Al | 3.3 | 9.98 | 7.02 | 3.67 | 24,298 | – | – | [27] |
| 1 | 1.3 | 25.3 | ITO/MoO3/NPB/TCTA/1/BmPyPB/LiF/Al | 3.1 | 3.21 | 3.15 | 2.46 | 2859 | 444 | (0.19,0.14) | [29] |
| 2 | 0.9 | 27.9 | ITO/MoO3/NPB/TCTA/2/BmPyPB/LiF/Al | 3.3 | 1.12 | 0.94 | 0.53 | 757 | 476 | (0.23,0.31) | [29] |
| 3 | 0.7 | 24.2 | ITO/MoO3/NPB/TCTA/3/BmPyPB/LiF/Al | 3.1 | 3.94 | 4.13 | 2.15 | 2918 | 468 | (0.19,0.24) | [29] |
| ITO/MoO3/NPB/TCTA/BmpyPB:3/BmPyPB/LiF/Al | 3.0 | 4.31 | 4.23 | 2.75 | 4277 | 452 | (0.17,0.16) | [29] | |||
| 4 | 0.7 | 11.8 | ITO/MoO3/NPB/TCTA/4/BmPyPB/LiF/Al | 2.8 | 1.71 | 1.84 | 0.6 | 506 | 516 | (0.30,0.51) | [29] |
| Py–TPE | – | – | ITO/HATCN/TAPC/TCTA/Py–TPE/BmPyPB/Liq/Al | 3.2 | 7.82 | 6.4 | 3.66 | 5453 | 484 | (0.22,0.34) | [31] |
| Py–TriPE | – | – | ITO/HATCN/TAPC/TCTA/Py–TriPE/BePP2/Liq/Al | 3.4 | 2.19 | 1.76 | 3.19 | 5797 | 492 | (0.20,0.31) | [31] |
| TTPE(1,3,5,9)Py | 2 | 78 | ITO/NPB/TCTA/TTPE(1,3,5,9)Py/TPBi/LiF/Al | 3.2 | 7.38 | 6.42 | 4.1 | 11,849 | 468 | (0.17,0.26) | [33] |
| Py(5,9)BTPE | 0.4 | 57.7 | ITO/NPB/TCTA/Py(5,9)BTPE/TPBi/LiF/Al | 3.2 | 6.51 | 6.24 | 3.35 | 11,450 | 488 | (0.19,0.28) | [34] |
| Py(5,9)BTriPE | 30.8 | 15.9 | ITO/NPB/TCTA/Py(5,9)BTriPE/TPBi/LiF/Al | 3.2 | 1.63 | 1.61 | 1.27 | 1271 | 456 | (0.16,0.17) | [34] |
| 9CzTPE | 0.53 | 63.4 | ITO/PEDOT:PSS/9CzTPE/TPBi/LiF/Al | 4.4 | 2.63 | – | 1.6 | 1646 | – | (0.18,0.32) | [35] |
| 3CzTPE | 0.63 | 65.5 | ITO/PEDOT:PSS/3CzTPE/TPBi/LiF/Al | 3.8 | 4.35 | – | 2.81 | 2548 | – | (0.18,0.29) | [35] |
| TPEPPI | 8 | 40 | ITO/HATCN/TAPC/TPEPPI/TPBi/LiF/Al | 2.7 | 4.25 | 3.35 | 2.36 | 16,750 | 467 | – | [36] |
| pTPI | 0.3 | 88.5 | ITO/HATCN/TAPC/TCTA/pTPI/TmPyPB/LiF/Al | 2.9 | 16.52 | 15.5 | 6.32 | 40,290 | 494 | (0.22,0.42) | [37] |
| mpCTPI | 0.5 | 67.2 | ITO/HATCN/TAPC/TCTA/mpCTPI/TmPyPB/LiF/Al | 2.9 | 18.27 | 19.12 | 6.71 | 36,030 | 506 | (0.24,0.44) | [37] |
| ppCTPI | 0.5 | 59.5 | ITO/HATCN/TAPC/TCTA/ppCTPI/TmPyPB/LiF/Al | 3.1 | 18.46 | 16.32 | 7.16 | 31,070 | 503 | (0.21,0.40) | [37] |
| mTPI | 0.5 | 47.6 | ITO/HATCN/TAPC/TCTA/mTPI/TmPyPB/LiF/Al | 3.1 | 8.87 | 8.7 | 3.98 | 12,310 | 492 | (0.20,0.33) | [37] |
| mmCTPI | 0.6 | 23.8 | ITO/HATCN/TAPC/TCTA/mmCTPI/TmPyPB/LiF/Al | 3.5 | 5.07 | 3.93 | 2.27 | 5836 | 493 | (0.21,0.34) | [37] |
| pmCTPI | 1.2 | 20.5 | ITO/HATCN/TAPC/TCTA/pmCTPI/TmPyPB/LiF/Al | 2.9 | 5.44 | 5.34 | 2.48 | 6748 | 489 | (0.19,0.34) | [37] |
| TPETPAPI | 1.3 | 73 | ITO/HATCN/TAPC(50)/TPETPAPI/TPBi/LiF/Al | 3.0 | 11.9 | 12.2 | 6.05 | 10,780 | 476 | (0.18,0.30) | [38] |
| TPE–NPPB | 16 | – | ITO/HATCN/NPB/TPE–NPPB/TPBi/LiF/Al | – | 4.32 | 4.01 | 3.2 | 10,231 | 444 | (0.18,0.21) | [39] |
| TPE–APPB | 46 | – | ITO/HATCN/NPB/TPE–APPB/TPBi/LiF/Al | – | 5.28 | 4.92 | 5.3 | 15,461 | 428 | (0.18,0.19) | [39] |
| NSPI–DVP | 9.2 | 70.7 | ITO/NPB/TCTA/NSPI–DVP/TPBi/LiF/Al | 2.7 | 5.61 | 4.99 | 5.09 | 8932 | 439 | (0.15,0.17) | [40] |
| ITO/NPB/CBP:20 wt%NSPI–DVP/TPBi/LiF/Al | 2.7 | 7.65 | 6.48 | 8.98 | 9812 | 416 | (0.15,0.08) | [40] | |||
| CNSPI–DVP | 7.8 | 40.8 | ITO/NPB/TCTA/CNSPI–DVP/TPBi/LiF/Al | 2.6 | 5.03 | 4.72 | 5.23 | 7623 | 427 | (0.14,0.13) | [40] |
| ITO/NPB/CBP:20 wt%CNSPI–DVP/TPBi/LiF/Al | 2.6 | 7.56 | 6.89 | 9.81 | 9967 | 409 | (0.14,0.06) | [40] | |||
| CNNPI | 6.5 | 21.7 | ITO/HATCN/TAPC/TCTA/CNNPI/TmPyPB/LiF/Al | 3.2 | 1.49 | 1.23 | 2.28 | 2745 | 432 | (0.15,0.07) | [41] |
| 2TriPE–CNNPI | 35 | 45.2 | ITO/HATCN/TAPC/TCTA/2TriPE–CNNPI/TmPyPB/LiF/Al | 3.0 | 3.7 | 2.98 | 2.75 | 5974 | 456 | (0.15,0.16) | [41] |
| 2CzPh–CNNPI | 33.7 | 25.5 | ITO/HATCN/TAPC/TCTA/2CzPh–CNNPI/TmPyPB/LiF/Al | 3.0 | 6.65 | 5.66 | 5.09 | 5980 | 454 | (0.15,0.14) | [41] |
| ITO/HATCN/TAPC/TCTA/CBP:xwt%2CzPh–CNNPI/TmPyPB/LiF/Al | 3.1 | 7.83 | 7.68 | 9.02 | 3652 | 436 | (0.15,0.10) | [41] | |||
| 2TriPE–BPI | 8.6 | 62.5 | ITO/HATCN/TAPC/TCTA/2TriPE–BPI/TPBi/LiF/Al | 2.8 | 4.87 | 5.21 | 3.74 | 8036 | 462 | (0.15,0.18) | [42] |
| 2TriPE–BPI–MCN | 5.8 | 45.8 | ITO/HATCN/TAPC/TCTA/2TriPE–BPI–MCN/TPBi/LiF/Al | 3.8 | 4.72 | 3.17 | 4.6 | 6129 | 452 | (0.15,0.14) | [42] |
| PIAnTPE | 8 | 65 | ITO/HATCN/TAPC/TCTA/PIAnTPE/TPBi/LiF/Al | 3.1 | 6.9 | 5 | 4.46 | 20,129 | 468 | (0.16,0.23) | [43] |
| TPAAnTPE | 10 | 70 | ITO/HATCN/TAPC/TCTA/TPAAnTPE/TPBi/LiF/Al | 3.8 | 7.5 | 4.3 | 4.13 | 18,414 | 476 | (0.16,0.28) | [43] |
| CzAnTPE | 7 | 46 | ITO/HATCN/TAPC/TCTA/CzAnTPE/TPBi/LiF/Al | 3.2 | 6.6 | 5.3 | 4.04 | 13,990 | 464 | (0.17,0.22) | [43] |
| TPEO1–PPI | 0 | 56.3 | ITO/HATCN/NPB/TCTA/TPEO1–PPI/TPBi/LiF/Al | 2.7 | 3.9 | 4.17 | 3.75 | 15,045 | 512 | (0.26,0.49) | [44] |
| TPEO4–PPI | 0 | 63.5 | ITO/HATCN/NPB/TCTA/TPEO4–PPI/TPBi/LiF/Al | 2.7 | 4.22 | 4.51 | 4.07 | 15,995 | 508 | (0.28,0.50) | [44] |
| TPEO6–PPI | 0 | 65.4 | ITO/HATCN/NPB/TCTA/TPEO6–PPI/TPBi/LiF/Al | 2.7 | 4.33 | 4.65 | 3.87 | 13,208 | 510 | (0.27,0.49) | [44] |
| TPE-1 | 1.5 | 98.7 | ITO/HATCN/NPB/TCTA/TPE-1/TPBi/LiF/Al | 3.0 | 10.74 | 8.29 | 3.97 | 19,408 | 508 | (0.23,0.45) | [46] |
| TPE-2 | 6.5 | 86.2 | ITO/HATCN/NPB/TCTA/TPE-2/TPBi/LiF/Al | 2.8 | 15.1 | 14.82 | 5.34 | 24,308 | 508 | (0.26,0.49) | [46] |
| TPE-3 | 6.4 | 84.0 | ITO/HATCN/NPB/TCTA/TPE-3/TPBi/LiF/Al | 2.7 | 13.27 | 13.9 | 4.8 | 14,427 | 508 | (0.24,0.47) | [46] |
| PPI–PIM–TPE | – | 61.9 | ITO/NPB/PPI–PIM–TPE/TPBi/LiF/Al | 3.0 | 5.2 | 3.39 | 2.41 | 5399 | 476 | (0.19,0.28) | [47] |
| ITO/NPB/CPB:PPI–PIM–TPE/TPBI/LiF/Al | 3.5 | 5.36 | 3.37 | 2.97 | 8604 | 465 | (0.17,0.22) | [47] | |||
| 2PPI–TPE | – | 73.4 | ITO/NPB/2PPI–TPE/TPBi/LiF/Al | 3 | 6.46 | 4.72 | 2.48 | 10,830 | 480 | (0.21,0.32) | [47] |
| ITO/NPB/CPB:2PPI–TPE/TPBI/LiF/Al | 3.5 | 6.67 | 5.52 | 3.55 | 12,930 | 465 | (0.17,0.22) | [47] | |||
| TCz | 0.1 | 17 | ITO/MoO3/NPB/TCTA/TCz/TPBi/LiF/Al | 5.7 | 2.1 | 1.2 | 1.2 | 4100 | 475 | (0.17,0.25) | [48] |
| Tac | 11 | 16 | ITO/MoO3/NPB/TCTA/TAc/TPBi/LiF/Al | 3.8 | 3.3 | 3 | 3.1 | 4900 | 472 | (0.16,0.21) | [48] |
| TAc-F | 2 | 29 | ITO/MoO3/NPB/TCTA/TAc-F/TPBi/LiF/Al | 4.7 | 4.6 | 2.8 | 3.2 | 5700 | 489 | (0.20,0.35) | [48] |
| TCz-F | 1 | 18 | ITO/HAT-CN/TCTA/mCP/TCz-F/TSPO1/TPBi/LiF/Al | 9.2 | 3.2 | 1.1 | 1.1 | 1900 | 490 | (0.19,0.32) | [48] |
| TPETAZ | – | 75.7 | ITO/HATCN/TAPC/TCTA/TPETAZ/TmPyPb/LiF/Al | 3.4 | 4.6 | 4.1 | 2.4 | 1020 | 468 | (0.20,0.27) | [49] |
| ITO/HATCN/TAPC/TCTA/CBP:20 wt%TPETAZ/TmPyPb/LiF/Al | 3.4 | 6.6 | 6 | 4.1 | 2220 | 466 | (0.18,0.22) | [49] |
- a ΦPL: total fluorescence quantum yield after nitrogen purging.
- b Voltage at 1 cd m−2.
- c Maximum current efficiency.
- d Maximum power efficiency.
- e Maximum external quantum efficiency.
- f Maximum luminance.
- g Maximum electroluminescence wavelength.
- h Commission internationale de l'eclairage.
| Emitter | ΦPLa (%) (sol.) | ΦPLa (%) (film) | Device configuration | Von (V)b | CEc (cd A−1) | PEd (lm W−1) | EQEe (%) | Lf (cd m−2) | λmaxg (nm) | CIE(x,y)h | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TPP–Cz | 0.8 | 21.1 | ITO/MoO3/HATCN/NPB/TCTA/TPP–Cz/Bphen/Liq/Al | 3.6 | 1.20 | 0.91 | 1.49 | 2264 | 436 | (0.16,0.11) | [52] |
| TPP–2Cz | 1.5 | 20.5 | ITO/MoO3/TAPC/TPP–2Cz/TPBi/LiF/Al | 3.8 | 1.01 | 0.63 | 0.77 | 2106 | 452 | (0.15,0.11) | [52] |
| TPP–PhCz | 0.9 | 23.6 | ITO/MoO3/TAPC/TPP–PhCz/TPBi/LiF/Al | 3.8 | 0.6 | 0.5 | 0.52 | 1931 | 448 | (0.16,0.12) | [52] |
| TPP–2PhCz | 1.5 | 14.8 | ITO/MoO3/TAPC/TPP–2PhCz/TPBi/LiF/Al | 4.2 | 0.82 | 0.62 | 0.7 | 2862 | 464 | (0.15,0.12) | [52] |
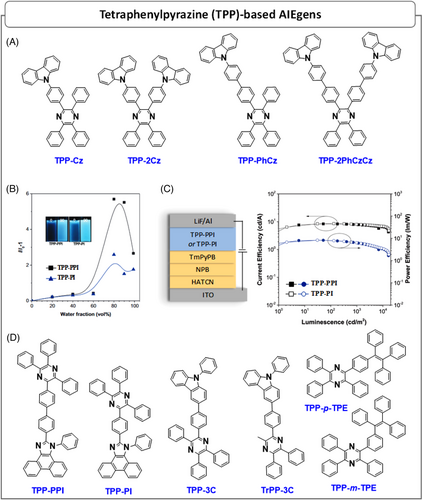
| TPP–PPI | 10 | 28.1 | ITO/HATCN/NPB/TcTA/TPP–PPI/TPBi/LiF/Al | 2.9 | 8.34 | 8.18 | 4.85 | 16,460 | 474 | (0.15,0.23) | [53] |
| TPP–PI | 13.8 | 20.2 | ITO/HATCN/NPB/TcTa/TPP–PI/TPBi/LiF/Al | 2.9 | 8.5 | 8.23 | 4.36 | 16,890 | 484 | (0.16,0.28) | [53] |
| TPP–3C | 3 | 36.6 | ITO/HATCN/TAPC/TCTA/TPP–3C/TPBi/LiF/Al | 3.3 | 3.09 | 2.43 | 2.81 | 6527 | 444 | (0.15,0.14) | [54] |
| TrPP–3C | 3.9 | 47.2 | ITO/HATCN/TAPC/TCTA/TrPP–3C/TPBi/LiF/Al | 3.3 | 1.88 | 1.49 | 2.89 | 2995 | 428 | (0.15,0.08) | [54] |
| TPP–p–TPE | – | 54.2 | ITO/NPB/TPP–p–TPE/TPBi/LiF/Al | 5.1 | 6.12 | 3.07 | 2.74 | 18,341 | 488 | (0.19,0.34) | [56] |
| TPP–m–TPE | – | 34.2 | ITO/NPB/TPP–m–TPE/TPBi/LiF/Al | 5.5 | 1.65 | 0.72 | 1.41 | 4571 | 454 | (0.16,0.15) | [56] |
- a ΦPL: total fluorescence quantum yield after nitrogen purging.
- b Voltage at 1 cd m−2.
- c Maximum current efficiency.
- d Maximum power efficiency.
- e Maximum external quantum efficiency.
- f Maximum luminance.
- g Maximum electroluminescence wavelength.
- h Commission internationale de l'eclairage.
| Emitter | ΦPLa (%) (sol.) | ΦPLa (%) (film) | Device configuration | Von (V)b | CEc (cd A−1) | PEd (lm W−1) | EQEe (%) | Lf (cd m−2) | λmaxg (nm) | CIE(x,y)h | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
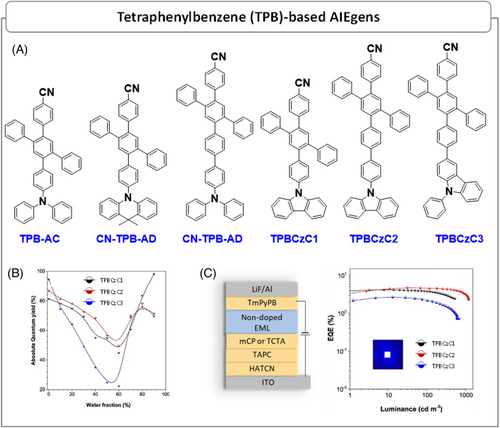
| TPB–AC | ∼70 | – | ITO/NPB/TPB–AC/TPBi/LiF/Al | 3.2 | 3.25 | 3.06 | 2.92 | 4629 | 458 | (0.15,0.12) | [57a] |
| TPB–AC | – | – | ITO/HAT–CN/TAPC/TCTA/TPB–AC/BmPyPB/LiF/Al | 2.8 | 5.2 | 5.3 | 7.0 | 3861 | 448 | (0.46,0.48) | [57b] |
| TPB–AC | – | 98.6 | ITO/HAT–CN/TAPC/TCTA/TPB–AC/BmPyPm/LiF/Al | 2.8 | 5.57 | 5.90 | 6.85 | – | – | (0.14,0.09) | [57c] |
| TPB–AC | – | – | ITO/HAT–CN/TAPC/TCTA/CBP: 50 wt% TPB–AC/BmPyPm/LiF/Al | 3.0 | 5.32 | 5.38 | 6.63 | – | – | (0.14,0.09) | [57c] |
| CN–TPB–AD | 14.7 | 55.3 | ITO/HATCN/TAPC/TCTA/CN–TPB–AD/TmPyPB/LiF/Al | 3.0 | 2.52 | 2.58 | 3.29 | 1821 | 449 | (0.15,0.09) | [60a] |
| CN–TPB–TPA | 88.3 | 93.2 | ITO/HATCN/TAPC/TCTA/CN–TPB–TPA/TmPyPB/LiF/Al | 3.0 | 5.59 | 5.35 | 7.27 | 4639 | 448 | (0.15,0.08) | [58a] |
| TPBCzC1 | 81.3 | 99.9 | ITO/HATCN/TAPC/mCP/TPBCzC1/TmPyPB/LiF/Al | 3.4 | 0.95 | 0.85 | 4.34 | – | 422 | (0.160,0.035) | [58b] |
| TPBCzC2 | 86.9 | 98.9 | ITO/HATCN/TAPC/TCTA/TPBCzC2/TmPyPB/LiF/Al | 3.4 | 2.01 | 1.57 | 4.78 | – | 423 | (0.159,0.060) | [58b] |
| TPBCzC3 | 94.5 | 98.6 | ITO/HATCN/TAPC/TCTA/TPBCzC3/TmPyPB/LiF/Al | 3.4 | 1.07 | 0.81 | 2.76 | – | 405 | (0.167,0.070) | [58b] |
- a ΦPL: total fluorescence quantum yield after nitrogen purging.
- b Voltage at 1 cd m−2.
- c Maximum current efficiency.
- d Maximum power efficiency.
- e Maximum external quantum efficiency.
- f Maximum luminance.
- g Maximum electroluminescence wavelength.
- h Commission internationale de l'eclairage.
| Emitter | ΦPLa (%) (sol.) | ΦPLa (%) (film) | Device configuration | Von (V)b | CEc (cd A−1) | PEd (lm W−1) | EQEe (%) | Lf (cd m−2) | λmaxg (nm) | CIE(x,y)h | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
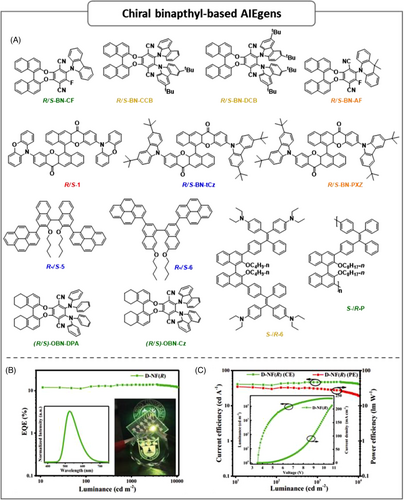
| S-BN-CF | – | 32 | ITO/HATCN/TAPC/TCTA/S-BN-CF/BmPyPB/Liq/Al | 3.8 | 10.3 | 6.7 | 3.5 | 2570 | 537 | (0.24,0.47) | [59] |
| TO/HATCN/TAPC:HATCN/TCTA/mCP:10%S-BN-CF/BmPyPB/BmPyPB:Liq/Liq/Al | 3.6 | 24.6 | 19.6 | 9.3 | 2948 | 496 | – | [59] | |||
| S-BN-CCB | – | 37 | ITO/HATCN/TAPC/TCTA/S-BN-CCB/BmPyPB/Liq/Al | 3.8 | 6.3 | 4.4 | 2.3 | 6633 | 563 | – | [59] |
| TO/HATCN/TAPC:HATCN/TCTA/mCP:10%S-BN-CCB/BmPyPB/BmPyPB:Liq/Liq/Al | 3.6 | 20.9 | 18.2 | 6.3 | 4199 | 527 | – | [59] | |||
| S-BN-DCB | – | 44 | ITO/HATCN/TAPC/TCTA/S-BN-DCB/BmPyPB/Liq/Al | 4.8 | 8.7 | 4.8 | 2.9 | 5729 | 550 | – | [59] |
| TO/HATCN/TAPC:HATCN/TCTA/mCP:10%S-BN-DCB/BmPyPB/BmPyPB:Liq/Liq/Al | 3.4 | 10.5 | 9.1 | 3.5 | 5056 | 547 | – | [59] | |||
| S-BN-AF | – | 12 | ITO/HATCN/TAPC/TCTA/S-BN-AF/BmPyPB/Liq/Al | 3.8 | 1.1 | 0.7 | 0.6 | 1473 | 597 | – | [59] |
| TO/HATCN/TAPC:HATCN/TCTA/mCP:10%S-BN-AF/BmPyPB/BmPyPB:Liq/Liq/Al | 4 | 4.5 | 2.9 | 1.7 | 3032 | 571 | – | [59] | |||
| R-1 | – | 18.5 | ITO/PEDOT:PSS/R-1/TPBI/Ca/Ag | 3.1 | 0.22 | – | 0.12 | 2726 | 604 | (0.57,0.43) | [60] |
| ITO/PEDOT:PSS/CBP:15 wt%R-1/TPBI/Ca/Ag | 3.4 | 9.1 | – | 4.1 | 40,470 | 596 | (0.56,0.44) | [60] | |||
| S-1 | – | – | ITO/PEDOT:PSS/S-1/TPBI/Ca/Ag | 3 | 0.36 | – | 0.22 | 4212 | 604 | (0.57,0.43) | [60] |
| ITO/PEDOT:PSS/TCTA:15 wt%S-1/TPBI/Ca/Ag | 2.7 | 4.2 | – | 1.8 | 11,783 | 580 | (0.50,0.48) | [60] | |||
| S-BN-tCz | 66.9 | 47.8 | ITO/PEDOT:PSS/S-BN-tCz/TPBi/Ca/Ag | 4.3 | 1.4 | – | 1.0 | 8442 | 488 | (0.20,0.37) | [61] |
| S-BN-PXZ | 68.0 | 47.9 | ITO/PEDOT:PSS/S-BN-tCz:0.1 wt% S-BN-PXZ/TPBi/Ca/Ag | 4.3 | 2.0 | – | 0.7 | 10,223 | White | (0.32,0.45) | [61] |
| R-BN-tCz | – | – | ITO/PEDOT:PSS/R-BN-tCz/TPBi/Ca/Ag | 4.3 | 1.5 | – | 0.8 | 7200 | 488 | (0.21,0.38) | [61] |
| R-BN-PXZ | – | – | ITO/PEDOT:PSS/R-BN-tCz:0.1 wt% R-BN-PXZ/TPBi/Ca/Ag | 4.3 | 2.3 | – | 1.6 | 10,397 | White | (0.31,0.45) | [61] |
| R-5 | – | – | ITO/PEDOT:PSS/R-5/TPBI/Ca/Ag | 3.18 | 3.91 | – | 2.79 | 11,336 | – | (0.16,0.18) | [62] |
| S-5 | 80 | 45.7 | ITO/PEDOT:PSS/S-5/TPBI/Ca/Ag | 3.43 | 3.86 | – | 2.17 | 10,886 | – | (0.16,0.16) | [62] |
| R-6 | – | – | ITO/PEDOT:PSS/R-6/TPBI/Ca/Ag | 3.18 | 5.17 | – | 3.09 | 24,806 | – | (0.17,0.26) | [62] |
| S-6 | 92 | 56.4 | ITO/PEDOT:PSS/S-6/TPBI/Ca/Ag | 3.18 | 4.26 | – | 2.27 | 22,880 | – | (0.17,0.26) | [62] |
| S-6 | 32.1 | 39.8 | ITO/PEDOT:PSS/S-6/TPBi/Ca/Ag | 3.18 | 1.32 | – | 0.48 | 8061 | 534 | (0.33,0.52) | [63] |
| R-6 | – | – | ITO/PEDOT:PSS/R-6/TPBi/Ca/Ag | 3.24 | 1.26 | – | 0.45 | 7946 | 534 | (0.31,0.52) | [63] |
| S-P | 0.6 | 14.8 | ITO/PEDOT:PSS/S-P/TPBI/Ca/Ag | 6 | 0.92 | 0.39 | – | 1669 | – | – | [64] |
| R-P | – | – | ITO/PEDOT:PSS/R-P/TPBI/Ca/Ag | 5.7 | 0.83 | 0.42 | – | 1270 | – | – | [64] |
| (R)-OBN-DPA | – | 84.67 | ITO/HATCN/TAPC/(R)-OBN-DPA/TmPyPB/Li/Al | 3.6 | 23 | 16.8 | 6.6 | 16,187 | – | – | [65] |
| ITO/HATCN/TAPC/TCTA:10 wt%(R)-OBN-DPA/26DCzPPy:10 wt%(R)-OBN-DPA/TmPyPB/LiF/Al | 4 | 45.2 | 30.7 | 12.3 | 25,418 | 544 | (0.39,0.57) | [65] | |||
| (S)-OBN-DPA | – | – | ITO/HATCN/TAPC/(S)-OBN-DPA/TmPyPB/Li/Al | 3.6 | 22.7 | 15.8 | 6.6 | 15,161 | – | – | [65] |
| ITO/HATCN/TAPC/TCTA:10 wt%(S)-OBN-DPA/26DCzPPy:10 wt%(S)-OBN-DPA/TmPyPB/LiF/Al | 3.9 | 45.3 | 30.6 | 12.4 | 24,518 | 544 | (0.39,0.57) | [65] | |||
| (R)-OBN-Cz | 58.2 | 92 | ITO/HATCN/TAPC/TcTa/(R)-OBN-Cz/TmPyPB/Li/Al | 3.5 | 47.8 | 34.6 | 14 | 35,633 | 526 | (0.33,0.59) | [66] |
| ITO/HATCN/TAPC:10 wt%(R)-OBN-Cz/26DCzPPy:10 wt%(R)-OBN-Cz/TmPyPB/LiF/Al | 3.8 | 93.7 | 59.3 | 32.6 | 46,651 | 501 | (0.22,0.53) | [66] |
- a ΦPL: total fluorescence quantum yield after nitrogen purging.
- b Voltage at 1 cd m−2.
- c Maximum current efficiency.
- d Maximum power efficiency.
- e Maximum external quantum efficiency.
- f Maximum luminance.
- g Maximum electroluminescence wavelength.
- h Commission internationale de l'eclairage.
| Emitter | S1 (eV)a | T1 (eV)b | ∆EST (eV)c | τd (μs)d | ΦPLe (%) (sol.) | ΦPLe (%) (film) | Device configuration | Von (V)f | CEg (cd A−1) | PEh (lmW−1) | EQEi (%) | Lj (cdm−2) | λmaxk (nm) | CIE(x,y)l | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
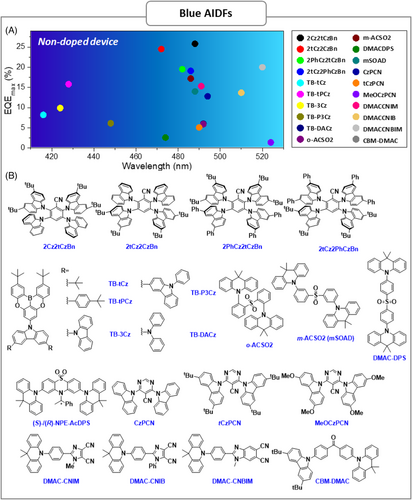
| 2Cz2tCzBn | – | – | 0.13 | 3.9 | – | 78 | ITO/PEDOT:PSS/PVK/2Cz2tCzBn/DPEPO/TmPyPb/LiF/Al | – | 64.8 | 41.5 | 25.8 | 9625 | 488 | (0.21,0.42) | [67] |
| 2tCz2CzBn | – | – | 0.13 | 15.7 | – | 66 | ITO/PEDOT:PSS/PVK/2tCz2CzBn/DPEPO/TmPyPb/LiF/Al | – | 41.9 | 26.3 | 24.5 | 4617 | 472 | (0.16,0.24) | [67] |
| 2PhCz2tCzBn | – | – | 0.14 | 5.7 | – | 55 | ITO/PEDOT:PSS/PVK/2PhCz2tCzBn/DPEPO/TmPyPb/LiF/Al | – | 42.5 | 26.7 | 19.5 | 9201 | 482 | (0.18,0.35) | [67] |
| 2tCz2PhCzBn | – | – | 0.15 | 5.6 | – | 53 | ITO/PEDOT:PSS/PVK/2tCz2PhCzBn/DPEPO/TmPyPb/LiF/Al | – | 43.7 | 29.2 | 19.1 | 10,480 | 486 | (0.19,0.38) | [67] |
| TB–tCz | 3.28 | 3.05 | 0.23 | – | 52.9 | 41.1 | ITO/PEDOT:PSS/PVK/TB-tCz/TPBi/LiF/Al | 3.6 | 2.19 | 1.97 | 8.21 | – | 416 | (0.17,0.06) | [68] |
| ITO/PEDOT:PSS/PVK/mCP:30 wt%TB-tCz/TPBi/LiF/Al | 3.8 | 2.72 | 2.14 | 15.9 | – | 412 | (0.17,0.06) | [68] | |||||||
| TB-tPCz | 3.26 | 3.09 | 0.17 | – | 56.4 | 51.9 | ITO/PEDOT:PSS/PVK/TB-tPCz/TPBi/LiF/Al | 5.6 | 5.61 | 5.03 | 15.8 | – | 428 | (0.16,0.05) | [68] |
| ITO/PEDOT:PSS/PVK/mCP:30 wt%TB-tPCz/TPBi/LiF/Al | 3.8 | 3.53 | 2.77 | 14.1 | – | 420 | (0.17,0.05) | [68] | |||||||
| TB-3Cz | – | – | – | 4.38 | 52 | 97 | ITO/PEDOT:PSS/PVK/TB-3Cz/TPBi/LiF/Al | 3.5 | 4 | 3.76 | 9.9 | 1224 | 424 | (0.17,0.07) | [69] |
| TB-P3Cz | – | – | – | 4.68 | 50.5 | 91.5 | ITO/PEDOT:PSS/PVK/TB-P3Cz/TPBi/LiF/Al | 3.0 | 4.59 | 4.12 | 6.13 | 3205 | 448 | (0.15,0.08) | [69] |
| TB-DACz | – | – | – | 5.43 | 52 | 89 | ITO/PEDOT:PSS/PVK/TB-DACz/TPBi/LiF/Al | 2.5 | 15 | 15.7 | 6.04 | 2355 | 492 | (0.18,0.40) | [69] |
| o-ACSO2 | 2.91 | 2.87 | 0.04 | 1.8 | – | 66 | ITO/PEDOT:PSS/o-ACSO2/DPEPO/TmPyPB/Liq/Al | 4.4 | 14.1 | 7.8 | 5.9 | – | 492 | (0.23,0.40) | [70] |
| ITO/PEDOT:PSS/CzSi:10 wt%o-ACSO2/DPEPO/TmPyPB/Liq/Al | 7.1 | 19 | 7.5 | 8.7 | – | 484 | (0.20,0.34) | [70] | |||||||
| m-ACSO2 | 3.01 | 2.94 | 0.07 | 3.2 | – | 76 | ITO/PEDOT:PSS/m-ACSO2/DPEPO/TmPyPB/Liq/Al | 4.1 | 37.9 | 23.8 | 17.2 | – | 486 | (0.21,0.34) | [70] |
| ITO/PEDOT:PSS/CzSi:10 wt%m-ACSO2/DPEPO/TmPyPB/Liq/Al | 6.7 | 19.2 | 9.1 | 9.7 | – | 480 | (0.19,0.30) | [70] | |||||||
| DMACDPS | 3.03 | 3 | 0.03 | 1.9 | – | 74 | ITO/PEDOT:PSS/DMACDPS/DPEPO/TmPyPB/Liq/Al | 5 | 4.6 | 1.8 | 2.6 | – | 474 | (0.18,0.26) | [70] |
| ITO/PEDOT:PSS/CzSi:10 wt%DMAC-DPS/DPEPO/TmPyPB/Liq/Al | 7.4 | 13.8 | 4.8 | 8.9 | – | 470 | (0.16,0.21) | [70] | |||||||
| (S)-NPE-AcDP | – | – | – | 3.4 | 86 | – | ITO/MoO3/TAPC/mCP/DPEPO:12 wt%S-NPE-AcDPS/3TPYMB/LiF/Al | 3 | – | – | 18.5 | – | – | – | [71] |
| mSOAD | 2.92 | 2.91 | 0.01 | 57.9 | – | 71.8 | ITO/MoO3/mCP/mSOAD/DPEPO/TPBI/LiF/Al | 3.1 | 31.7 | 28.4 | 14 | – | 488 | (0.18,0.32) | [72] |
| ITO/MoO3/mCP/DPEPO:36 wt%mSOAD/DPEPO/TPBI/LiF/Al | 3.6 | 34.6 | 27.1 | 16.6 | – | 480 | (0.18,0.30) | [72] | |||||||
| CzPCN | 3.17 | 3.03 | 0.14 | 2.1 | 15 | 53 | ITO/HAT-CN/TCTA/mCP/CzPCN/TSPO1/TPBi/LiF/Al | – | 32.4 | 18.8 | 12.8 | 13,100 | 494 | (0.20,0.36) | [73] |
| ITO/HAT-CN/TCTA/mCP:10 wt%CzPCN/TSPO1/TPBi/LiF/Al | – | 33.3 | 20.2 | 14 | 23,100 | 489 | (0.18,0.33) | [73] | |||||||
| tCzPCN | 3.02 | 2.93 | 0.09 | 2.3 | 12 | 25 | ITO/HAT-CN/TCTA/mCP/tCzPCN/TSPO1/TPBi/LiF/Al | – | 12.3 | 4.6 | 5.1 | 13,200 | 490 | (0.19,0.35) | [73] |
| ITO/HAT-CN/TCTA/mCP:10 wt%tCzPCN/TSPO1/TPBi/LiF/Al | – | 33.7 | 18 | 13.7 | 14,800 | 490 | (0.18,0.35) | [73] | |||||||
| MeOCzPCN | 2.89 | 2.88 | 0.01 | 1 | 1 | 2 | ITO/HAT-CN/TCTA/mCP/MeOCzPCN/TSPO1/TPBi/LiF/Al | – | 4 | 2.1 | 1.4 | 9300 | 524 | (0.30,0.49) | [73] |
| ITO/HAT-CN/TCTA/mCP:10 wt%MeOCzPCN/TSPO1/TPBi/LiF/Al | – | 3.8 | 1.2 | 1.4 | 13,700 | 500 | (0.21,0.430 | [73] | |||||||
| DMACCNIM | 3.01 | 2.9 | 0.11 | 5.8 | 9.9 | 81.7 | ITO/TAPC/TCTA/DMACCNIM/TmPyPb/LiF/Al | 2.8 | – | 36.8 | 15.3 | – | 491 | (0.18,0.33) | [74] |
| DMACCNIB | 2.89 | 2.83 | 0.06 | 14.1 | 14.3 | 60.2 | ITO/TAPC/TCTA/DMACCNIB/TmPyPb/LiF/Al | 2.7 | – | 43.8 | 13.7 | – | 510 | (0.25,0.50) | [74] |
| DMACCNBIM | 2.87 | 2.81 | 0.06 | 4.5 | 20.6 | 92.3 | ITO/TAPC/TCTA/DMACCNBIM/TmPyPb/LiF/Al | 2.6 | – | 61.1 | 20 | – | 520 | (0.30,0.53) | [74] |
| CBM-DMAC | 2.81 | 2.71 | 0.1 | 0.99 | 1.2 | 46 | ITO/PEDOT:PSS/CBM-DMAC/TPBi/Cs2CO3/Al | 4.5 | 14.3 | 6.4 | 6.7 | 2599 | 499 | (0.28,0.47) | [75] |
- a S1: lowest singlet energy.
- b T1: lowest triplet energy.
- c ∆EST: energy gap between S1 and T1.
- d ιDF: delayed fluorescence lifetime.
- e ΦPL: total fluorescence quantum yield after nitrogen purging.
- f Voltage at 1 cd m−2.
- g Maximum current efficiency.
- h Maximum power efficiency.
- i Maximum EQE.
- j Maximum luminance.
- k Maximum electroluminescence wavelength.
- l Commission internationale de l'eclairage.
| Emitter | S1 (eV)a | T1 (eV)b | ∆EST (eV)c | τd (μs)d | ΦPLe (%) (sol.) | ΦPLe (%) (film) | Device configuration | Von (V)f | CEg (cd A−1) | PEh (lm W−1) | EQEi (%) | Lj (cd m−2) | λmaxk (nm) | CIE(x,y)l | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
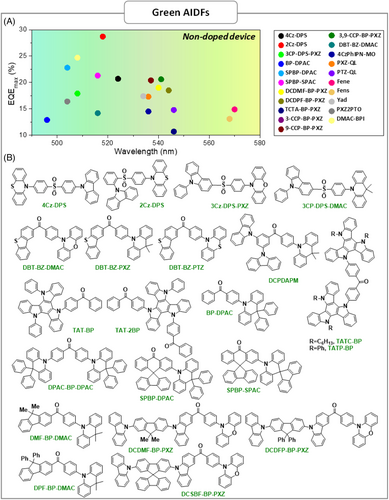
| 4Cz-DPS | – | – | 0.25 | 62.2 | – | 97.3 | ITO/PEDOT:PSS/MCP/4Cz-DPS/TPBI/LiF/Al | 3.5 | 61.2 | 38.4 | 20.7 | 11,310 | 524 | – | [76] |
| 2Cz-DPS | – | – | 0.32 | 19.1 | – | 91.9 | ITO/PEDOT:PSS/MCP/2Cz-DPS/TPBI/LiF/Al | 4.1 | 82.3 | 51.8 | 28.7 | 4220 | 518 | – | [76] |
| 3CP-DPS-PXZ | 2.69 | 2.66 | 0.03 | 0.96 | – | 52 | ITO/MoO3/TAPC/TCTA/3CP-DPS-PXZ/TmPyPB/LiF/Al | 2.5 | 52.6 | 63.5 | 17.9 | – | 508 | (0.28,0.5) | [77] |
| 3CP-DPS-DMAC | 2.97 | 2.9 | 0.07 | 2.36 | – | 65 | ITO/MoO3/TAPC/TCTA/3CP-DPS-DMAC/TmPyPB/LiF/Al | 2.9 | 17 | 15.7 | 9.1 | – | 484 | (0.16,0.28) | [77] |
| DBT-BZ-DMAC | – | – | 0.08 | 2.9 | 8.3 | 80.2 | ITO/TAPC/DBT-BZ-DMAC/TmPyPB/LiF/Al | 2.7 | 43.3 | 35.7 | 14.2 | 27,270 | 516 | (0.26,0.55) | [78] |
| ITO/TAPC/CBP:6 wt%DBT-BZ-DMAC/TmPyPB/LiF/Al | 3.3 | 51.7 | 50.7 | 17.9 | 11,200 | 508 | (0.23,0.51) | [78] | |||||||
| DBT-BZ-PXZ | – | – | 0.09 | 1.3 | 0.5 | 57.5 | ITO/TAPC/DBT-BZ-PXZ/TmPyPB/LiF/Al | 2.9 | 26.6 | 27.9 | 9.2 | – | 557 | (0.43,0.54) | [78] |
| ITO/TAPC/CBP:6 wt%DBT–BZ–PXZ/TmPyPB/LiF/Al | 3.2 | 60.6 | 59.2 | 19.2 | – | 528 | (0.34,0.57) | [78] | |||||||
| DBT–BZ–PTZ | – | – | 0.05 | 1.8 | 0.5 | 76.8 | ITO/TAPC/DBT-BZ-DMAC/TmPyPB/LiF/Al | 2.7 | 26.5 | 29.1 | 9.7 | – | 563 | (0.44,0.53) | [78] |
| ITO/TAPC/CBP:6 wt%DBT–BZ–PTZ/TmPyPB/LiF/Al | 3.2 | 46 | 43.3 | 15.1 | – | 538 | (0.37,0.56) | [78] | |||||||
| DCPDAPM | 2.82 | 2.72 | 0.1 | 8.1 | 6.2 | 76.1 | ITO/HATCN/TAPC/DCPDAPM/Tmpypb/LiF/Al | 3.2 | 26.8 | 15.6 | 8.15 | 123,371 | 522 | (0.28,0.59) | [79] |
| ITO/HATCN/TAPC/CBP:20 wt%DCPDAPM/TmPyPB/LiF/Al | 3.6 | 61.8 | 40.4 | 19.6 | 116,100 | 522 | (0.26,0.56) | [79] | |||||||
| TATC-BP | – | – | 0.12 | 0.94 | 0.8 | 22 | ITO/PEDOT:PSS/TATC-BP/TmPyPB/LiF/Al | 2.6 | 17.8 | 20 | 5.9 | – | 549 | (0.41,0.54) | [80] |
| ITO/PEDOT:PSS/H2:30 wt%TATC-BP/TmPyPB/LiF/Al | 2.8 | 48.1 | 47.8 | 15.9 | – | – | (0.37,0.53) | [80] | |||||||
| TATP-BP | – | – | 0.12 | 0.91 | 1.9 | 24.2 | ITO/PEDOT:PSS/TATP-BP/TmPyPB/LiF/Al | 2.7 | 18.9 | 19.2 | 6 | – | 541 | (0.38,0.55) | [80] |
| ITO/PEDOT:PSS/H2:30 wt%TATC-BP/TmPyPB/LiF/Al | 2.8 | 46.4 | 47.2 | 15.4 | – | – | (0.34,0.53) | [80] | |||||||
| TAT-BP | – | – | 0.06 | 0.79 | 14 | 51 | ITO/PEDOT:PSS/TAT-BP/TmPyPB/LiF/Al | 2.5 | 20.9 | 21.8 | 6.4 | – | 530 | – | [81] |
| ITO/PEDOT:PSS/H2:30 wt%TAT-BP/TmPyPB/LiF/Al | 2.9 | 37.1 | 38.2 | 12.1 | – | – | – | [81] | |||||||
| TAT-2BP | – | – | 0.09 | 0.54 | 1.2 | 44 | ITO/PEDOT:PSS/TAT-2BP/TmPyPB/LiF/Al | 2.5 | 32.3 | 33 | 9.8 | – | 530 | – | [81] |
| ITO/PEDOT:PSS/H2:30 wt%TAT-2BP/TmPyPB/LiF/Al | 2.9 | 40.4 | 34.8 | 13.2 | – | – | – | [81] | |||||||
| BP-DPAC | – | – | 0.03 | 4.34 | – | 50.4 | ITO/HATCN/TAPC/DPEPO:16 wt%BP-DPAC/TPBi/LiF/Al | – | 15.08 | 13.52 | 6.82 | 5087 | – | (0.18,0.36) | [82] |
| DPAC-BP-DPAC | – | – | 0.09 | 4.08 | – | 62.5 | ITO/HATCN/TAPC/DPEPO:16 wt%DPAC-BP-DPAC/TPBi/Li/Al | – | 43.7 | 35.19 | 18.67 | 5453 | – | (0.17,0.41) | [82] |
| BP-DPAC | 2.97 | 2.93 | 0.04 | 5.3 | – | 88 | ITO/TAPC/TCTA/mCP/BP-DPAC/TmPyPb/LiF/Al | 3.0 | 32.7 | 34.3 | 12.9 | – | 496 | (0.21,0.42) | [83] |
| SPBP–DPAC | 2.95 | 2.88 | 0.07 | 2.5 | – | 98 | ITO/TAPC/TCTA/mCP/SPBP-DPAC/TmPyPb/LiF/Al | 3.1 | 63.7 | 54.1 | 22.8 | – | 504 | (0.23,0.50) | [83] |
| SPBP-SPAC | 2.92 | 2.84 | 0.08 | 1.3 | 99 | ITO/TAPC/TCTA/mCP/SPBP-SPAC/TmPyPb/LiF/Al | 3.1 | 65.7 | 51.6 | 21.3 | – | 516 | (0.27,0.56) | [83] | |
| DMF-BP-DMAC | – | – | 0.14 | 4.9 | 21.9 | 31.9 | ITO/HATCN/NPB/mCP/DMF-BP-DMAC/TPBi/LiF/Al | 3.9 | 21.6 | 14.6 | 6.4 | 32,460 | 526 | (0.29,0.59) | [84] |
| DPF–BP-DMAC | – | – | 0.09 | 7.8 | 21.7 | 62.3 | ITO/HATCN/NPB/mCP/DPF-BP-DMAC/TPBi/LiF/Al | 3.1 | 42.3 | 30.2 | 14.4 | 52,560 | 524 | (0.30,0.56) | [84] |
| DCDMF-BP-PXZ | – | – | 0.04 | 2.1 | 3 | 88.5 | ITO/TAPC/DCDMF-BP-PXZ/TmPyPB/LiF/Al | 2.6 | 62.2 | 63.7 | 19 | 51,652 | 540 | (0.39,0.57) | [85] |
| ITO/TAPC/CBP:10 wt%DCDMF-BP-PXZ/TmPyPB/LiF/Al | 3.2 | 69.2 | 59.3 | 21.7 | 66,205 | 538 | (0.36,0.57) | [85] | |||||||
| DCDPF–BP-PXZ | – | – | 0.05 | 1.7 | 2.8 | 89.0 | ITO/HATCN/TAPC/TCTA/DCDPF-BP-PXZ/TmPyPB/LiF/Al | 2.5 | 61.1 | 67.4 | 18.5 | 80,630 | 544 | (0.38,0.57) | [85] |
| ITO/TAPC/CBP:10 wt%DCDPF-BP-PXZ/TmPyPB/LiF/Al | 3.1 | 78.8 | 77.9 | 24.4 | 59,249 | 532 | (0.33,0.57) | [85] | |||||||
| DCSBF-BP-PXZ | – | – | 0.11 | 0.9 | 3 | 39 | ITO/HATCN/TAPC/TCTA/DCSBF-BP-PXZ/TmPyPB/LiF/Al) | 2.7 | 10.8 | 8.6 | 3.3 | 21,928 | 548 | (0.40,0.56) | [85] |
| ITO/TAPC/CBP:10 wt%DCSBF-BP-PXZ/TmPyPB/LiF/Al | 3.1 | 72.2 | 70.9 | 22.3 | 52,581 | 528 | (0.33,0.57) | [85] | |||||||
| CC6-DBP-PXZ | – | – | 0.07 | 1.6 | 4.1 | 59.0 | ITO/PEDOT:PSS/PVK/CC6-DBP-PXZ/TmPyPB/LiF/Al | 2.9 | 22.23 | 16.11 | 7.73 | 30,644 | 568 | (0.45,0.52) | [86] |
| ITO/PEDOT:PSS/PVK/CBP:10 wt%CC6-DBP-PXZ/TmPyPB/LiF/Al | 4.0 | 37.62 | 17.76 | 12.1 | 33,286 | 524 | (0.34,0.56) | [86] | |||||||
| CC6-DBP-DMAC | – | – | 0.05 | 6.4 | 11.7 | 69.1 | ITO/PEDOT:PSS/PVK/CC6-DBP-DMAC/TmPyPB/LiF/Al | 4.2 | 25.08 | 11.25 | 9.02 | 14,366 | 505 | (0.26,0.50) | [86] |
| ITO/PEDOT:PSS/PVK/CBP:10 wt%CC6-DBP-DMAC/TmPyPB/LiF/Al | 3.7 | 27.07 | 14.73 | 10.0 | 11,247 | 499 | (0.22,0.48) | [86] | |||||||
| TCTA-BP-PXZ | – | – | 0.06 | 2.12 | 4.9 | 53.5 | ITO/PEDOT:PSS/PVK/TCTA-BP-PXZ/TmPyPB/LiF/Al | 3.0 | 34.4 | 24.6 | 10.7 | 12,524 | 546 | (0.40,0.56) | [87] |
| ITO/mPEDOT:PSS/mCP:50 wt%TCTA-BP-PXZ/DPEPO/TmPyPB/Liq/Al | 5.0 | 25.7 | 13.9 | 9.3 | 12,910 | 534 | – | [87] | |||||||
| TCTA-BP-DMAC | – | – | 0.09 | 2.87 | 7.2 | 57.6 | ITO/PEDOT:PSS/PVK/TCTA-BP-DMAC/TmPyPB/LiF/Al | 3.1 | 22.8 | 14.9 | 9.8 | 2040 | 488 | (0.21,0.41) | [87] |
| ITO/mPEDOT:PSS/mCP:50 wt%TCTA-BP-DMAC/DPEPO/TmPyPB/Liq/Al | 4.0 | 21.2 | 12.5 | 8.7 | 5032 | 492 | – | [87] | |||||||
| 3-CCP-BP-PXZ | – | – | 0.016 | 0.76 | 3 | 73 | ITO/HATCN/TAPC/TCTA/3-CCP-BP-PXZ/TmPyPB/LiF/Al | 2.8 | 76.6 | 75.2 | 21.7 | 88,750 | 540 | (0.38,0.58) | [88] |
| ITO/HATCN/TAPC/TCTA/20 wt%3-CCP-BP-PXZ:CBP/TmPyPB/LiF | 3.0 | 100.1 | 104.8 | 29.1 | 76,820 | 523 | (0.32,0.59) | [88] | |||||||
| 9-CCP-BP-PXZ | – | – | 0.018 | 0.68 | 2.8 | 70 | ITO/HATCN/TAPC/TCTA/9-CCP-BP-PXZ/TmPyPB/LiF/Al | 2.6 | 72.5 | 53.5 | 20.4 | 29,510 | 537 | (0.37,0.59) | [88] |
| ITO/HATCN/TAPC/TCTA/20 wt%9-CCP-BP-PXZ:CBP/TmPyPB/LiF | 3.4 | 77.6 | 64.1 | 23.6 | 36,970 | 520 | (0.28,0.59) | [88] | |||||||
| 3,9-CCP-BP-PXZ | – | – | 0.019 | 0.42 | 2.7 | 72 | ITO/HATCN/TAPC/TCTA/3,9-CCP-BP-PXZ/TmPyPB/LiF/Al | 2.8 | 72.1 | 65.4 | 20.6 | 35,580 | 541 | (0.39,0.58) | [88] |
| ITO/HATCN/TAPC/TCTA/20 wt%3,9-CCP-BP-PXZ:CBP/TmPyPB/LiF | 3.0 | 92.1 | 90.4 | 26.5 | 39,730 | 528 | (0.34,0.59) | [88] | |||||||
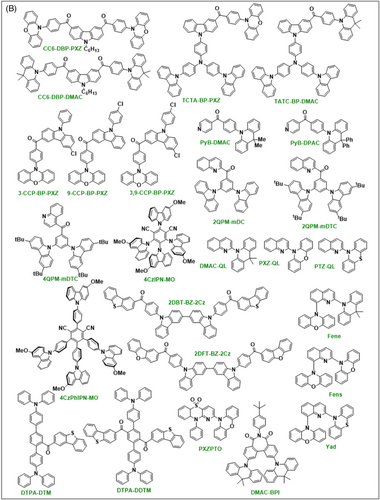
| 2QPM–mDC | 2.84 | 2.65 | 0.19 | 362 | – | 90 | ITO/HAT-CN/TAPC/mCBP:7 wt%2QPM-mDC/TmPyPB/LiF/Al | 3.5 | 46.6 | 41.6 | 17.5 | 14,866 | 498 | (0.19,0.46) | [89] |
| 2QPM-mDTC | 2.7 | 2.63 | 0.07 | 90.2 | – | 98 | ITO/HAT-CN/TAPC/mCBP:7 wt%2QPM-mDTC/TmPyPB/LiF/Al | 3.5 | 79.5 | 62.3 | 24 | 26,447 | 514 | (0.25,0.56) | [89] |
| 4QPM-mDTC | 2.8 | 2.62 | 0.18 | 357 | – | 80 | ITO/HAT-CN/TAPC/mCBP:7 wt%4QPM-mDTC/TmPyPB/LiF/Al | 3.8 | 45.7 | 40.8 | 16.2 | 10,025 | 502 | (0.20,0.47) | [89] |
| PyB-DMAC | 2.59 | 2.54 | 0.05 | 1.25 | – | – | ITO/TAPC/TCTA/PyB-DMAC/TmPyPB/Liq/Al | 7.0 | 23.8 | 8.3 | 8.4 | – | 538 | (0.39,0.54) | [90] |
| PyB-DPAC | 2.68 | 2.61 | 0.07 | 1.71 | – | – | ITO/TAPC/TCTA/PyB-DPAC/TmPyPB/Liq/Al | 4.0 | 28.8 | 12.9 | 9.7 | – | 522 | (0.32,0.55) | [90] |
| 4CzIPN-MO | 2.55 | 2.46 | 0.09 | – | – | 35 | ITO/PEDOT:PSS/4CzIPN-MO/TPBi/Cs2CO3/Al | 3.1 | 15.6 | 12.8 | 5.6 | 7615 | 574 | (0.48,0.54) | [91] |
| 4CzPhIPN-MO | 2.72 | 2.7 | 0.01 | – | – | 86 | ITO/PEDOT:PSS/4CzPhIPN-MO/TPBi/Cs2CO3/Al | 3.4 | 45.1 | 36 | 14.5 | 16,682 | 536 | (0.34,0.56) | [91] |
| ITO/PEDOT:PSS/CBP:10 wt%4CzPhIPN-MO/TPBi/Cs2CO3/Al | 4.8 | 44.2 | 16.9 | 16.2 | 7711 | 496 | (0.22,0.39) | [91] | |||||||
| 2DBT-BZ-2Cz | 2.45 | 2.43 | 0.02 | 0.38 | – | – | ITO/PEDOT:PSS/2DBT-BZ-2CZ/TPBI/Cs2CO3/Al | 3.6 | 20.7 | 12.4 | 6.8 | 10,000 | 536 | (0.37,0.58) | [92] |
| 2DFT-BZ-2Cz | 2.44 | 2.43 | 0.01 | 0.44 | – | – | ITO/PEDOT:PSS/2DFT-BZ-2CZ/TPBI/Cs2CO3/Al | 3.9 | 7.6 | 7.6 | 4.5 | 6700 | 540 | (0.38,0.59) | [92] |
| DTPA-DTM | 2.91 | 2.74 | 0.18 | – | – | 38.6 | ITO/HATCN/TAPC/DTPA-DTM/TmPyPB/LiF/Al | 3.8 | 9.7 | 6.3 | 4.4 | 2736 | 494 | (0.21,0.42) | [93] |
| ITO/HATCN/TAPC/30 wt%DTPA-DTM:mCP/TmPyPB/LiF/Al | 3.7 | 15.6 | 11.8 | 7.1 | 4332 | 489 | (0.18,0.39) | [93] | |||||||
| DTPA-DDTM | 2.64 | 2.48 | 0.17 | – | – | 60.5 | ITO/HATCN/TAPC/DTPA-DDTM/TmPyPB/LiF/Al | 3.9 | 25.6 | 15.2 | 8.2 | 3432 | 555 | (0.41,0.55) | [93] |
| ITO/HATCN/TAPC/30 wt%DTPA-DDTM:mCP/TmPyPB/LiF/Al | 3.8 | 42.2 | 28.2 | 13.6 | 4788 | 540 | (0.36,0.59) | [93] | |||||||
| DMAC-QL | 2.71 | 2.65 | 0.06 | 2.15 | – | 32.6 | ITO/HATCN/TAPC/DMAC–QL/DPEPO/TmPyPB/LiF/Al | 3.2 | 27.2 | 24.6 | 7.7 | – | 522 | (0.31,0.51) | [94] |
| PXZ-QL | 2.65 | 2.65 | 0.1 | 1.86 | – | 64.7 | ITO/HATCN/TAPC/PXZ-QL/DPEPO/TmPyPB/LiF/Al | 2.6 | 55.9 | 64.6 | 17.3 | – | 536 | (0.36,0.55) | [94] |
| PTZ-QL | 2.57 | 2.53 | 0.04 | 15.7 | – | 52.3 | ITO/HATCN/TAPC/PTZ-QL/DPEPO/TmPyPB/LiF/Al | 2.8 | 51.5 | 56.7 | 14.8 | – | 546 | (0.39,0.56) | [94] |
| Fene | 2.19 | 2.15 | 0.04 | 2.75 | – | 58.2 | ITO/TAPC/TCTA/Fene/TmPyPB/LiF/Al | 3.2 | 42.2 | 31.57 | 14.9 | – | 570 | – | [95] |
| Fens | 2.2 | 2.17 | 0.03 | 3.27 | – | 36.1 | ITO/TAPC/TCTA/Fens/TmPyPB/LiF/Al | 3.2 | 36.8 | 30.4 | 13.1 | – | 568 | – | [95] |
| Yad | 2.28 | 2.24 | 0.04 | 16.0 | – | 79.6 | ITO/TAPC/TCTA/Yad/TmPyPB/LiF/Al | 3.0 | 58.14 | 57.01 | 17.4 | – | 534 | – | [95] |
| PXZ2PTO | 2.75 | 2.73 | 0.02 | – | 61.5 | – | ITO/MoO3/TAPC/mCP/PXZ2PTO/DPEPO/TPBi/LiF/Al | 4.3 | 44.9 | 32 | 16.4 | 1568 | 504 | (0.27,0.50) | [96] |
| ITO/MoO3/TAPC/mCP/80 wt%PXZ2PTO:DPEPO/DPEPO/TPBi/LiF/Al | 3.8 | 43.8 | 35.2 | 16.3 | 4444 | 500 | (0.24,0.44) | [96] | |||||||
| DMAC-BPI | 3.93 | 2.91 | 0.02 | 3.1 | 16.2 | 95.8 | ITO/TAPC/TCTA/DMAC-BPI/TmPyPb/LiF/Al | 2.9 | – | 59.7 | 24.7 | – | 508 | (0.24,0.49) | [97] |
| BPI-PhPXZ | 2.67 | 2.64 | 0.03 | 20.5 | 8.3 | 96 | ITO/HATCN/TAPC/TCTA/20 wt % BPI-PhPXZ:CBP/TmPyPB/LiF/Al | 3.2 | 58.7 | 54.2 | 18.1 | 10,040 | 542 | (0.38, 0.57) | [98] |
| BPI-PhDMAC | 2.9 | 2.92 | 0.02 | 14.9 | 28 | 64 | ITO/HATCN/TAPC/TCTA/10 wt % BPI-PhDMAC:CBP/TmPyPB/LiF/Al | 3.1 | 37.1 | 34.3 | 16.2 | 2990 | 506 | (0.19, 0.34) | [98] |
- a S1: lowest singlet energy.
- b T1: lowest triplet energy.
- c ∆EST: energy gap between S1 and T1.
- d τDF: delayed fluorescence lifetime.
- e ΦPL: total fluorescence quantum yield after nitrogen purging.
- f Voltage at 1 cd m−2.
- g Maximum current efficiency.
- h Maximum power efficiency.
- i Maximum EQE.
- j Maximum luminance.
- k Maximum electroluminescence wavelength.
- l Commission internationale de l'eclairage.
| Emitter | S1 (eV)a | T1 (eV)b | ∆EST (eV)c | τd (μs)d | ΦPLe (%) (sol.) | ΦPLe (%) (film) | Device configuration | Von (V)f | CEg (cd A−1) | PEh (lm W−1) | EQEi (%) | Lj (cd m−2) | λmaxk(nm) | CIE(x,y)l | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
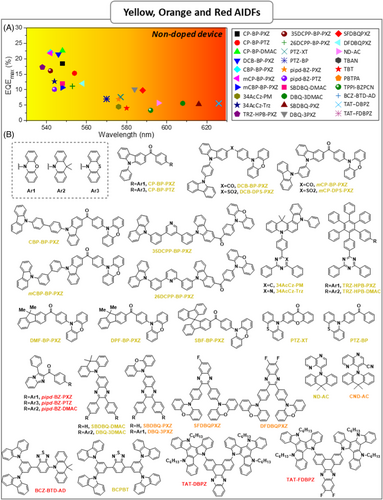
| CP-BP-PXZ | – | – | 0.024 | 2.1 | 0.02 | 74 | ITO/TAPC/CP-BP-PXZ/TmPyPB/LiF/Al | 2.5 | 59.1 | 65.7 | 18.4 | 100,290 | 548 | (0.40,0.57) | [99] |
| ITO/TAPC/CBP:6 wt%CP-BP-PXZ/TmPyPB/LiF/Al | 3.4 | 71.9 | 65.3 | 22.3 | 53,640 | 528 | (0.32,0.57) | [99] | |||||||
| CP-BP-PTZ | – | – | 0.033 | 5.7 | 0.01 | 55 | ITO/TAPC/CP-BP-PTZ/TmPyPB/LiF/Al | 2.5 | 46.1 | 55.7 | 15.3 | 46,820 | 554 | (0.42,0.55) | [99] |
| ITO/TAPC/CBP:6 wt%CP-BP-PTZ/TmPyPB/LiF/Al | 3.1 | 73.3 | 69.6 | 22.7 | 64,490 | 528 | (0.33,0.58) | [99] | |||||||
| CP-BP-DMAC | – | – | 0.016 | 5.5 | 0.18 | 40 | ITO/TAPC/CP-BP-DMAC/TmPyPB/LiF/Al | 2.7 | 41.6 | 37.9 | 15 | 37,680 | 502 | (0.23,0.49) | [99] |
| ITO/TAPC/CBP:6 wt%CP-BP-DMAC/TmPyPB/LiF/Al | 3.4 | 61.6 | 55.5 | 19.1 | 27,540 | 542 | (0.37,0.57) | [99] | |||||||
| DCB-BP-PXZ | – | – | 0.024 | 2.6 | 3.9 | 69 | ITO/TAPC/DCB-BP-PXZ/TmPyPB/LiF/Al | 2.5 | 72.9 | 81.8 | 22.6 | 95,577 | 548 | (0.39,0.57) | [100] |
| CBP-BP-PXZ | – | – | 0.02 | 2.4 | 3 | 71.6 | ITO/TAPC/CBP-BP-PXZ/TmPyPB/LiF/Al | 2.5 | 69 | 75 | 21.4 | 98,089 | 546 | (0.39,0.57) | [100] |
| mCP–BP-PXZ | – | – | 0.024 | 2.3 | 3.1 | 66 | ITO/TAPC/mCP-BP-PXZ/TmPyPB/LiF/Al | 2.5 | 72.3 | 79 | 22.1 | 100,126 | 542 | (0.39,0.57) | [100] |
| mCBP-BP-PXZ | – | – | 0.016 | 2.4 | 2.8 | 71.2 | ITO/TAPC/mCBP-BP-PXZ/TmPyPB/LiF/Al | 2.5 | 70.4 | 76.5 | 21.8 | 96,815 | 542 | (0.38,0.57) | [100] |
| mPhDCzDPSPXZ | 2.75 | 2.67 | 0.08 | 0.624 | – | 0.4 | ITO/MoO3/TAPC/TCTA/DCB-DPS-PXZ/TmPyPB/LiF/Al | 2.5 | 40.5 | 48.2 | 13.9 | – | 520 | (0.32,0.56) | [101] |
| mPhDCzDPSPXZ | 2.7 | 2.65 | 0.05 | 0.667 | – | 0.47 | ITO/MoO3/TAPC/TCTA/mCP-DPS-PXZ/TmPyPB/LiF/Al | 2.5 | 45.6 | 55 | 14.7 | – | 520 | (0.32,0.56) | [101] |
| mPhDCzDPSPXZ | 2.68 | 2.65 | 0.03 | 0.75 | – | 0.55 | ITO/MoO3/TAPC/TCTA/pPhDCzDPSPXZ/TmPyPB/LiF/Al | 2.5 | 53 | 59.9 | 17.1 | – | 523 | (0.34,0.56) | [101] |
| mPhDCzDPSPXZ | 2.68 | 2.65 | 0.03 | 0.765 | – | 0.56 | ITO/MoO3/TAPC/TCTA/mPhDCzDPSPXZ/TmPyPB/LiF/Al | 2.5 | 56.3 | 63.9 | 18.1 | – | 521 | (0.34,0.56) | [101] |
| 34AcCz-PM | 2.72 | 2.57 | 0.15 | – | – | 67 | ITO/MoO3/TAPC/TCTA/34AcCz-PM/TmPyPB/LiF/Al | 3.1 | 45.2 | 40.0 | 14.1 | – | 548 | (0.42,0.55) | [102] |
| ITO/MoO3/TAPC/TCTA/10 wt%34AcCz-PM:CBP/TmPyPB/LiF/Al | 2.84 | 73.3 | 72.4 | 22.6 | – | 520 | (0.33,0.58) | [102] | |||||||
| 34AcCz-Trz | 2.62 | 2.5 | 0.12 | – | – | 42 | ITO/MoO3/TAPC/TCTA/34AcCz-Trz/TmPyPB/LiF/Al | 3.35 | 18.0 | 15.5 | 7.3 | – | 576 | (0.50,0.49) | [102] |
| ITO/MoO3/TAPC/TCTA/5 wt%34AcCz-Trz:CBP/TmPyPB/LiF/Al | 3.15 | 44.7 | 38.0 | 14.4 | – | 540 | (0.40,0.56) | [102] | |||||||
| TRZ-HPB-PXZ | – | – | 0.02 | 2.1 | 61.5 | 5.5 | ITO/HATCN/TAPC/TCTA/TRZ-HPB-PXZ/TmPyPB/LiF/Al | 2.5 | 41.2 | 44.9 | 12.7 | 40,382 | 544 | (0.39,0.57) | [103] |
| TRZ-HPB-DMAC | – | – | 0.09 | 4.7 | 51.8 | 9.1 | ITO/HATCN/TAPC/TCTA/TRZ-HPB-DMAC/TmPyPB/LiF/Al | 3.1 | 221.4 | 17.6 | 6.5 | 15,460 | 521 | (0.28,0.58) | [103] |
| 35DCPP-BP-PXZ | – | – | 0.021 | 1.58 | 66.5 | – | ITO/HATCN/TAPC/TCTA/35DCPP-BP-PXZ/TmPyPB/LiF/Al | 3.0 | 57.6 | 49.7 | 17.3 | 38,237 | 538 | – | [104] |
| 26DCPP-BP-PXZ | – | – | 0.013 | 1.68 | 67.9 | – | ITO/HATCN/TAPC/TCTA/26DCPP-BP-PXZ/TmPyPB/LiF/Al | 3.0 | 53.2 | 37 | 16.1 | 25,106 | 542 | – | [104] |
| DMF-BP-PXZ | – | – | – | 1.4 | 73 | 2.6 | ITO/TAPC/DMF-BP-PXZ/TmPyPB/LiF/Al | 2.7 | 39.9 | 38 | 13.3 | 27,331 | – | (0.44,0.54) | [105] |
| ITO/TAPC/30 wt%DMF-BP-PXZ:CBP/TmPyPB/LiF/Al | 2.7 | 60.6 | 55.6 | 18.6 | 53,013 | – | (0.40,0.56) | [105] | |||||||
| DPF-BP-PXZ | – | – | – | 1.1 | 69 | 2.8 | ITO/TAPC/DPF-BP-PXZ/TmPyPB/LiF/Al | 2.6 | 41.6 | 45 | 14.3 | 31,422 | – | (0.45,0.53) | [105] |
| ITO/TAPC/30 wt%DPF-BP-PXZ:CBP/TmPyPB/LiF/Al | 2.7 | 62.3 | 59.9 | 19 | 77,480 | – | (0.39,0.56) | [105] | |||||||
| SBF-BP-PXZ | – | – | – | 1.1 | 73 | 1.8 | ITO/TAPC/SBF-BP-PXZ/TmPyPB/LiF/Al | 2.5 | 36.8 | 37.9 | 12.3 | 33,990 | – | (0.45,0.52) | [105] |
| ITO/TAPC/30 wt%SBF-BP-PXZ:CBP/TmPyPB/LiF/Al | 2.7 | 62.3 | 62.9 | 19.4 | 111,145 | – | (0.41,0.55) | [105] | |||||||
| PTZ-XT | – | – | 0.071 | 1.9 | – | 53 | ITO/α-NPD/PTZ-XT/B3PyPB/Liq/Al | – | – | – | 11.1 | – | 553 | – | [106] |
| PTZ-BP | – | – | 0.065 | 1.4 | – | 31 | ITO/α-NPD/PTZ-BP/B3PyPB/Liq/Al | – | – | – | 7.6 | – | 577 | – | [106] |
| pipd-BZ-PXZ | – | – | 0.071 | 2.2 | 55.8 | 3.3 | ITO/HATCN/TAPC/TCTA/pipd-BZ-PXZ/TmPyPB/LiF/Al | 3.2 | 19.8 | 17.3 | 7.04 | 24,474 | 570 | (0.42,0.55) | [107] |
| ITO/HATCN/TAPC/TCTA/CBP:6 wt%pipd-BZ-PXZ/TmPyPB/LiF/Al | 3.0 | 55.4 | 58.0 | 15.7 | 42,574 | 545 | (0.31,0.57) | [107] | |||||||
| pipd-BZ-PTZ | – | – | 0.035 | 35.5 | 38.2 | 1.2 | ITO/HATCN/TAPC/TCTA/pipd-BZ-PTZ/TmPyPB/LiF/Al | 2.6 | 14.35 | 18.6 | 6.91 | 18,006 | 576 | (0.51,0.48) | [107] |
| ITO/HATCN/TAPC/TCTA/CBP:6 wt%pipd-BZ-PTZ/TmPyPB/LiF/Al | 3.2 | 37.7 | 33.2 | 11.7 | 27,271 | 548 | (0.42,0.57) | [107] | |||||||
| pipd-BZ-DMAC | – | – | 0.299 | 42.3 | 13.5 | 8.8 | ITO/HATCN/TAPC/TCTA/pipd-BZ-DMAC/TmPyPB/LiF/Al | 3.0 | 7.16 | 6.25 | 2.58 | 4436 | 528 | (0.39,0.54) | [107] |
| ITO/HATCN/TAPC/TCTA/CBP:6 wt%pipd-BZ-DMAC/TmPyPB/LiF/Al | 3.0 | 14.83 | 14.0 | 5.05 | 4448 | 513 | (0.28,0.57) | [107] | |||||||
| SBDBQ-DMAC | – | – | 0.06 | 8.3 | – | 74 | ITO/MoO3/TAPC/mCP/SBDBQ-DMAC/Bphen/LiF/Al | 2.8 | 35.4 | 32.7 | 10.1 | 14,578 | 544 | (0.39,0.58) | [108] |
| ITO/MoO3/TAPC/mCP/CBP:10 wt%SBDBQ-DMAC/Bphen/LiF/Al | 3.0 | 45 | 39.9 | 13 | 33,586 | 532 | (0.34,0.60) | [108] | |||||||
| DBQ-3DMAC | – | – | 0.06 | 6.5 | – | 83 | ITO/MoO3/TAPC/mCP/DBQ-3DMAC/Bphen/LiF/Al | 2.6 | 41.2 | 45.4 | 12 | 29,843 | 548 | (0.40,0.57) | [108] |
| ITO/MoO3/TAPC/mCP/CBP:10 wt%DBQ-3DMAC/Bphen/LiF/Al | 3.4 | 80.3 | 64.1 | 22.4 | 31,099 | 536 | (0.35,0.59) | [108] | |||||||
| SBDBQ-PXZ | – | – | 0.07 | 2.4 | – | 73 | ITO/MoO3/TAPC/mCP/SBDBQ-PXZ/Bphen/LiF/Al | 2.4 | 10.5 | 12 | 5.6 | 21,050 | 608 | (0.56,0.43) | [108] |
| ITO/MoO3/TAPC/mCP/CBP:10 wt%SBDBQ-PXZ/Bphen/LiF/Al | 3.1 | 29.1 | 23.4 | 11.1 | 30,039 | 572 | (0.49,0.50) | [108] | |||||||
| DBQ-3PXZ | – | – | 0.03 | 1.9 | – | 76 | ITO/MoO3/TAPC/mCP/DBQ-3PXZ/Bphen/LiF/Al | 2.8 | 7.5 | 6.2 | 5.3 | 13,167 | 616 | (0.60,0.40) | [108] |
| ITO/MoO3/TAPC/mCP/CBP:10 wt%DBQ-3PXZ/Bphen/LiF/Al | 3.4 | 36.1 | 28.1 | 14.1 | 25,375 | 572 | (0.50,0.49) | [108] | |||||||
| SFDBQPXZ | 2.27 | 2.23 | 0.04 | 3.4 | 43.4 | 13.8 | ITO/MoO3/TAPC/mCP/SFDBQPXZ/Bphen/LiF/Al | 3.4 | 24.3 | 22.5 | 10.1 | 21,102 | 584 | – | [109] |
| ITO/MoO3/TAPC:MoO3/TAPC/CBP:10 wt%SFDBQPXZ/Bphen/LiF/Al | 2.7 | 78.3 | 91.1 | 23.5 | 31,790 | 548 | – | [109] | |||||||
| DFDBQPXZ | 2.25 | 2.21 | 0.04 | 3.3 | 33.2 | 11.2 | ITO/MoO3/TAPC/mCP/DFDBQPXZ/Bphen/LiF/Al | 3.2 | 21 | 20.6 | 9.8 | 16,497 | 588 | – | [109] |
| ITO/MoO3/TAPC:MoO3/TAPC/CBP:DFDBQPXZ/Bphen/LiF/Al | 2.8 | 55.9 | 57.8 | 16.8 | 31,099 | 548 | – | [109] | |||||||
| ND-AC | 2.61 | 2.58 | 0.03 | – | – | 55 | ITO/TAPC/TCTA/ND-AC/TmPyPB/LiF/Al | – | 38.5 | 30.2 | 12.0 | – | 558 | (0.43,0.54) | [110] |
| ITO/TAPC/TCTA/9 wt%ND-AC:CBP/TmPyPB/LiF/Al | – | 58.1 | 50.7 | 16.8 | – | 542 | (0.38,0.57) | [110] | |||||||
| CND-AC | 2.33 | 2.32 | 0.01 | – | – | – | ITO/TAPC/TCTA/1.5 wt%CND-AC:CBP/TmPyPB/LiF/Al | – | 21.3 | 14.6 | 8.4 | – | 588 | (0.43,0.54) | [110] |
| BCZ-BTD-AD | 2.32 | 2.16 | 0.16 | 0.12 | 82 | 50 | ITO/MoO3/TAPC/TCTA/BCZ-BTD-AD/TmPyPb/LiF/Al | 3.6 | 1.6 | 1.2 | 2.3 | 1728 | 652 | (0.63,0.32) | [111] |
| ITO/MoO3/TAPC/TCTA/CBP:20 wt%BCZ-BTD-AD/TmPyPb/LiF/Al | 3.2 | 6.8 | 5 | 5.6 | 1883 | 628 | (0.53,0.37) | [111] | |||||||
| BCPBT | – | – | – | – | – | – | ITO/MoO33nm/TAPC/BCPBT/TmPyPb/LiF/Al | 3.9 | 0.9 | 0.5 | 0.3 | 1182 | 540 | (.038,0.52) | [111] |
| ITO/MoO3/TAPC/CBP:25 wt%BCPBT/TmPyPb/LiF/Al | 3.9 | 1.8 | 1 | 0.6 | 2328 | 516 | (0.27,0.48) | [111] | |||||||
| TAT–DBPZ | 2.44 | 2.28 | 0.16 | 2.3 | 76 | – | ITO/PEDOT:PSS/TAT–DBPZ/TmPyPB42nm/LiF/Al | 3 | 7.3 | 7.6 | 5.6 | – | 626 | (0.61,0.38) | [112] |
| ITO/PEDOT:PSS/CBP:20 wt%TAT–DBPZ/TmPyPB/LiF/Al | 3.2 | 29.7 | 23.3 | 15.4 | – | 604 | – | [112] | |||||||
| TAT–FDBPZ | 2.38 | 2.28 | 0.1 | 1.51 | 62 | – | ITO/PEDOT:PSS/TAT–FDBPZ/TmPyPB/LiF/Al | 3 | 2.5 | 2.6 | 2.9 | – | 641 | (0.64,0.35) | [112] |
| ITO/PEDOT:PSS/CBP:20 wt%TAT–FDBPZ/TmPyPB/LiF/Al | 3 | 15.6 | 14 | 9.2 | – | 611 | – | [112] |
- a S1: lowest singlet energy.
- b T1: lowest triplet energy.
- c ∆EST: energy gap between S1 and T1.
- d τDF: delayed fluorescence lifetime.
- e ΦPL: total fluorescence quantum yield after nitrogen purging.
- f Voltage at 1 cd m−2.
- g Maximum current efficiency.
- h Maximum power efficiency.
- i Maximum EQE.
- j Maximum luminance.
- k Maximum electroluminescence wavelength.
- l Commission internationale de l'eclairage.
| Emitter | S1 (eV)a | T1 (eV)b | ∆EST (eV)c | τd (μs)d | ΦPLe (%) (sol.) | ΦPLe (%) (film) | Device configuration | Von (V)f | CEg (cd A−1) | PEh (lm W−1) | EQEi (%) | L j (cd m−2) | λmaxk (nm) | CIE(x,y)l | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
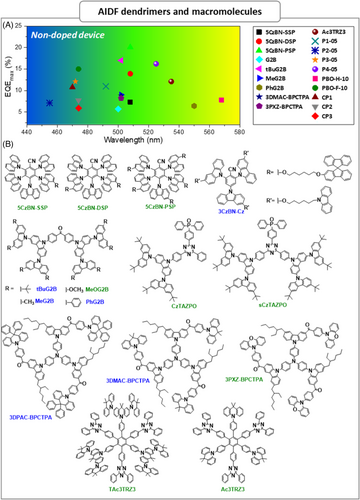
| 5CzBN-SSP | 2.96 | 2.81 | 0.15 | 3.1 | – | 38 | ITO/PEDOT:PSS/5CzBN-SSP/POT2T/Cs2CO3/Al | 3.4 | 21.9 | 17.2 | 7.3 | 5400 | 508 | (0.28,0.54) | [113] |
| 5CzBN-DSP | 2.93 | 2.78 | 0.15 | 3.8 | – | 45 | ITO/PEDOT:PSS/5CzBN-DSP/POT2T/Cs2CO3/Al | 3.2 | 40.7 | 31.9 | 13.9 | 14,800 | 508 | (0.27,0.54) | [113] |
| 5CzBN-PSP | 2.87 | 2.74 | 0.13 | 4.5 | – | 69 | ITO/PEDOT:PSS/5CzBN-PSP/POT2T/Cs2CO3/Al | 3.1 | 58.7 | 46.2 | 20.1 | 13,700 | 508 | (0.27,0.53) | [113] |
| 3CzBN-Cz | 3.18 | 2.88 | 0.3 | 2.3 | 0.63 | 89 | ITO/PEDOT:PSS/3CzBN-Cz:PY-Cz/TPBI/Cs2CO3/Al | 4.1 | 13.92 | 7.6 | 10.16 | 6200 | 468 | (0.15,0.14) | [115] |
| tBuG2B | 2.8 | 2.72 | 0.08 | 2.2 | 47 | 74 | ITO/PEDOT:PSS/PVK/tBuG2B/TPBi/Ca/Al | 2.7 | 46.6 | 40.7 | 17 | 4639 | 502 | (0.25,0.48) | [117] |
| MeG2B | 2.82 | 2.73 | 0.09 | 0.5 | 48 | 34 | ITO/PEDOT:PSS/PVK/MeG2B/TPBi/Ca/Al | 2.6 | 23.5 | 25 | 9 | 661 | 502 | (0.27,0.47) | [117] |
| MeOG2B | 2.65 | 2.54 | 0.11 | 0.06 | 74 | 17 | ITO/PEDOT:PSS/PVK/MeOG2B/TPBi/Ca/Al | 2.5 | 17.7 | 19 | 6.4 | 1017 | 550 | (0.42,0.52) | [117] |
| PhG2B | 2.83 | 2.69 | 0.14 | 1.2 | 32 | 41 | ITO/PEDOT:PSS/PVK/PhG2B/TPBi/Ca/Al | 2.7 | 22.7 | 20 | 8.8 | 965 | 502 | (0.30,0.48) | [117] |
| CzTAZPO | 2.7 | 2.62 | 0.08 | 1.1 | 71 | 3.2 | ITO/PEDOT:PSS/CzTAZPO/TmPyPB/Ca/Al | 4.5 | 29.1 | 28.6 | 12.8 | 9776 | – | (0.27,0.56) | [118] |
| sCzTAZPO | 2.75 | 2.65 | 0.1 | 0.81 | 57 | 4.1 | ITO/PEDOT:PSS/sCzTAZPO/TmPyPB/Ca/Al | 4.1 | 20.6 | 20.4 | 9.6 | 8283 | – | (0.36,0.56) | [118] |
| 3DPAC-BPCTPA | 2.94 | 2.87 | 0.07 | – | 2.4 | 46.6 | ITO/PEDOT:PSS/3DPAC-BPCTPA/TPBi/Cs2CO3/Al | 4.4 | 13.3 | 7.3 | 4.8 | 1267 | 486 | (0.22,0.31) | [119] |
| 3DMAC-BPCTPA | 2.84 | 2.78 | 0.06 | – | 1.9 | 64.8 | ITO/PEDOT:PSS/3DMAC-BPCTPA/TPBi/Cs2CO3/Al | 4.5 | 23.3 | 12.2 | 8.2 | 3913 | 502 | (0.27,0.46) | [119] |
| 3PXZ-BPCTPA | 2.67 | 2.66 | 0.04 | – | 1.1 | 66.5 | ITO/PEDOT:PSS/3PXZ-BPCTPA/TPBi/Cs2CO3/Al | 4.2 | 37.2 | 14.6 | 12.1 | 13,708 | 535 | (0.38,0.56) | [119] |
| TAc3TRZ3 | 2.7 | 2.66 | 0.04 | 3.16 | 63 | 63 | ITO/PEDOT:PSS/TAc3TRZ3/TSPO1/TmPyPB/LiF/Al | 2.9 | 10.2 | – | 3.1 | 7860 | 538 | (0.26,0.48) | [120] |
| ITO/PEDOT:PSS/AC6:10 wt%TAc3TRZ3/TSPO1/TmPyPB/LiF/Al | 2.9 | 40.6 | – | 14.2 | 9689 | 503 | (0.25,0.47) | [120] | |||||||
| Ac3TRZ3 | 2.77 | 2.69 | 0.08 | 3.54 | – | 54 | ITO/PEDOT:PSS/Ac3TRZ3/TSPO1/TmPyPB/LiF/Al | 3.4 | 11.4 | – | 3.5 | 6910 | 520 | (0.30,0.54) | [120] |
| ITO/PEDOT:PSS/AC6:10 wt%Ac3TRZ3/TSPO1/TmPyPB/LiF/Al | 2.9 | 30.3 | – | 11 | 6175 | 492 | (0.22,0.42) | [122] | |||||||
| P1-05 | 3.06 | 3.02 | 0.04 | 0.36 | – | 29 | ITO/PEDOT:PSS/P1-05/TSPO1/TmPyPB/LiF/Al | 3.2 | 10.6 | – | 7.1 | 1902 | 455 | (0.17,0.17) | [121] |
| P1-50 | 3.02 | 2.97 | 0.05 | 0.36 | – | 32 | ITO/PEDOT:PSS/P1-50/TSPO1/TmPyPB/LiF/Al | 4.6 | 1.1 | – | 0.5 | 960 | 473 | (0.22,0.31) | [121] |
| P2-05 | 2.91 | 2.89 | 0.02 | 1.91 | – | 51 | ITO/PEDOT:PSS/P2-05/TSPO1/TmPyPB/LiF/Al | 3.2 | 24.8 | – | 12.1 | 6150 | 472 | (0.18,0.27) | [121] |
| P2-50 | 2.84 | 2.82 | 0.02 | 1.42 | – | 6 | ITO/PEDOT:PSS/P2-50/TSPO1/TmPyPB/LiF/Al | 3.4 | 8.5 | – | 3.1 | 7989 | 497 | (0.22,0.43) | [121] |
| P3-05 | 2.73 | 2.69 | 0.04 | 1.98 | – | 74 | ITO/PEDOT:PSS/P3-05/SPPO13/LiF/Al | 3 | 50.3 | – | 16.2 | 10,273 | 525 | (0.34,0.55) | [121] |
| P3-50 | 2.66 | 2.63 | 0.03 | 1.56 | – | 64 | ITO/PEDOT:PSS/P3-50/SPPO13/LiF/Al | 3.8 | 10.9 | – | 3.7 | 6140 | 552 | (0.42,0.53) | [121] |
| P4-05 | 2.53 | 2.48 | 0.05 | 0.82 | – | 34 | ITO/PEDOT:PSS/P4-05/SPPO13/LiF/Al | 3.2 | 21.1 | – | 7.8 | 5339 | 568 | (0.46,0.50) | [121] |
| P5-05 | 2.3 | 2.25 | 0.05 | 0.94 | – | 06 | ITO/PEDOT:PSS/P4-05/SPPO13/LiF/Al | 4.6 | 1.7 | – | 1 | 1283 | 616 | (0.57,0.41) | [121] |
| PH-5 | – | – | 0.076 | 0.19 | – | 26 | ITO/PEDOT:PSS/PH-5/TSPO1/TmPyPB/LiF/Al | 3.4 | 5.5 | – | 2.9 | – | ∼480 | (0.23,0.27) | [122] |
| PH-10 | – | – | 0.076 | 0.19 | – | 26 | ITO/PEDOT:PSS/PH-10/TSPO1/TmPyPB/LiF/Al | 3.4 | 5.5 | – | 2.9 | – | ∼480 | (0.23,0.27) | [122] |
| PH-20 | – | – | 0.083 | 0.22 | – | 27 | ITO/PEDOT:PSS/PH-20/TSPO1/TmPyPB/LiF/Al | 3.4 | 7.7 | – | 3.6 | – | ∼480 | (0.23,0.31) | [122] |
| PF-5 | – | – | 0.096 | 0.21 | – | 27 | ITO/PEDOT:PSS/PF-5/TSPO1/TmPyPB/LiF/Al | 3.4 | 7.7 | – | 3 | – | ∼480 | (0.27,0.36) | [122] |
| PF-10 | – | – | 0.07 | 0.49 | – | 27 | ITO/PEDOT:PSS/PF-10/TSPO1/TmPyPB/LiF/Al | 3.2 | 5.7 | – | 2.9 | – | ∼460 | (023,0.26) | [122] |
| PF-20 | – | – | 0.073 | 0.44 | – | 30 | ITO/PEDOT:PSS/PF-20/TSPO1/TmPyPB/LiF/Al | 3.2 | 6.4 | – | 3.1 | – | ∼460 | (0.25,0.29) | [122] |
| PTF-5 | – | – | 0.09 | 0.46 | – | 38 | ITO/PEDOT:PSS/PTF-5/TSPO1/TmPyPB/LiF/Al | 3.2 | 6.3 | – | 2.8 | – | ∼460 | (0.28,0.34) | [122] |
| PTF-10 | – | – | 0.068 | 0.86 | – | 34 | ITO/PEDOT:PSS/PTF-10/TSPO1/TmPyPB/LiF/Al | 3 | 16.3 | – | 5.7 | – | – | (0.25,0.39) | [122] |
| PTF-20 | – | – | 0.067 | 0.95 | – | 44 | ITO/PEDOT:PSS/PTF-20/TSPO1/TmPyPB/LiF/Al | 3 | 17.4 | – | 6.7 | – | – | (0.25,0.41) | [122] |
| PBO-TB-5 | – | – | 0.09 | 0.12 | – | 32 | ITO/PEDOT:PSS/PBO-TB-5/TSPO1/TmPyPB/LiF/Al | 3.8 | 4.6 | 3.6 | 3.5 | 405 | – | (0.17,0.13) | [123] |
| PBO-TB-10 | – | – | 0.09 | 0.11 | – | 37 | ITO/PEDOT:PSS/PBO-TB-10/TSPO1/TmPyPB/LiF/Al | 3.8 | 5.8 | 5.1 | 3.8 | 578 | 451 | (0.18,0.17) | [123] |
| PBO-H-5 | – | – | 0.06 | 0.18 | – | 44 | ITO/PEDOT:PSS/PBO-H-5/TSPO1/TmPyPB/LiF/Al | 3.4 | 8.1 | 7.5 | 5.2 | 1190 | – | (0.17,0.16) | [123] |
| PBO-H-10 | – | – | 0.07 | 0.15 | – | 48 | ITO/PEDOT:PSS/PBO-H-10/TSPO1/TmPyPB/LiF/Al | 3.4 | 10.3 | 9.5 | 6.1 | 1409 | 455 | (0.18,0.20) | [123] |
| PBO-F-5 | – | – | 0.01 | 0.25 | – | 65 | ITO/PEDOT:PSS/PBO-F-5/TSPO1/TmPyPB/LiF/Al | 3.2 | 25.3 | 24.9 | 14.4 | 1532 | – | (0.15,0.21) | [123] |
| PBO-F-10 | – | – | 0.02 | 0.23 | – | 70 | ITO/PEDOT:PSS/PBO-F-10/TSPO1/TmPyPB/LiF/Al | 3.2 | 30.7 | 30.2 | 15 | 1650 | 474 | (0.16,0.27) | [123] |
| P1 | 2.63 | 2.54 | 0.09 | 0.031 | 19.8 | 10 | ITO/PEDOT:PSS/P1:TCTA:TAPC(10:65:25 wt%)/TmPyPB/LiF/Al | 3.1 | 3.1 | 2.3 | 1.1 | 1552 | – | (0.46,0.43) | [124] |
| P2 | 2.63 | 2.57 | 0.06 | 0.026 | 28.3 | 23.5 | ITO/PEDOT:PSS/P2:TCTA:TAPC(10:65:25 wt%)/TmPyPB/LiF/Al | 2.9 | 11 | 12.3 | 4.5 | 1764 | – | (0.45,0.40) | [124] |
| P3 | 2.64 | 2.6 | 0.04 | 0.164 | 52.9 | 19.5 | ITO/PEDOT:PSS/P3:TCTA:TAPC(10:65:25 wt%)/TmPyPB/LiF/Al | 2.9 | 23 | 32.8 | 10.4 | 1653 | – | (0.37,0.38) | [124] |
| CP1 | 3.05 | 3.08 | −0.03 | – | 2.8 | 50.1 | ITO/PEDOT:PSS/mCP:5 wt%CP1/DPEPO/TmPyPB/Liq/Al | 4.4 | 9.74 | 16.96 | 10.75 | 2441 | 470 | (0.17,0.24) | [125] |
| CP2 | 3.05 | 3.13 | −0.08 | – | 43.1 | 26.5 | ITO/PEDOT:PSS/mCP:5 wt%CP2/DPEPO/TmPyPB/Liq/Al | 4.6 | 6.51 | 12.32 | 7.75 | 3224 | 474 | (0.19,0.28) | [125] |
| CP3 | 3.05 | 3.1 | −0.05 | – | 48.9 | 43.8 | ITO/PEDOT:PSS/mCP:5 wt%CP3/DPEPO/TmPyPB/Liq/Al | 4.7 | 4.87 | 9.38 | 5.89 | 2673 | 474 | (0.19,0.28) | [125] |
| pTPE–DPA–Cz | – | – | – | – | 0.4 | 63.3 | ITO/PEDOT:PSS/pTPE–DPA–Cz/TmPyPB/LiF/Al | 3.2 | 6.47 | 4.73 | 2.98 | 5967 | 482 | (0.209,0.316) | [126] |
| pTPE–DPA–Flu | – | – | – | – | 1.5 | 56.1 | ITO/PEDOT:PSS/pTPE–DPA–Flu/TmPyPB/LiF/Al | 3.6 | 6.32 | 4.62 | 3.26 | 4578 | 480 | (0.208,0.275) | [126] |
- a S1: lowest singlet energy.
- b T1: lowest triplet energy.
- c ∆EST: energy gap between S1 and T1.
- d τDF: delayed fluorescence lifetime.
- e ΦPL: total fluorescence quantum yield after nitrogen purging.
- f Voltage at 1 cd m−2.
- g Maximum current efficiency.
- h Maximum power efficiency.
- i Maximum EQE.
- j Maximum luminance.
- k Maximum electroluminescence wavelength.
- l Commission internationale de l'eclairage.
2 FLUORESCENT AIEGENS
2.1 Silole-based AIEgens
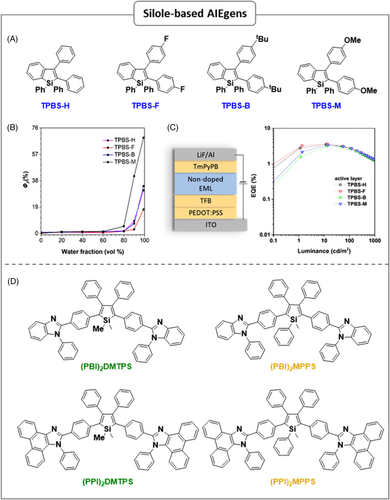
In 2001, Tang's group first observed AIE phenomenon in “1-methyl-1,2,3,4,5-pentaphenylsilole (MPPS)”,9 which is nonemissive in the solution state, but highly emissive in the aggregated (solid) state. Silole derivatives have high electron affinity and fast electron mobility because of the unique σ*–π* conjugation effect from the σ* orbital of the two exocyclic Si-C bonds and the π* orbital of the butadiene unit. MPPS can show an EQE of up to 8%, which exceeds the theoretical limit.22 In general, silole derivatives shown strong emission in green and yellow regions, but seldom in blue region. In 2016, Li's group reported green to blue AIEgens without the σ*–π* conjugation effect, by replacing the Si atom with a C atom, thereby, achieving a limited degree of intramolecular conjugation. In these AIEgens, a new core, tetraphenylcyclopentadiene, was connected with TPE or TriPE as peripheral moieties.23 Furthermore, a blue or deep-blue AIEgens were achieved by controlling the conjugative effect via the adjustment of the linkage mode. Nondoped devices based on these resulting new luminogens could show good performance with blue emission (λEL = 440 nm) rather than the green or yellow-green emission of siloles. In order to construct blue/deep-blue AIEgens based on silole derivatives, recently Tang's research group synthesized a new building block, tetraphenylbenzosilole (TPBS), by replacing the two phenyl groups of hexaphenylsiole (HPS) at the 2,3-positions with one phenyl group consist with a silole core via a silyl radical cascade process with intermolecular radical cyclization.24 TPBS core can effectively increase the skeleton rigidity and reduce the number of excited-state conformations, which is beneficial for obtaining practical deep-blue emission. As expected, the resulting four new TPBS derivatives not only inherited the AIE feature from HPS but also exhibited high-efficiency blue/deep-blue emission in the aggregated state. The remarkable performance of these derivatives was attributed to their tunable highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), increased skeleton rigidity, and reduced the vibrational–rotational motions of peripheral phenyl groups. Thus, nonradiative channel of excited state was gradually blocked, and radiative channels were activated. Thanks to the hydrophobic nature of resulting luminogens, which are gathered into aggregated states when adding water (fw ≥ 80%) to THF solution; therefore, the resulting spatial limitation reduced the motion of rotational units and the nonradiative channels of excited state were gradually blocked. Consequently, as shown in Figure 2B, the TPBS-based derivatives exhibited drastically enhanced PL intensity and increased their PLQYs (ΦF) in the aggregated state, which reveals a typical AIE feature. As a result, the resultant blue nondoped OLEDs with the configuration of ITO/PEDOT:PSS/TFB (40 nm)/emitting layer (about 15 nm)/TmPyPB (30 nm)/LiF (1.5 nm)/Al (120 nm) displayed excellent performance with high EQEs (3.1–3.6%) at CIE coordinates of (0.15, 0.10) (Figure 2C). These results demonstrate the effectiveness of a fused benzo-based strategy to obtain building blocks for deep-blue AIEgens that could overcome the deficiency of HPS derivatives for constructing blue/deep-blue OLED emitters.
As known, TPSs are excellent solid-state light emitters with AIE characteristics, but the bifunctional materials capable of both light emitting and electron transporting in OLEDs are much rare. To create bifunctional materials, Tang's group reported three derivatives of 2,3,4,5-TPS, connected with dimesitylboryl groups.25 Owing to the synergistic effect between the silole ring and dimesitylboryl moieties, the new derivatives exhibited lower LUMO energy levels than the corresponding parent moiety 2,3,4,5-TPSs and improved electron-transporting ability, which offered them to serve as dual functioning of light-emitting layers and electron-transporting layers (ETL) in a single device. In addition, the branched conformation of dimesitylboryl unit greatly suppresses close π–π stacking, which helps to exhibit AIE characteristics for these silole derivatives with high PLQY values of 56–62% in solid film state. The nondoped double-layered configuration of these devices revealed excellent performances with high electroluminescence efficiencies up to 4.35%, 13.9 cd A−1, and 11.6 lm W−1 relative to the corresponding triple-layer configured devices. Therefore, these are behaving as excellent bifunctional materials with both light-emitting and electron-transporting properties. Furthermore, to improve the electron-transporting ability of light emitters, Tang's group synthesized another set of four AIE active n-type emitters based on silole derivatives, (PBI)2DMTPS, (PBI)2MPPS, (PPI)2DMTPS, and (PPI)2MPPS, by introducing 1-phenyl-1H-benzo[d]imidazole (PBI) and 1-phenyl-1H-phenanthro[9,10-d]imidazole (PPI) groups to 2,3,4,5-TPS core.26 The resulting four luminogens revealing AIE feature by enhancing PL emission when fw exceeded 50% in THF–water system because the intramolecular rotations were restricted by the steric constraint, and gradually the nonradiative internal conversion (IC) channels were deactivated, it helps the strong emission of the molecules in aggregated state with a high PLQY values of 49.5–62.1%. Thanks to the synergistic effect of the silole and PBI units of new silole derivatives, which showed better electron-transporting abilities compared with 2,2′,2-(1,3,5-benzinetriyl)-tris(1-phenyl-1-H-benzimidazole) (TPBi). Impressively, a double-layer OLED based on (PBI)2DMTPS without an additional ETL exhibited a maximum EQE (EQEmax) of 4.25%, which is a comparable result with the EQEmax (4.22%) of its corresponding trilayer OLED. Nondoped trilayer-configured light-emitting device based on (PPI)2DMTPS displayed a high EQEmax of 4.84%, which is close to the theoretical limit of fluorescent OLEDs. This work provided an effective strategy to achieve bifunctional materials (i.e., n-type light emitters) for highly efficient, simplified, and low-cost OLEDs. The relevant data from the aforementioned representative reports of silole-based AIEgens are summarized in Table 1, and their corresponding chemical structures are shown in Figure 2.
2.2 TPE-based AIEgens
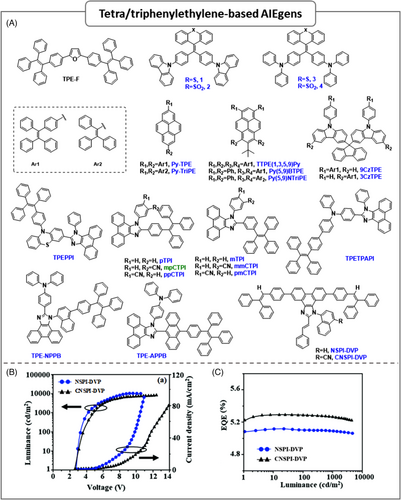
Amongst the various AIEgens, TPE and TriPE are the most common and versatile AIEgens, which can be incorporated into several chromophores. As is well known, the highly twisted conformation, restriction of intramolecular motion (RIM), and weak intermolecular interactions of AIEgens containing a TPE core efficiently suppress ACQ in solid films of OLEDs. As a result, AIEgens exhibit great potential for fabricating nondoped OLEDs with high efficiency and small efficiency roll-off. AIEgens containing TPE/TriPE cores have been widely used to develop high-performance nondoped OLED materials so far.
In 2017, Tang et al.27 synthesized thiophene/furan-cored AIEgens via the cascaded cyclization of diyne, abbreviated as TPE-T and TPE-F. In dilute THF solution, both luminogens were weakly emissive due to their active intramolecular rotations that acted as the fast IC channels and efficiently deactivated the excited state via nonradiative relaxation. The addition of water into THF solution (aggregated state) enhanced their PL emission; the maximum emission was achieved at a fw of 99%, due to the greater restriction in the intramolecular rotations and the nonradiative IC channels were blocked. Owing to its higher restriction of RIM in TPE-F, stronger PL emission and higher PLQY (50%) than TPE-T (18%) were shown. As well, OLED devices of TPE-F displayed high EL efficiencies comparable with the TPE-T-based device. The nondoped OLED device using TPE-F as the emitter exhibited low turn-on voltage (Von), the maximum luminescence (Lmax), current efficiency (CEmax), power efficiency (PEmax), and EQEmax values of 3.3 V, 24,298 cd m−2, 9.98 cd A−1, 7.02 lm W−1, and 3.67%, respectively. These results are better than those of other similar hetero aromatic AIEgens containing benzo-2,1,3-thiadiazole and silole with the same device structure reported earlier by the same group.28 The authors also reported a new series of AIE-active sulfur/sulfone-bridged TPE derivatives connected with carbazole (Cz) and diphenylamine (DPA) units that exhibited a wide energy band gap and pure deep-blue color narrow emission with full width at half maximum (FWHM) of 55–65 nm at 434–473 nm in the solid state. A nondoped device based on the sulfur-bridged TPE–DPA derivative exhibited excellent performances (Lmax: 2918 cd m−2, CEmax: 3.94 cd A−1, PEmax: 4.13 lm W−1 and EQEmax: 2.15% at λEL: 468 nm) comparable with that of doped OLEDs.29
However, the fluorophores using TPE as AIEgens with emission peaks below 470 nm are still rare. The first report of such fluorophores was by Tang et al.30 based on TPE substituted on pyrene (TTPEPy). These fluorophores exhibited excellent EL performance with an EQE of 4.95% and sky-blue EL emission at 488 nm; the same group reported two sky-blue AIEgens, by substituting TPE or TriPE at 2,7-positions of pyrene, named Py–TPE and Py–TriPE, respectively.31 Both luminogens exhibiting AIE feature in THF/water mixture, for example, Py–TPE has weak fluorescence with a maximum peak at 450 nm in THF solution, while the emission intensity was enhanced by ∼60-fold when increasing water fraction until 60%, then PL emission intensity gradually increased to ∼200-fold and a large red-shifted emission peak at 491 nm when the fw reached 99%. A nondoped device with Py–TPE as emitter showed a EQEmax of 3.19%, but its emission extended beyond the blue region. Later on, some reports were devoted based on TPE–pyrene, exhibiting red-shifted emission due to extended π-conjugation with pyrene. In order to achieve blue emission, a few strategies have been developed to control the degree of π-conjugation in AIEgen by changing the linkage pattern or break up the π-conjugation or to make the twisted structure.32 Based on these strategies, Hu et al.33 reported a twisted TPE-substituted pyrene-based blue AIEgen, abbreviated as TTPE(1,3,5,9)Py by modifying TTPEPy. Similar to TTPEPy, the TTPE(1,3,5,9)Py showed robust AIE features in fw from 40% to 90% and enhanced its PLQY value by about 10-fold in fw > 40%, this value further increased to 65% when the fw reached 90%. A nondoped device based on TTPE(1,3,5,9)Py showed pure-blue emission (λEL: 468 nm) with EQEmax and CEmax values of 4.10% and 7.38 cd A−1, respectively, as well as exhibited negligible efficiency roll-off and good color purity. In addition, the same research group integrated two TPE or TriPE units into 5,9-positions of pyrene moiety in the shape of butterfly, namely, Py(5,9)BTPE and Py(5,9)BTriPE.34 Py(5,9)BTPE displayed significant AIE characteristics upon aggregation in a THF/water system, as well as high PLQY of 57.7% in the thin film state. A nondoped OLED based on Py(5,9)BTPE exhibited a sky-blue emission with an EQEmax of 3.35%, whereas a nondoped device with Py(5,9)BTriPE displayed a pure-blue emission (CIE: 0.16, 0.17) with a moderate EQEmax of 1.27% and a small efficiency roll-off. On the other hand, Xu et al.35 synthesized two sky-blue AIEgens, namely, 9CzTPE and 3CzTPE, by tuning the linkage modes between TPE and the Cz unit. Both compounds exhibited remarkable AIE behavior with PLQY values of 63.4 and 65.5%, respectively, in a solid state. A nondoped OLED based on 3CzTPE displayed an EQEmax of 2.81%, high luminous efficiency of 4.35 cd A−1 and with sky-blue emission at 479 nm.
In 2018, Xue and coworkers,36 reported TPEPPI as a deep-blue AIEgen, using phenothiazine (PTZ) as a donor and TPE as the AIEgen. TPEPPI is hardly luminescent in THF solution, but its PL intensity increased by 5.5-fold when fw reached 95%. A nondoped device with TPEPPI as the emitter showed EQEmax, CEmax, PEmax, and λEL values 2.36%, 4.25 cd A−1, 3.35 lm W−1, and 467 nm, respectively, as well, the device showed a negligible efficiency roll-off of 3.3% with increasing luminance. As known, phenanthroimidazole (PI) core is one of the most widely used π-conjugated rigid structure that can be used to explore deep-blue to green electroluminescence when combined with AIE blocks. For instance, in 2019, Tang's group37 synthesized six TPE-PI-based derivatives with different conjugation patterns at the C2 and N1 substituent positions of the PI moiety according to HLCT strategy, which is another strategy to harvest 75% of nonemissive triplet excitons for light emission. In which, hot excitons (excitons in second or higher excited state) are injected to the high-lying triplet state and are transferred to the singlet state via RISC process. The resulting TPE-PI-based derivatives revealed AIE characteristics by enhancing PL emission as water fraction (fw) increased, due to the restriction of rotational motions by the spatial constraint in the aggregated state and leads to suppression of nonradiative decay of the excited state. A nondoped OLED employing ppCTPI as the emitter exhibited excellent performance with a Lmax, CEmax, and EQEmax values of up to 31,070 cd m−2, 18.46 cd A−1, and 7.16%, respectively, as well this device exhibited a very small efficiency roll-off of 4.0% at 1000 cd m−2, which is the best reported thus far for nondoped OLEDs based on TPE-substituted AIE molecules. However, the EL peak wavelength of blue fluorophores based on TPE-substituted AIE cannot be controlled in the blue-light-emission region, thus, fabricating efficient AIE-active organic blue light-emitting materials is fairly difficult. Encouragingly, Xue et al.38 synthesized TPE-substituted efficient AIE-active blue-fluorescent D–A molecule, TPETPAPI, composed with TPA and PI as the donor and acceptor units, respectively. TPETPAPI revealed AIE feature with the emission intensity increased by up to ∼100 folds upon aggregation, as well, showed higher PLQY of 73% in film state than in the THF solution (1.3%). A nondoped device bearing TPETPAPI as the emitter exhibited excellent EL performance with EQEmax, Lmax, CEmax, and PEmax values of 6.05% 10,780 cd m−2, 12.2 cd A−1, and 11.9 lm W−1, respectively; these values are among the best reported to date for small AIEgens used in TPE-substituted nondoped blue OLEDs in λEL of 480 nm.
Jayabharathi and coworkers39 reported two D–A-based positional isomers of TPE-substituted blue AIEgens, namely, TPE–NPPB and TPE–APPB, containing PI and TPA cores. A nondoped OLEDs using them as emitters exhibited high EQE of 3.2 and 5.3%, CE of 4.32 and 5.28 cd A−1, and PE of 4.01 and 4.92 lm W−1, respectively. These materials also showed reversible mechanochromism between the colors blue and green. In very recent work, the same research group synthesized TPE-based AIE active PIs, namely, NSPI–DVP and CNSPI–DVP.40 These materials not only exhibited HLCT states, but also showed reversible mechanochromism between the colors blue and green. The nondoped devices based on NSPI–DVP and CNSPI–DVP as the emitters displayed EQEmax of 5.09 and 5.23%, CEmax of 5.61 and 5.03 cd A−1, and PEmax of 4.99 and 4.72 lm W−1, respectively (Figures 3B and 3C), as well exhibited negligible efficiency roll-off [NSPI–DVP 1.76% and CNSPI–DVP 0.96%] due to the effective h+–e− recombination and reduced exciton quenching. In comparison, CNSPI–DVP shows higher EQE than NSPI–DVP under the same device configuration because the special excited state of CNSPI–DVP can trigger the fast kRISC with a hot exciton process and harvesting more triplet excitons, which leads to a higher exciton utilization efficiency of 64.0% than NSPI–DVP (36.0%). In 2020, Tang's research group also explored PI-based three blue-emissive AIEgens, CNNPI, 2TriPECNNPI, and 2CzPh–CNNPI, with HLCT characteristics.41 A nondoped OLED based on 2CzPh–CNNPI exhibited pure blue emission with an impressive EQEmax of 5.09%. Moreover, the material could simultaneously be employed as an emitter, emissive dopant, and sensitizing host. The nondoped/doped deep-blue OLEDs and HLCT-sensitized green fluorescent OLEDs prepared from this material are among the most efficient OLEDs prepared via the “hot exciton” approach reported thus far. 2TriPE-BPI-MCN was developed as a blue AIEgen by inserting para-cyano and ortho-methyl groups to the phenyl unit at the C2 position of PI moiety from its matrix 2TriPE–BPI by adopting HLCT approach.42 Interestingly, the excited state of 2TriPE–BPI–MCN explored RISC channel to obtain high exciton utilization efficiency during the EL process. A nondoped blue OLED based on 2TriPE–BPI–MCN exhibited excellent EL performance with a high EQE of 4.60% and high exciton utilization efficiency of 50.2%; these values are better than those of its matrix part 2TriPE–BPI (3.74 and 29.9%). Lu et al.43 developed three blue aggregation-induced emission enhancement (AIEE) type luminogens, namely, PIAnTPE, TPAAnTPE, and CzAnTPE, based on TriPE core substituted PI/TPA/Cz moieties at 9,10-positions of anthracene, respectively. The resultant materials displayed AIE characteristics upon aggregation and significant PLQY values of 65, 70, and 46%, respectively, in the film state. The nondoped OLEDs using them as emitters showed the EQEmax values of 4.46, 4.13, and 4.04%, respectively, as well, realized negligible efficiency roll-offs. Huo et al.44 reported three TPE–PI derivatives with different alkoxy chain lengths, namely, TPEO1–PPI, TPEO4–PPI, and TPEO6–PPI, observed high solid-state PL efficiencies along with AIE and mechanochromic luminescence (MCL) characteristics in these materials. A nondoped OLED based on TPEO4–PPI showed excellent EL performance with a EQEmax and Lmax values of 4.07% and 15995 cd m−2, respectively, as well as the device showed negligible efficiency roll-off.
Owing to the poor charge carrier mobility of TPE core, issue still exists to make high performance of TPE-based devices; some researchers believe that this issue could be overcome by balancing the charge carriers in the device rather than the addition of carrier transporting layer to the OLED device. Previous reports noted the favorable ability of bipolar materials based on the donor–acceptor (D–A) model inject and transport both holes and electrons.45 These bipolar materials have the ability of balance charge carrier transportation in the device, as a result can reduce production costs and simplify the manufacturing process. For instance, Ge et al.46 reported three bipolar AIEgens with a D–A configuration by combining TPE with Cz and benzimidazole, named TPE-1, TPE-2, and TPE-3. These three derivatives exhibited weak PL emission in THF solution because of nonradiative decay through strong intramolecular motion of TPE units, which is gradually reduced and enhanced PL emission intensity in the aggregated state; upon addition of water to its THF solutions, it indicates typical AIE feature of resulting luminogens. As well as PLQY values were high as 98.7, 86.2, and 84.0% respectively, in the solid state. A nondoped OLED using TPE-2 as the emitter exhibited good performances with CEmax, PEmax, and EQEmax values of 15.1 cd A−1, 14.8 lm W−1, and 5.34%, respectively. Tong and coworkers47 also synthesized two bipolar blue molecules, PPI–PIM–TPE and 2PPI–TPE, the resultant materials showed AIE and mechanochromic characteristics, as well as prominent PLQY values of as 61.9 and 73.4%, respectively, in the solid state. A nondoped blue OLED using 2PPI–TPE as the emitter exhibited an EQE, CE, and PE values of 2.48%, 6.46 cd A−1, and 4.72 lm W−1, respectively. In addition, TriPE substituted with Cz or acridan units featuring fluorinated or nonfluorinated fragments (TCz, TCz-F, TAc, and TAc-F) have been reported as bipolar charge transporting AIEE emitters for blue nondoped OLEDs.48 TPE–triazole hybrids with AIE characteristics could simultaneously function as intrinsic blue-light emission and electron-transport layer in OLEDs.49 TPETAZ exhibited PLQYs of 75.7% in the film state. A nondoped device with ETL 1,3,5-tris(3-pyridyl-3-phenyl)benzene (TmPyPB) showed EQEmax and Lmax of 2.4% and 1020 cd m−2, respectively, with an EL emission peak of 468 nm. Whereas, nondoped device without the TmPyPB layer exhibited EQEmax values of up to 2.0%, which is only 18% lower than that of the device with TmPyPB as ETL, and Lmax of 1171 cd m−2. These findings could be attributed to the ability of TPETAZ to function as both light-emitting layers and ETLs in OLEDs. The relevant data from the aforementioned representative reports of tetra/TPE-based AIEgens are summarized in Table 2, and their corresponding chemical structures are shown in Figure 3.
2.3 TPP-based AIEgens
TPP is a new AIE-active unit introduced by Tang's group in 2015 with the benefits of facile synthesis, easy modification, and excellent thermal stability.50 In addition, TPP unit exhibits narrow emission at 390 nm, which is more beneficial for fabricating deep-blue AIE emitters for OLED applications. However, the previous report based on TPA–TPP derivatives are limited to the sky-blue region.51 Owing to the strong interactions between the TPA donor and TPP acceptor, as well as the increased rotational freedom of the TPA moiety in the excited state, red-shifted emission with widened spectra, which is unfavorable for the construction of efficient deep-blue OLEDs, was observed. To address this issue, the group developed TPP–Cz, TPP–PhCz, TPP–2Cz, and TPP–2PhCz, by employing the Cz unit instead of TPA unit; Cz features a good donor capacity and can restrict rotation via a locked single bond.52 The four TPP-based luminogens revealed AIE phenomenon, by increasing their PL emission intensities when formation of aggregates with increasing water fraction to THF solutions, due to the RIM of TPP unit in the molecules. Thanks to the formation of stable quinone conformation in the excited state of these derivatives that led to great enhancements on their radiative transition decay rate, which contrasts the behavior of traditional AIEgens. A nondoped OLED based on TPP-Cz exhibited the optimal EL performance with an EQE of 1.49% and ideal roll-off properties with a retaining CIE coordinate of (0.16, 0.11).
In general, large torsion has been observed between the pyrazine center and peripheral phenyl groups in the ground state, when forms quinone conformation in the excited state, its excited conjugation is expanded for planarization, which is referred to as excited-state quinone-conformation-induced planarization. Taking advantage of the restricted intramolecular rotation mechanism of TPP and its ability to form a stable quinone conformation in the excited state, Tang's group tailored two AIEgens, using the strategy of planarized ICT (PLICT), namely, TPP–PPI and TPP–PI.53 Both the materials exhibited blue emission with the peak at 452 and 463 nm in the dilute THF solution, along with fluorescence quantum yields (ΦF) of 10.0 and 13.8%, respectively. Their PL intensities remain low up to the water fraction (fw) is less than 60% in the THF/water mixture, but it increases gradually when the fw is more than 80%, which indicates its AIE feature. Due to the formation of nanoaggregates in the THF/water mixture, which restricts intramolecular motions and blocks the nonradiative decay channel, the radiative decay rate (Kr) increase and surely contributes to the enhanced emission (Figure 4B). Nondoped devices bearing TPP–PPI and TPP–PI with the configuration of ITO/HATCN (5 nm)/NPB (40 nm)/TCTA (5 nm)/EML (20 nm)/TPBi (40 nm)/LiF (1 nm)/Al exhibited stable sky-blue emission with the peaks at 474 and 484 nm, respectively, close to their PL emission in the neat film. These nondoped devices displayed significant EQEmax values of 4.85 and 4.36%, respectively, and exhibited small efficiency roll-off of 5% (EQEs remain to 4.51 and 4.12%, respectively, at the luminescence of 1000 cd cm−2) (Figure 4C). On the other hand, tetra/triphenylpyrazine–3Cz derivatives, namely, TPP–3C and TrPP–3C, were synthesized by replacing the phenyl group with a methyl group at the 3-position of pyrazine to obtain deep-blue emitters; meanwhile, 3-Cz was melded instead of 9-phenyl-Cz in TPP–PhCz to improve the luminescence efficiency of the resultant devices.54 Compared with an OLED bearing TPP–3C, a nondoped OLED employing TrPP–3C as the emitter showed better EL performance with a significant EQEmax of 2.89%.
Furthermore, the same group synthesized three blue fluorophores by inserting a phenyl group at the ortho-, meta-, and para-positions of a pyrazine unit containing 3-Cz, to investigate the role of each phenyl group in the TPP core.55 AIE characteristics were observed in the derivative with a phenyl group at ortho-position (23-B3C) but not in those with phenyl groups at the meta- and para-positions (i.e., 26-B3C, 25-B3C) because of the large steric hindrance between the ortho-phenyl group and pyrazine core, which distorts the planarity of pyrazine. Among the nondoped OLED devices, 26-B3C exhibited the best EL performance with an EQEmax of 3.32% and deep-blue emission at CIE coordinates of (0.16, 0.07). Moreover, to investigate AIE features inherited from TPE and TPP, the same group tailored three AIE isomers by connecting TriPE at the para, meta, and ortho-position to TPP unit, respectively, namely, TPP-p-TPE, TPP-m-TPE, and TPP-o-TPE.56 The isomerism effect of the AIEgens shortened their conjugation length, and their emission gradually weakened from the para to the ortho isomer, owing to decrease in RIM due to the their loose intermolecular packing. Meanwhile, the molecular porosity increases, which showed low emission efficiency but improved sensitivity as fluorescent probes. Based on distinct properties, the resultant isomers investigated not only in nondoped blue OLEDs, but also in nanofluorescent probes, and molecule-capturing porous crystals. Nondoped devices using TPP–p–TPE and TPP–m–TPE as EMLs exhibited EQEmax values of 2.74 and 1.14%, respectively. Moreover, the device prepared with TPP–p–TPE showed sky-blue emission at 488 nm, whereas TPP–m–TPE revealed deep-blue emission at 454 nm. The relevant data from the aforementioned representative reports of TPP-based AIEgens are summarized in Table 3, and their corresponding chemical structures are shown in Figure 4.
2.4 TPB-based AIEgens
Qin and Tang et al. introduced another AIE core, TPB, and significant research has been conducted on it in the past few years. TPB is similar to TPE and TPP, but the nitrogen atom is replaced with a carbon atom in TPP. TPB emits at 380 nm in the aggregated state with good thermal stability and is more suitable for constructing stable deep-blue emitters in OLEDs. The same group has reported many deep-blue AIEgens based on the TPB core by extending the conjugation or introduction of donors and acceptors. For example, TPB–AC was synthesized by melting a DPA moiety and a cyano group on the TPB core.[57] The TPA core in TPB–AC enhances the hole-transport properties of the device, whereas the cyano group reduces the LUMO energy level, diminishes the electron injection barrier, and improves the intermolecular interactions. It emits intensely at 479 nm in a THF solution. With an increase in the water fraction (fw) from 0 to 70%, the emission intensity gradually decreased, along with a red shift in the emission peak, owing to a TICT process. With a further increase in the water fraction (fw > 70%), the emission intensity gradually increased, with a blue shift in the peak wavelength, owing to the formation of aggregates and restriction of the intramolecular rotation of the phenyl rings in TPB–AC. In addition, it exhibited a high PLQY of 69.4% in the aggregated state. A nondoped OLED based on TPB–AC exhibited deep-blue electroluminescence with its Von as low as 3.2 V and ηext,max up to 2.92% with the device configuration of ITO/NPB (40 nm)/TPB–AC (20 nm)/TPBi (40 nm)/LiF (1 nm)/Al. The low performance of the device based on TPB–AC may be due to the unbalanced electron–hole transport system provided by the device structure. After optimization of the device structure as ITO/HAT–CN(5 nm)/TAPC(50 nm)/TCTA(5 nm)/TPB–AC(20 nm)/BmPyPB(40 nm)/LiF(1 nm)/Al(120 nm), high-efficiency deep-blue EL emission with a ηext,max of 7.0% was obtained, which exceeded the theoretical limit and was ascribed to the high horizontal dipole orientation of TPB–AC. Furthermore, the EQEs remained at 6.3% at a luminance of 1000 cd m−2, exhibiting a very low roll-off. In addition, TPB–AC can also be used as a good host for highly efficient green/orange/red phosphorescent OLEDs and hybrid WOLEDs because of its high triplet energy level.[57] Moreover, the same research group studied the luminescence mechanisms in TPB–AC-based OLEDs using magnetic field effect measurements to determine the reason for the efficiency of nondoped OLEDs exceeding the theoretical limitation.[59] They found that the theoretical efficiency was exceeded owing to the energy conversion process of T2→S1 in TPB–AC. Low-field rise and high-field reduction line shapes of magneto-electroluminescence were realized, which cannot be ascribed to the TTA process. In addition, time-dependent density functional theory (TD–DFT) studies proved the presence of an extra T2→S1 conversion in TPB–AC, which is directly related to the high EQE and characteristic line shapes of magneto-electroluminescence. Furthermore, the efficiency and efficiency roll-off of TPB–AC AIEgen-based blue OLEDs were investigated by introducing a phosphorescent (FIrpic)-doped layer adjacent to the TPB–AC layer separated by 2,6-bis(3-(9H-carbazol-9-yl)phenyl)pyridine (26DCZPPY). The maximum EQE reached 7.93% and remained at 7.57% at a luminance of 1000 cd m−2. Thus, this study provided physical insight for designing high-performance blue OLEDs using AIE materials. Inspired by the high horizontal orientation of TPB–AC molecules and to improve the device performance, the same group attempted to increase the molecular length and developed two deep-blue AIEgens, CN–TPB–AD and CN–TPB–TPA, by attaching TPA or DMAC as donors in the direction of the long molecular axis of TPB–AC.[58] The two AIEgens exhibited deep-blue emission with PLQY values of 55.3 and 93.2% in the film state, respectively, and CN–TPB–TPA showed a good horizontal dipole orientation of 83.6%; thus, a device with ηout > 30% was obtained. Notably, a nondoped OLED based on CN–TPB–TPA exhibited a maximum external quantum efficiency of 7.27%, with a low efficiency roll-off and CIE coordinates of (0.15, 0.08). Additionally, CN–TPB–TPA also acts as a good host for hybrid warm WOLEDs, with the maximum current, power, and external quantum efficiency of 58.0 cd A−1, 60.7 lm W−1, and 19.1%, respectively. To further blue-shift the emission of TPB-based AIEgens, Qin et al.[58] developed three TPB-based violet-blue AIE emitters, namely, TPBCzC1, TPBCzC2, and TPBCzC3, by altering the donor group from DPA or TPA to Cz with weak electron-donating ability, and the cyano group was used as an acceptor. In these derivatives, TPB acts as a π-conjugate bridge and helps to reduce the intramolecular interference between the donor and acceptor groups; further, it decreases the intermolecular charge transfer (ICT) to enhance the emission efficiency in the solid state, which is a desirable feature for emitters in the aggregate state. These three AIEgens exhibited excellent PL quantum yields (ΦF) of over 98% in their film states. They also showed the AIE feature by gradually enhancing the PL intensity along with blue-shifted emission in THF:water mixtures, because of the restriction of intermolecular π–π stacking interactions and inhibition of intramolecular motions (vibration and rotation) in the aggregated state. Consequently, violet–blue nondoped OLEDs were constructed using these three AIEgens as emitters. Among them, the TPBCzC1-based device exhibited a maximum EQE of 4.34% with CIE coordinates of (0.160, 0.035), which is the first example of a nondoped violet–blue AIEgen-based OLED with a CIEy smaller than 0.046. The relevant data from the aforementioned representative reports of TPB-based AIEgens are summarized in Table 4, and their corresponding chemical structures are shown in Figure 5.
2.5 Chiral binaphthyl-based AIEgens
A few reports have been devoted to circularly polarized OLEDs (CP–OLEDs) based on small chiral organic molecules with TADF and AIE characteristics. The first report of such materials were published in 2018; Ma, Tang and coworkers59 explored four pairs of chiral AIEgens with DF, namely, R/S–BN–CF, R/S–BN–CCB, R/S–BN–DCB, and R/S–BN-F, for CP–OLEDs, by using a binaphthalene unit as the chiral source, cyano group as the acceptor, and Cz or tert-butyl Cz (tCz) and dimethylacridine (DMAC) as donor units. The resulting chiral luminogens revealed AIE features along with TICT behavior, for example, the emission intensity of S-BN–CF gradually increased by as much as 48-fold as fw increased from 70 to 99%, and the material showed a large blue-shift of as high as 46 nm with an emission peak centered at 506 nm. Nondoped and doped CP–OLEDs fabricated using these materials as emitters exhibited EQEmax values of up to 3.5 and 9.3%, respectively, with multicolor EL emissions ranging from 537 to 597 nm and from 496 to 571 nm, respectively. More importantly, the roll-off of current efficiency for nondoped OLED (11.7%) is much smaller than that of the roll-off efficiency for doped OLED (53.3%). Cheng's research group60 synthesized two pairs of chiral AIEgens based on binaphthalene-TADF emitters with xanthenone or benzophenone units as acceptor and phenoxazine (PXZ) as the donor; these luminogens were named, R/S-1 and R/S-2, respectively. The PL emission values of R-1 at 590 nm and R-2 at 542 nm were enhanced by 59 and 40-fold, respectively, when fw was increased to 99%, which is indicative of AIE characteristics. However, owing to the fixed conjugation structures of R/S-1, the AIEgens revealed CP–EL signals; R/S-2 revealed no CP–EL signals because of the rotatable nature of its benzophenone structure, which can limit the chirality transfer process. Thus, doped and nondoped CP-OLEDs using R/S-1 as the emitter exhibited EQEmax of 4.1% and 0.22% respectively, with orange-red emission.
Besides, the same group developed a pair of sky-blue chiral D–A type emitters(S-/R-BN-tCz) by using tCz, instead of PXZ as the donor in R/S-1.61 The resultant nondoped blue CP–OLEDs exhibited an EQEmax of 1.0%; furthermore, solution-processed white CP–OLEDs are fabricated by combining the aforementioned blue and orange CPL AIEgens. The properties of the resultant OLEDs were optimized by adjusting the doping ratios of the orange emitters. The device exhibited a low Von of 4.3 V, Lmax of 10,200 cd m−2, and CEmax of 2.0 cd A−1 with intense CP–EL signals in the range of 450–650 nm. Two pairs of blue chiral AIEgens, namely, R-/S-5 and R-/S-6, developed by introducing pyrene groups on BINOL at 3,3ʹ or 6,6ʹ-positions. Nondoped CP–OLEDs using them as emitters exhibited EQEmax of 2.79 and 3.09%, respectively, with blue and sky-blue emission peaks at 460 and 480 nm, respectively.62
In addition, a few reports have been devoted about CP–EL of AIE-active chiral polymer emitters based on chiral binaphthyl core and AIE-active diphenylethane linker or TPE unit. For instance, Cheng's group63 reported a pair of AIE-active chiral binaphthyl enantiomers (S-/R-6) containing a TPE core. The resulting chiral luminogens showed excellent AIE characteristics upon aggregation in a THF–water system, for example, S-6 showed almost no fluorescence emission in pure THF solution and no change was observed until fw reached 80%. When fw increased up to 99%, the emission intensity of S-6 gradually increased by as much as 262-fold, the luminogen exhibited bright yellow fluorescence emission appears at 532 nm and a PLQY of 32.1%, these features indicate that S-6 is an excellent AIE-active emitter in the aggregated state. Nondoped CP–OLEDs using S-/R-6 exhibited bright yellow CP–EL emission at 534 nm with EQEmax values of 0.48 and 0.45%, respectively, as well as CEmax values of 1.32 and 1.26 cd A−1, respectively. Another pair of chiral polymer enantiomers (S-/R-P) were also reported by Cheng et al.,64 these materials showed typical AIE features with up to ∼16-fold enhancements in emission intensity upon aggregation at fw of 90%; such enhancement is due to the restricted vibration of phenyl rings in the trans-1,2-diphenylethane linker. A nondoped device using S-/R-P luminogens emitted green CP–EL at 512 nm with CEmax values of 0.92/0.83 cd A−1 and the PEmax values of 0.39/0.42 lm W−1, respectively.
However, the development of CP-OLEDs with high EQE and small efficiency roll-off remains challenging. To overcome this issue, a few attempts were made by Zheng et al.,65 who reported a pair of octahydro-binaphthyl (OBN)-based chiral AIEgens, namely, (R/S)–OBN–DPA, by merging chiral (R/S)–OBN units with the TADF skeleton of cyan CN–DPA derivatives. Owing to the twisted structure of (R/S)-OBN, there was a small ΔEST of 0.09 eV, which suggested the fast RISC leading to improved radiative efficiency. Both doped and nondoped CP–OLEDs fabricated by employing them as emitters displayed high EL performances with EQEmax values of 12.4 and 6.6%, respectively, as well observed low efficiency roll-offs with an EQE of 11.5% at 1000 cd m−2 for doped one. Owing to the steric hindrance of 16 peripheral hydrogen atoms in the cyclohexane unit, exciton annihilation was effectively suppressed by reducing the intermolecular interactions of the molecules, resulting in small efficiency roll-off at high current density in the OLEDs. Soon afterward, the same group successfully constructed doped and nondoped CP–OLEDs with EQEmax values of 32.6 and 14.0%, respectively, by using Cz, instead of DPA, as the donor.66 The resultant isomers, (R/S)–OBN–Cz, displayed a small ΔEST of 0.037 eV, and a high PLQY of 92%. Furthermore, in particular, the efficiency roll-off of doped CP–OLEDs were extremely low (i.e., 2.5, 3.0, and 5.9% at 1000, 3000, and 5000 cd m−2, respectively), and their EQE was maintained at 30.6% even at a high luminance of 5000 cd m−2. As well as nondoped OLEDs exhibited milder efficiency roll-off with an EQE of 13.8% maintained at 3000 cd m−2 are almost the same as the maximum values. The roll-off ratios of the nondoped OLEDs is 1.0%, which is smaller than that of the doped OLEDs (i.e., 3.0%), owing to their shorter delayed lifetime and the AIE effect (Figures 6B and 6C). The relevant data from the aforementioned representative reports of chiral binaphthyl-based AIEgens are summarized in Table 5, and their corresponding chemical structures are shown in Figure 6.
3 DELAYED FLUORESCENT AIEGENS
Different photophysical mechanisms, such as fluorescence, phosphorescence, TTA, TADF, singlet fission (SF), and HLCT, have been explored to develop high-performance OLEDs. Among these mechanisms, TADF is considered as the most potential strategy and applied to OLEDs by Adachi in 2012, later, work on this concept led to more research activities focusing on the design of AIEgens. As is well known, TADF emitters are designed with donor and acceptor moieties, to minimize the ΔEST, by providing a clear distribution of HOMO and LUMO orbitals, respectively, in the corresponding units of a single molecule. Thus, TADF emitters can harvest both singlet (25%) and triplet (75%) excitons through RISC from the lowest excited triplet state (T1) to the lowest excited singlet state (S1) upon thermal activation. In theory, TADF emitters can achieve an IQE of 100%, and greatly enhanced electroluminescent efficiencies compared with traditional organic emitters.
However, the common TADF emitters often exhibits ACQ-related problems because of their strong intermolecular π–π interactions. Melding AIE units to the ACQ chromophores with TADF characteristics could lead to the development of AIDF emitters, which can overcome this issue by RIM in the aggregated state. AIDF materials also feature reduced current efficiency roll-off, which can improve device performance. The AIDF strategy was first reported by Chi's research group in 2015.18 Specifically, the team explored the advantages of AIDF emitters based on the D–A–D and D–A–Dʹ configurations. Since then, many more studies have been devoted to the preparation of AIDF emitters for OLED applications.
3.1 Blue AIDF emitters
In general, blue luminogens are very needful for constructing commercialized OLEDs. However, the development of efficient blue luminogens is limited by the wide band gap and low PL efficiency of these materials in the solid state. The features of AIDF emitters offer rich opportunities for developing efficient blue-emitting luminogens for OLED applications. The development of blue AIDF emitters with stable and high color purity for OLEDs is a challenging endeavor because of the difficulty of injecting charge carriers from the adjacent layers of a device due to the wide bandgap (>3.0 eV) of blue-emitting materials. Thus, the design and synthesis of blue-emitting systems with AIDF materials must be investigated further.
Tang, Cheng, and coworkers67 developed two pairs of AIE active efficient blue TADF isomers and named them 2Cz2tCzBn, 2tCz2CzBn, 2PhCz2tCzBn, and 2tCz2PhCzBn, respectively, these materials featured a twisted acceptor–hetero–donor configuration with cyanobenzene as the acceptor unit and Cz or 3,6-diphenylcarbazole as one donor, and 3,6-di-tert-butylcarbazole as an another donor. Owing to the large twisted dihedral angle between the donor and acceptor units of 55.4° to 83.9°, the materials showed increased molecular rigidity and reduced nonradiative decay, resulting in high RISC rate constant (kRISC) for the DF. The ΔEST values for these emitters are calculated to be 0.13–0.15 eV, which are small enough for efficient RISC process and TADF characteristics. The incorporation of tert-butyl groups in the molecules could effectively increase the solubility and reduce the ACQ effect by inhibiting the intramolecular vibrational relaxation and the intermolecular π–π stacking. Nondoped OLEDs bearing 2Cz2tCzBn, 2tCz2CzBn, 2PhCz2tCzBn, and 2tCz2PhCzBn, exhibited record-high EL performances with EQEs of 25.8, 24.5, 19.5, and 19.1%, respectively. High-performance white OLEDs were obtained by employing a single EML with this blue TADF emitter as a host to orange-red 3DMAC-BP as a TADF dopant, which exhibited EQEmax and CE values of 27.3% and 67.0 cd A−1, respectively.
Our group recently developed two ultra-deep-blue AIDF emitters, namely, TB–tCz and TB–tPCz, based on the D–A configuration using 3,6-substituted Czs as the donor and an oxygen-bridged boron core (TB) as the acceptor.68 The hetero atom (O or N) bridged aryl boron core structure restricted the bathochromic shift of these materials. Both materials revealed significant TADF characteristics with AIE features. Compared with TB–tCz, TB–tPCz was found to be a superior AIDF material because of its stronger AIE behavior (≈24-fold greater), lower ΔEST, larger SOC constant, and faster RISC rate. The corresponding nondoped devices exhibited ultra-deep-blue EL emissions at 416–428 nm and high color purity with a narrow FWHM of 42.2–44.4 nm. The TB–tCz-based nondoped device showed excellent EL performance with an EQEmax of 15.8% and excellent color purity close to the National Television System Committee (NTSC) deep-blue standard of (0.14, 0.08). Moreover, both AIDF emitters exhibited outstanding device performances with EQEmax of 14.1–15.9% in doped OLEDs. We also prepared a series of deep-blue to sky-blue OLEDs based on D–A-type AIDF emitters, namely TB–3Cz, TB–P3Cz, and TB–DACz, using the same TB acceptor and Cz or DPA substituted on the Cz derivatives as different donor entities.69 The AIDF emitters showed high PLQYs of 90–99%, and high kRISC values in the neat-film state, as well as AIE characteristics with 11–25-fold enhancements in PL emission intensity when fw reached 90%; such enhancements could help suppress the nonradiative decay process. Nondoped device based on TB–3Cz, TB–P3Cz, and TB–DACz exhibited a EQEmax values of 9.90, 6.13, and 6.04%, respectively. Interestingly, among these emitters, TB–3Cz and TB–P3Cz exhibited deep-blue emission at CIE coordinates of (0.17, 0.07) and (0.15, 0.08), which are close to the NTSC blue standards.
In 2018, Yang et al.70 explored two blue AIDF isomers, namely o-ACSO2 and m-ACSO2, which were based on diphenyl sulfone (DPS) as the acceptor and DMAC as the donor. The engineering design of these isomers was inspired by the DMAC–DPS emitter reported by Adachi et al.[5] Both luminogens exhibit good TADF and AIE characteristics owing to the large twisted angles between donors and acceptor unit. The meta-isomer, m-ACSO2, displayed small ΔEST of 0.07 eV, high PLQY of 76%, and excellent morphology of neat films. As a result, a nondoped solution-processed sky-blue OLED employing m-ACSO2 as the emitter achieved high EL performance with a EQEmax, CEmax, and PEmax values of 17.2%, 37.9 cd A−1, and 23.8 lm W−1, respectively. The same group developed another pair of blue chiral emitters, namely, (S)-NPE–AcDPS and (R)-NPE–AcDPS, with CPL, TADF, and AIEE characteristics by fusing chiral amines at the ortho-positions of the DPS unit in DMAC–DPS emitter.71 A doped deep-blue OLED based on (S)-NPE–AcDPS exhibited a EQE of up to 18.5%. Indeed, Guo's group initially reported m-ACSO2 as bis(3-(9,9-dimethyl-9,10-dihydroacridine)phenyl)sulfone (mSOAD) based on DMAC and DPS units with a highly twisted zig-zag configuration, and compared with its linear isomer, DMAC–DPS.72 The twisted zig-zag structure of mSOAD led to a very low ΔEST of 0.01 eV, compared to para- substituted DMAC–DPS (ΔEST of 0.08 eV), as well as the resulting luminogen concurrently exhibits both AIE and TADF characteristics. The EL performance of a nondoped blue OLED employing mSOAD was higher than that of an OLED bearing DMAC–DPS, with EQEmax, CEmax, and PEmax values of 14.0%, 31.7 cd A−1, and 28.4 lm W−1, respectively.
Grazulevicius and coworkers73 developed three multifunctional luminescent materials composed of pyrimidine-5-carbonitrile with different substituted Czs, and named the materials CzPCN, tCzPCN, and MeOCzPN. The resulting molecules simultaneously exhibit both AIE and TADF properties. These materials could be used not only as blue emitters for OLEDs but also as oxygen probes for optical sensors. Nondoped and doped devices based on CzPCN, exhibited EQEmax values of 12.8 and 14%, respectively. You and coworkers74 employed a series of imidazole derivatives with TADF and AIE characteristics for the first time to prepare AIEgens namely, DMAC–CNIM, DMAC–CNIB, and DMAC–CNBIM. Owing to attachment of two cyano groups with electron-withdrawing properties to the imidazole moiety, TADF property was induced with small ΔEST values (0.06–0.10 eV); while, the insertion of methyl/phenyl at N1 position of imidazole moiety can twist the geometry between imidazole and phenyl bridge, thus the imidazole derivatives transformed from ACQ emitter into AIEgens. To obtaining insight into the AIE mechanism, DMAC–CNIH (hydrogen at N1 position of imidazole moiety) is also synthesized for comparison, which gets a planar geometry with strong N-H···N intermolecular hydrogen bonding interaction, thus leads to severe ACQ effect. Meanwhile, the presence of a steric hindrance group at the N1 position of imidazole moiety induced AIE feature in THF/water system. As a result, nondoped OLED based on DMAC–CNBIM AIEgen as emitter exhibited a high EQE of 20.0% and low efficiency roll-off (EQE at 1000 cd m−2, 16.1%). These results represented the state-of-the-art performance for all imidazole-based OLEDs. Qi's research group75 explored a series of AIDF luminogens based on D–A–Dʹ configuration featuring phenyl(pyridyl)methanone as the acceptor and DMAC or PXZ as the donor. Interestingly, the molecules became rigid on account of the formation of intramolecular hydrogen bonds with the nitrogen atom in the acceptor. Thus, the resultant AIEgens demonstrated small ΔEST (0.03–0.10 eV), suppressed nonradiative decay and increased luminescence efficiency in the solid state. The PLQYs were realized to be 46.7, 66.8, and 53.3% for CBM–DMAC, 3CPyM–DMAC, and 2CPyM–DMAC, respectively, these enhanced emission values can ascribe to be the suppressed nonradiative transition by virtue of the formation of intramolecular hydrogen-bonding interactions and the intramolecular rotation can be locked. Nondoped devices using these emitters exhibited EL emission from sky-blue to orange, and the device employing CBM–DMAC as the emitter revealed an EQEmax of 6.7 %.
The relevant data from the aforementioned representative reports of blue AIDF emitters are summarized in Table 6, and their corresponding chemical structures are shown in Figure 7.
3.2 Green AIDF emitters
Many green AIDF materials have been reported as EML in OLEDs, and some of these materials show very high EL performance. For example, in 2019 Chi's group reported a green AIDF emitter 2Cz–DPS, which was obtained by inserting DPS between Cz and PTZ donors with an asymmetric D–A–Dʹ configuration.76 The resultant material showed dual-charge transfer pathways, that is, intramolecular electrostatic attraction and through-space charge transfer (TSCT) because the Cz donor and DPS acceptor groups were connected at the ortho-position to achieve the spatially close D–A interaction. The dual charge transfer process significantly increased the oscillator strength (f = 0.0207) for faster radiative decay, in comparison with the linearly linked conformation of 4Cz–DPS (f = 0.0074), exhibiting a high PLQY of 91.9% in solid state. In addition, strong intramolecular interactions created in 2Cz–DPS molecule, which assist the increase in molecular rigidity, suppress nonradiative decay channels, and increase the radiative transition rate led to effective AIE and TADF characteristics. A nondoped OLED constructed based on 2Cz–DPS with the configuration of ITO/PEDOT:PSS (30 nm)/mCP (20 nm)/2Cz–DPS (30 nm)/TPBI (40 nm)/LiF (1 nm)/Al exhibited a record-high EL performances with EQEmax, CEmax, and PEmax values of 28.7%, 82.3 cd A−1, and 51.8 lm W−1, respectively. Two D–A–Dʹ-configured AIDF luminogens based on DPS derivatives, namely, 3CP–DPS–PXZ and 3CP–DPS–DMAC, were explored by Wang et al.77 The materials were prepared with an asymmetric configuration to understand the effect of donor on their luminescence properties with different donors Dʹ, namely, PXZ and DMAC, herein, 9-phenylcarbazole was as the fixed donor D. Both emitters exhibited clear AIE behavior, high PLQY in the neat film, as well, observed a very small ΔEST of 0.03–0.07 eV. They also demonstrated TADF features with microsecond-scale lifetimes of 0.96–2.36 μs. The molecule containing PXZ featured stronger electron-donating capability compared with 9,9-DMAC, thus 3CP–DPS–PXZ exhibited a red-shift of emission and enhanced AIE effect and higher RISC (kRISC) rate constant in comparison with 3CP–DPS–DMAC. Nondoped devices using 3CP–DPS–PXZ and 3CP–DPS–DMAC as EMLs exhibited EQEmax values of 17.9 and 9.1%, respectively. As well, 3CP–DPS–PXZ showed low efficiency roll-off that EQE remained 14.5% at the luminance of 1000 cd m−2.
In 2015, Adachi's group constructed nondoped OLEDs with a symmetrical D–A–D configuration and obtained excellent EL performances (EQEmax = 18.9%) and negligible efficiency roll-off by using benzophenone, instead of DPS as the acceptor.[5] In 2017, the same group developed three AIDF emitters with an asymmetric D–A–Dʹ configuration by using dibenzothiophene (DBT) as the donor, benzophenone as the acceptor, and PXZ, PTZ, and DMAC as another donor; these emitters were named DBT−BZ−PXZ, DBT−BZ−PTZ, and DBT–BZ–DMAC.78 Indeed, they initially reported DBT–BZ–DMAC[78] revealed AIE feature by gradually enhancing its PL intensity along with blue-shifted emission in THF:water mixtures because intramolecular rotational and vibrational motions are inactive in aggregated state, resulting in suppressed nonradiative decay of the excited state, leading to enhanced radiative decay in the form of luminance. DBT–BZ–DMAC showed its PL maximum at 505 nm in the neat film, as well realized PLQY of 80.2% and smallest ΔEST of 0.08 eV in the neat film. A nondoped OLED fabricated using DBT–BZ–DMAC exhibited excellent EL performances with CEmax and EQEmax values of 25.6 cd A−1 and 14.2%, respectively, and high value of small CE roll-off of 0.46%. By comparison, nondoped devices based on DBT−BZ−PTZ and DBT–BZ–DMAC as emitters displayed green/yellow EL emissions with EQEmax values of 9.2 and 9.7%, respectively, as well as small efficiency roll-off.[78] The corresponding, doped devices also exhibited good EL performances, with EQEmax values up to 19.2%. Soon afterward, the same group tailored a green AIDF emitter (DCPDAPM) with a twisted D–A–Dʹ configuration by replacing the DBT with two Czs as D in the aforementioned report, benzophenone as the acceptor, and DMAC as another donor Dʹ.79 Owing to its twisted conformation, the ΔEST of DCPDAPM in the solid state was calculated to be 0.10 eV and showed a high solid-state PLQY of 76.1%. The resulting green emitter simultaneously exhibits AIE and TADF characteristics. A nondoped device fabricated using DCPDAPM as the emitter exhibited EQEmax and CEmax values of 8.15% and 26.8 cd A−1, respectively. By comparison, a doped OLED with 20 wt% DCPDAPM in CBP showed EQEmax and CEmax values of 19.6% and 61.8 cd A−1, respectively.
To develop more twisted D–A–D-based AIDF emitters, Wang's group introduced a triazatruxene (TAT) unit as strong donor on both sides of benzophenone and named the resultant materials TATC–BP and TATP–BP.80 Owing to the different substituents on TAT unit, the material showed different AIE and MCL characteristics. TATC–BP with flexible hexyl substituents showed color-changing AIE and clear MCL behaviors with a large red-shift of 59 nm. By contrast, TATP-BP, which features rigid phenyl substituents, exhibited no color-changing AIE and MCL behavior with a smaller red-shift of 36 nm. Nondoped OLEDs prepared with TATP–BP and TATC–BP showed good EL performance with EQEmax values of 5.9 and 6.0%, respectively, and exhibited remarkable small efficiency roll-off. In particular, TATP–BP showed the roll-off ratio of 3.3% in EQE at 1000 cd m−2 was much lower than that of TATC–BP (18.6%). To further develop TAT-based AIDF luminogens, the same group explored two green AIDF emitters based on TAT as the donor and benzophenone as the acceptor with D–A configuration, namely, TAT–BP and TAT–2BP.81 Owing to their highly twisted rigid structures with large dihedral angles, the emitters showed ΔEST smaller than 0.1 eV, thus both emitters displayed not only typical AIE behavior, but also a distinct TADF nature along with high PLQY and a relatively short DF lifetimes (<1 μs). Nondoped devices bearing TAT–2BP and TAT–BP showed EQEmax values of 9.8 and 6.4%, respectively. In addition, these devices exhibited small efficiency roll-off characteristics, in particular, the TAT–2BP-based devices showed a roll-off ratio of 1.0% in EQE at 1000 cd m−2.
Unfortunately, AIDF emitters are insufficient to obtain ideal nondoped OLED emitters. Indeed, previous reports indicated that AIDF emitters suffer from Dexter energy transfer (DET), which is mainly involved in the complete quenching process of TADF in neat films. Triplet exciton-related quenching may also explain the occurrence of efficiency roll-off in practical brightness at high current densities, which is a major issue in EL process. To address this problem, Zhang and coworkers82 tailored two green AIDF emitters by reshaping the D–A-based TADF emitter BP–DPAC and named the resultant materials SPBP–DPAC and SPBP–SPAC.83 These emitters were uniquely designed by locking the phenyl rings of benzophenone unit via a rigid fluorine moiety. Thus, the materials showed not only enhanced molecular rigidity but also reduced the intermolecular interaction, resulting suppressed ACQ and showed potential AIE characteristics by enhancing their PLQYs. The ΔEST of both luminogens was calculated to be less than 0.1 eV and PLQYs of SPBP–DPAC and SPBP–SPAC in neat films, at 93 and 98%, respectively, were twice that of BP–DPAC of 47%. Thus, the materials demonstrated TADF with very short delayed lifetimes of 2.5 and 1.3 μs, respectively, such a short lifetime are favorable to suppress the device efficiency roll-off by accelerating triplet conversion to DF. Nondoped OLEDs using SPBP–DPAC and SPBP–SPAC exhibited excellent EQEmax values of 22.8 and 21.3%, respectively, and a very negligible efficiency roll-offs owing to the short delayed lifetimes of 2.5 and 1.3 μs for both emitters, respectively.
Tang's group prepared the same type of twisted D–A–Dʹ-based green AIDF emitters by using carbonyl group as the acceptor and DMAC as an electron donor. The authors melded 9,9-dimethylfluorene (DMF) and 9,9-diphenylfluorene (DPF) to the carbonyl group to decrease intermolecular π–π stacking and increase the steric hindrance and named the resultant products DMF–BP–DMAC and DPF–BP–DMAC, respectively.84 The ΔEST values of these materials were calculated to be to be 0.14 and 0.09 eV, respectively, which are sufficiently low to achieve effective TADF feature. In particular, DPF–BP–DMAC revealed a high PLQY of 62.3%, and its nondoped device exhibited good EL performances, with an EQEmax of up to 14.4%, and retained an EQE of 12.6% at 1000 cd m−2, indicating a very small efficiency roll-off. Another series of D–A–Dʹ-based AIDF emitters were synthesized by Zhao and coworkers,85 by introduction of 4-(phenoxazin-10-yl) benzoyl group to the common chromophores of Cz-substituted fluorene derivatives, namely, DCDMF–BP–PXZ, DCDPF–BPPXZ, and DCSBF–BP–PXZ. DCDMF–BP–PXZ and DCDPF–BP–PXZ showed very high PLQY values of 88.5 and 89.0%, respectively, films than DCSBF–BP–PXZ of 39.6% because of poor molecular π-conjugation as well as strong intermolecular π–π interaction of DCSBF–BP–PXZ. Consequently, nondoped OLEDs using DCDMF–BP–PXZ and DCDPF–BP–PXZ exhibited high EL performances with high EQEmax values of 19.0 and 18.5%, respectively. A nondoped OLED using DCSBF–BP–PXZ displayed a low EQEmax value of 3.3%, owing to its low PLQY and unbalanced charge-carrier transport ability. Doped OLEDs based on DCDMF–BP–PXZ and DCDPF–BP–PXZ and DCSBF–BP–PXZ with a CBP host exhibited high EQEmax values of 21.7, 24.4, and 22.3%, respectively.
The same research group employed two D–A–D-based AIDF emitters, CC6–DBP–PXZ and CC6–DBP–DMAC, containing PXZ or DMAC as donor units and benzoyl (BZ) as the central acceptor and successfully achieved extremely small efficiency roll-offs in both vacuum-deposited and solution-processed OLEDs.86 Serious efficiency loss at high voltages is an important issue in TADF–OLEDs because severe exciton annihilation occurs by bimolecular quenching processes (TTA, STA, etc.) at high voltages. To overcome this problem, the researchers designed emitters with long alkyl chains to increase their film-forming ability during the solution-processing technique, and elongating the distance between excitons, which are mainly located on BZ unit. The resultant materials exhibited a high EQEmax of 9.02% and negligible efficiency roll-off in solution-processed nondoped OLEDs; in solution-processed doped OLEDs with 4,4′-bis(N-carbazolyl)-1,1′-biphenyl (CBP), the materials displayed a high EQEmax of up to 12.1%. Zhao and coworkers87 subsequently tailored two D–A–Dʹ-based AIDF emitters, namely, TCTA–BP–PXZ and TCTA–BP–DMAC, to address the problem of serious efficiency roll-offs at high voltages. The BZ acceptor melded with PXZ or DMAC units could form D–A structure to generate DF and dendritic moiety of tris(4-carbazoyl-9-ylphenyl)amine (TCTA) could increase the film-forming ability and charge transport of the emitters during solution processing. Because of the RIM of the twisted dendritic model structures, both materials showed significant AIDF features and high PLQY values of 53.5 and 57.6% in the neat film. Nondoped OLEDs based on TCTABP–PXZ and TCTA–BP–DMAC emitted yellow light with an EQEmax of 10.7% and sky-blue light with an EQEmax of 9.8%, respectively, as well as negligible efficiency roll-offs in solution-processed OLEDs. Moreover, nondoped and doped OLEDs fabricated using TCTA–BPPXZ exhibited high EQE values of 16.9 and 17.6%, respectively, as well as very small roll-off values of 0.6% in vacuum-deposited OLEDs. In 2019, the same research group explored three AIDF emitters, 3-CCP–BP–PXZ, 9-CCP–BP–PXZ, and 3,9-CCP–BP–PXZ; here, heavy atoms (Cl) were inserted into these emitters to increase their SOC.88 In the emitters, the chlorine atom is chosen because the chlorine−carbon bond is stronger than bromine–carbon and iodine–carbon bonds in terms of chemical stability, as well carbonyl and chlorine already have been proved to be capable of enhancing SOC. Thus, the materials exhibit fast RISC rate, high photoluminescence (PL) quantum yield (ΦPL) and short delayed lifetime values in neat films. Nondoped OLEDs using these emitters exhibited EQEmax values of 20.4−21.7%, whereas doped OLEDs exhibited EQEmax values of up to 29.1% with very small efficiency roll-offs.
Another attempt was made by Cheng et al.89 in 2019 to synthesize emitters based on D–A configuration; these emitters composed of benzoylquinoline as the acceptor and Cz or tert-butylcarbazole as the donor and were named 2QPM–mDC, 2QPM–mDTC, and 4QPM–mDTC. Interestingly, the quinoline moiety in 2QPM–mDC could form intramolecular hydrogen bonding between the o-hydrogen on the central phenyl and the nitrogen of quinolone, thus the resulting molecule was rigidified to certain degree, leading to narrow emission peak and enhanced emission intensity. The ΔEST values of these emitters were calculated to be 0.19, 0.07, and 0.18 eV, respectively, which indicated that they possessed TADF property through RISC. The 2QPM–mDTC-based OLED displayed an EQEmax of 24.0% with narrow emission bandwidth and high color purity due to the formation of intramolecular H-bonding in 2QPM–mDTC molecule, which endowed it with good rigidity, and high PLQY of 98%. Yang et al.90 synthesized two AIDF emitters, namely, PyB–DPAC and PyB–DMAC, based on the D–A configuration; these emitters were composed of a 4-benzoylpyridine unit as the electron acceptor and DPAC or DMAC as the electron donor. Vacuum-deposited nondoped devices using PyB–DPAC and PyB–DMAC exhibited EQEmax values of 8.4 and 9.7%, respectively. A solution-processed nondoped device based on PyB–DPAC exhibited an EQEmax of 11.1%. Sun et al.91 recently reported two robust luminogens featuring AIE and TADF characteristics; these materials were obtained by substituting the methoxy groups and inserting the phenyl-spacer into the twisted molecular configuration of 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene, 2,4,5,6-tetrakis(9H-carbazol-9-yl) isophthalonitrile (4CzIPN)[8] and named 4CzIPN–MO and 4CzPhIPN–MO. Especially, 4CzPhIPN–MO revealed remarkable AIE performance because its phenyl bridge alleviate the sterically hindrance effect of the donor units, which, in turn, inhibits nonradiative decay. The materials showed a shallow HOMO level because of the methoxy group, which led to reductions in the injection barrier of the device. A solution-processed OLED based on 4CzPhIPN–MO exhibited an EQEmax of 14.5% with remarkable small efficiency roll-offs.
To suppress nonradiative decay process effectively via increased molecular rigidity, Tang and coworkers91 explored two green AIDF emitters consisting of a dual emitting core with the A–D–D–A molecular configuration based on Cz as the donor unit and dibenzo [b,d] thiophen-2-yl (phenyl) ketone and dibenzo [b,d] furan-2-yl (phenyl) ketone are as two different acceptor units, namely, 2DBT–BZ–2Cz and 2DFT–BZ–2Cz.[92] Owing to the highly twisted molecular structure of the dual emitting cores, they observed small ΔEST (0.01–0.02), efficient RISC, suppressed nonradiative decay and TADF–AIE characteristics. Solution-processed nondoped OLEDs based on 2DBT–BZ–2Cz and 2DFT–BZ–2Cz displayed a green emission with EQEmax values of 6.8 and 4.5%, respectively. In 2019, Qi and coworkers93 reported highly twisted AIDF emitters with the same dual-functional acceptor moieties (i.e., dibenzo [b,d] thiophen-2-yl (phenyl) ketone) and TPA as the donor to obtain DTPA–DTM and DTPA–DDTM, respectively. These emitters demonstrated dual charge transfer (i.e., TBCT and TSCT); in addition, small ΔEST of 0.17 and 0.18 eV respectively, and high radiative efficiency simultaneously revealed for these emitters. In particular, DTPA–DDTM showed higher PLQY of 60.5% and its nondoped OLED exhibited EQEmax and CEmax values of 8.2% and 25.6 cd A−1, respectively.
Chen et al.94 explored three AIDF emitters based on D–A configuration with quinolone as the acceptor and DAMC, PXZ, and PTZ as donors. Owing to their twisted conformation, DMAC–QL, PXZ–QL, and PTZ–QL displayed small ΔEST values of 0.06, 0.10, and 0.04 eV, respectively, as well as excellent TADF behavior with DF lifetimes of 2.15, 1.86, and 15.76 μs, respectively. Nondoped OLEDs using them as emitters exhibited EQEmax values of 7.7, 17.3, and 14.8%, respectively. In addition, the PXZ–QL-based nondoped device displayed very small efficiency roll-off owing to its short delay lifetime of 1.86 μs. The same research group synthesized three AIDF emitters by melding another donor to the above emitters to achieve the D–A–D configuration and named the resultant materials Fene, Fens, and Yad.95 Nondoped OLEDs using them as emitters exhibited high EL performance with EQEmax values of 14.9, 13.1, and 17.4%, respectively, and relatively low Von values ranging from 3.0 to 3.2 V. Wang and coworkers96 synthesized a green emitter by introducing 10-phenyl-10H-PTZ 5,5-dioxide (2PTO) as a novel acceptor to develop high performance nondoped OLED with AIE and TADF features, named, PXZ2PTO. A nondoped OLED using this green AIDF emitter displayed a high EQEmax and CEmax values of 16.4% and 44.9 cd A−1, respectively. Moreover, doped OLEDs fabricated with this emitter demonstrated results comparable with those of nondoped OLEDs, with EQEmax and CEmax values of 16.3% and 43.8 cd A−1, respectively.
An interesting strategy to overcome the excessive rotational or twisting motions even in the aggregated state with retaining AIDF features was reported by Bin, You, and coworkers.97 The group tailored a green AIDF emitter (DMAC-BPI) based on D–A–D structure by introducing a heptagonal diimide ((N-(4-(tert-butyl)phenyl)-1,1′-biphenyl-2,2′-dicarboximide (BPI)) with well-balanced rigidity and rotatability nature as the acceptor and DMAC as the donor unit.97 The acceptor can restrict excessive intramolecular rotation and inhibit close intermolecular π–π stacking, thereby simultaneously enhancing the radiative transition rate and reducing the exciton quenching in the aggregated state. DMAC–BPI showed a high PLQY of 95.8% in the neat film. A nondoped OLED based on DMAC–BPI as emitter displayed excellent EL performances with an EQEmax of 24.7% and remarkably small efficiency roll-off of 1.0% at 1000 cd m−2. Zhao, He, and coworkers98 adopted the same strategy and developed two D–A–D-based emitters by introducing a long alkyl chain on the N atom of central acceptor core and melding PO or DMAC to the BPI core through the π-bridge of phenyl unit as the donor. The resultant materials were named BPI–PhPXZ and BPI–PhDMAC, respectively.98 Furthermore, doped yellow and green OLEDs using them as emitters exhibited a high EQEmax values of 18.1 and 16.2%, respectively. The relevant data from the aforementioned representative reports of green AIDF emitters are summarized in Table 7, and their corresponding chemical structures are shown in Figure 8.
3.3 Yellow, orange, and red AIDF emitters
Several excellent reports exist on yellow, orange, and red emissive AIEgens for highly efficient nondoped and doped OLEDs. Nonetheless, research of highly efficient long-wavelength TADF emitters with small efficiency roll-off remains a major challenge because it is difficult to simultaneously achieve a high fluorescence radiative rate (krS) and a small ΔEST for long-wavelength TADF emitters. As is well known from the energy gap law, the nonradiative IC rate (kIC) is appreciably enhanced with the increase in emission wavelength, whereas fluorescence radiative rate is not high enough to control kIC, which results in low PLQY.
Although there are many reports on AIDF luminogens, only some of them showed very high performance. For instance, in 2017, Tang's research group99 briefly explained an intrinsic relationship between AIE and TADF features through the exploration of three luminogens, CP–BP–PXZ, CP–BP–PTZ, and CP–BP–DMAC. The transient PL curve of CP–BP–PXZ and CP–BP–PTZ only exhibited prompt fluorescence with short lifetimes of 11.8 and 13.5 ns, respectively in THF solution. However, upon aggregation, the fluorescence lifetime of CP–BP–DMAC increased to 116.4 ns with prominent DF at 681.5 ns, revealing that DF is induced by AIDF, which enables high PL efficiencies and efficient exciton utilization in neat films. In addition, smaller ΔEST values and faster RISC processes were observed for the resultant luminogens in neat films than in doped films. As a result, high EL efficiencies with an EQEmax value of up to 18.4% with negligible efficiency roll-off in nondoped OLEDs was observed. Furthermore, the same research group developed a series of luminogens by incorporating the AIDF core (4-(phenoxazin-10-yl) BZ (BP–PXZ)) within the host materials (DCB, CBP, mCP, and mCBP) to explore efficient luminescent materials for nondoped OLEDs.100 In these twisted structures, the HOMO is mainly centered on PXZ donor, whereas the LUMO stays on BZ acceptor, resulting in small ΔEST values (0.016–0.024 eV), that enables the harvesting of both singlet and triplet excitons for light emission via fast RISC in neat film. Consequently, nondoped OLEDs using AIDF luminogens as emitters exhibited excellent EL efficiencies with an EQEmax of up to 22.6% and negligible efficiency roll-off at 1000 cd m−2 luminance.
Inspired by the above reports, Wang and coworkers also developed a series of luminogens, namely, DCB–DPS–PXZ, mCP–DPS–PXZ, pPhDCzDPSPXZ, and mPhDCzDPSPXZ, by adopting DPS–PXZ instead of BP–PXZ as the AIDF core and grafting with host substituents (DCB, mCP, pPhDCz, and mPhDCz).101 Owing to the large twist angle between the host substituents and AIDF core in the pPhDCz- and mPhDCz-substituted materials, stronger AIE effect was observed, resulting in the higher neat-film PLQYs values of 56 and 55% than the other two materials. The nondoped devices based on mPhDCzDPSPXZ and pPhDCzDPSPXZ exhibited high EL performances with EQEmax values of 18.1 and 17.1%, with small efficiency roll-offs of 7.7 and 9.9%, respectively. Furthermore, an elegant hybrid donor was introduced by merging DMAC and Cz, which is attached to pyrimidine and triazine (TRZ) acceptors based on D–A configuration, resulting in two AIDF molecules, 34AcCz–PM and 34AcCz–Trz, respectively.102 These materials could be employed in both nondoped and doped OLEDs, and high EQEs of 22.6 and 14.1% were observed for 34AcCz–PM in doped and nondoped OLEDs, respectively. Whereas Tang's research group103 tailored two AIDF luminogens, TRZ–HPB–PXZ and TRZ–HPB–DMAC, by adopting TSCT strategy based on the D–A configuration, wherein the donor (PXZ or DMAC) and acceptor (TRZ) are connected through a bridge (hexaphenylbenzene). Hexaphenylbenzene not only exhibits strong toroidal delocalization of π-electrons but also has significant AIE features. Both luminogens exhibited TSCT between donor and acceptor units, resulting in a small ΔEST and prominent DF upon aggregation, which leads to high exciton utilization of the emitter in the device. As a result, nondoped OLEDs using the aforementioned luminogens as EMLs afforded excellent EL performance with EQEmax values of 12.7 and 6.5%, respectively, and a very small efficiency roll-off of 2.7% at 1000 cd m−2. This work had suggested that TSCT-capable AIDF luminogens could achieve high exciton utilization and suppressed exciton annihilation at high luminance for nondoped OLEDs.
In addition, in order to increase the carrier injection and transporting ability of AIDF emitters, bipolar carrier transport materials were incorporated in the same AIDF core (4-(phenoxazin-10-yl) BZ), resulting in two new AIEgens, 35DCZPP–BP–PXZ and 26DCPP–BP–PXZ,104 based on 3,5-bis((9H-carbazol-9-yl)-3,1phenylene) pyridine (35DCPP) and 2,6-bis(3-(9H-carbazol-9-yl) phenyl) pyridine (26DCPP) as bipolar materials. Nondoped OLEDs constructed using 35DCZPP–BP–PXZ and 26DCPP–BP–PXZ as emitters exhibited bright yellow emission with EQEmax values of 17.3 and 16.1%, respectively, and a small efficiency roll-off of 0.6% and 1.2% at 1000 cd m−2, respectively, based on 1,1-bis[(di-4-tolylamino)phenyl]cyclohexane (TAPC)–TmPyPB transport system. However, nondoped OLEDs fabricated with the N,N′-di(1-naphthyl)-N,N′-diphenyl-(1,1′-biphenyl)-4,4′-diamine (NPB)–2,2′,2″-(1,3,5-benzinetriyl)-tris(1-phenyl-1-H-benzimidazole) (TPBi) transport system also exhibited nearly the same EQEmax values (16.3 and 15.5% for 35DCZPP–BP–PXZ and 26DCPP–BP–PXZ, respectively), indicating the versatility of the emitters in OLEDs applications. Besides, in 2019, three AIDF luminogens (DMF–BP–PXZ, DPF–BP–PXZ, and SBF–BP–PXZ) were developed based on the D–A–Dʹ configuration using benzophenone (BZ) as an acceptor and PXZ and fluorene derivatives as electron donors.105 Upon aggregation, DMF–BP–PXZ showed anti-Kasha fluorescence behavior, which means that the emission originated from the higher-energy excited-state S2, rather than the lowest excited state of S1. This is attributable to competition between the electron-donating PXZ and DMF units and the electron-accepting BZ unit in forming excited CT state and excited-state geometry. Moreover, DMF–BP–PXZ, DPF–BP–PXZ, and SBF–BP–PXZ could be used in both nondoped and doped OLEDs; nondoped OLEDs employing these emitters exhibited EQEmax values of 13.3, 14.3, and 12.3%, respectively, with small efficiency roll-offs of 6.0, 1.4, and 0.8%, respectively, at 1000 cd m−2 luminance.
In 2017, Yasuda et al.106 reported two simple D–A-based yellow emissive AIDF molecules using xanthone or benzophenone as the acceptor unit and PTZ as the donor unit. Upon aggregation, both materials, PTZ–XT and PTZ–BP, exhibited a strong yellow DF with moderate PL efficiencies. Nondoped OLEDs using PTZ–XT and PTZ–BP as emitters showed EQEmax values of 11.1 and 7.6%, respectively. Su, Zhao, and coworkers107 tailored three stimuli-responsive AIDF luminogens, pipd–BZ–PXZ, pipd–BZ–PTZ, and pipd–BZ–DMAC, based on D–A configuration comprising a fused N-heterocycle diarylketone (imidazo[1,2-a] pyridin-2-yl(phenyl)methanone (pipd)) as the acceptor and PXZ, PTZ, and DMAC as donors. A nondoped OLED based on pipd–BZ–PXZ offered an EQEmax and CE of 7.04% and 19.8 cd A−1, respectively, with orange to red emission above 570 nm and a high EQE of 6.91% at a high luminance of 1000 cd m−2 with efficiency roll-off of 2.3%. A doped device using pipd–BZ–PXZ showed better performance with a CEmax and EQEmax of up to 55.41 cd A−1 and 15.77%, respectively.
Other research groups have also developed highly efficient long wavelength emissive AIDF materials for nondoped OLEDs, and achieved excellent performances with good stability. For example, Yang's group108 developed four luminogens with TADF and AIE characteristics, namely, SBDBQ–DMAC, DBQ–3DMAC, SBDBQ–PXZ, and DBQ–3PXZ, by incorporated DMAC or PXZ as donors into a quinoxaline framework. These luminogens exhibited varied PL emissions from green to red depending on the strength and quantity of the donor units. Fine tuning the twisted structures of the resultant luminogens led to good separation of the HOMO and the LUMO, resulting in small ΔEST values of 0.06, 0.06, 0.07, and 0.03 eV, respectively, and a high fluorescence radiative rate (krS) for TADF molecules. As a result, nondoped device based on DBQ–3DMAC exhibited excellent EL performances with a maximum CE, PE, and EQE of up to 41.2 cd A−1, 45.4 lm W−1, and 12.0%, respectively, and small efficiency roll-offs. Doped OLEDs comprising DBQ–3DMAC and DBQ–3PXZ exhibited yellow and orange emission with EQEmax values of 22.4 and 14.1%, respectively, and extremely small efficiency roll-offs. Subsequently, Yang's group109 introduced a weak electron-withdrawing fluorine atom into the same quinoxaline framework to achieve an efficient exciton harvesting for highly efficient OLEDs, resulting in the development of new AIDF luminogens, namely, SFDBQPXZ and DFDBQPXZ. Both orange emitters simultaneously exhibit both AIE and TADF features with delayed lifetimes of 4.6 and 2.6 μs. More importantly, monofluoro-substituted SFDBQPXZ showed high efficiencies in both doped and nondoped OLEDs with a EQEmax values of 23.5 and 10.1%, respectively, and observed small efficiency roll-offs.
Furthermore, recently, Yang and coworkers110 developed two AIDF luminogens, ND–AC and CND–AC, based on twisted D–A configuration consisting of naphthyridine or cyano-naphthyridine as the acceptor and DMAC as the donor. Due to the orthogonal twisted structures of these luminogens, their FMO distributions are well separated, resulting in small ΔEST values of 0.03 and 0.01 eV. The luminogens also exhibited distinct TADF and AIE characteristics with considerable PLQYs in both doped and neat films. AIE feature revealed by increasing their PL emission intensities when the water fraction increased to 98 and 95% for ND–AC and CND–AC, respectively, because of restriction of intermolecular π–π stacking interactions and inhibit intramolecular motions (vibration and rotation) in the aggregation state to some extent, which is derived from their orthogonal molecular conformation with large steric hindrance. Consequently, doped OLEDs based on ND–AC and CND–AC as dopants exhibited yellow and orange emission with EQEmax values of 16.8 and 8.4%, respectively. More importantly, nondoped device employing ND–AC as the emitter exhibited excellent EL performances of 12.0%, 38.5 cd A−1, and 30.2 lm W−1.
Tang, Miao, and coworkers111 also explored a red AIDF emitter based on D–A–Dʹ framework, named, BCZ–BTD–AD, by melding a DMAC donor to benzothiadiazole core. These two emitters exhibit AIE feature when the water fraction (fw) reaches to 40 and 60%, which is derived from its orthometric molecular conformation with large steric hindrance. Upon aggregation, the large torsion angle is created in the resulting molecule due to the N-connection of Cz or 9,10-dihydro-9,9-dimethylacridin, which inhibits the nonradiative decay process. Nondoped and doped OLEDs using BCZ–BTD–AD showed EQEmax of 2.3 and 5.6%, respectively. Meanwhile, Tong, Wang, and coworkers112 explored solution-processed two red AIDF emitters, namely, TAT−DBPZ and TAT−FDBPZ, by introducing a strong donor, TAT, into the planar acceptors, that is, dibenzo-phenazine (DBPZ) or fluorine-substituted dibenzo-phenazine (FDBPZ). Due to their rigid structures and the large steric hindrance between the acceptor and donor, small ΔEST values of 0.16 for TAT–DBPZ and 0.10 eV for TAT–FDBPZ were achieved, resulting in short DF lifetimes of 2.30 and 1.51 μs, respectively. Meanwhile, their large planar conjugated structures favored high PLQYs (62–76%) in the solid states. Consequently, solution-processed nondoped devices employing TAT−DBPZ and TAT−FDBPZ as emitters exhibited red and deep-red EL spectra with emission peaks at 626 and 641 nm, respectively and corresponding EQEmax values of 5.6 and 2.9%, respectively. In contrast, doped OLEDs employing TAT−DBPZ and TAT−FDBPZ as dopants showed EQEmax values of 15.4 and 9.2%, respectively, with a red emission peak at 604 and 611, respectively.
The relevant data from the aforementioned representative reports of yellow, orange, and red AIDF emitters are summarized in Table 8, and their corresponding chemical structures are shown in Figure 9.
3.4 AIDF dendrimers and macromolecules
Dendrimers are highly structured macromolecules having molecular weight of over 1000 that provide a high degree of surface functionality and versatility. Apart from AIDF small molecules, dendrimers and polymers with AIDF feature also shown potential to construct efficient nondoped OLEDs, and a great deal of research has been devoted to the use of several dendrimers and polymers as nondoped emitters in OLEDs through solution processing. For example, recently, Tang's research group introduced a core–dendron system into nondoped emissive layers by furnishing the familiar TADF core 5CzBN with alkyl chain-linked spirobifluorene dendrons to synthesize dendritic molecules, 5CzBN–SSP, 5CzBN–DSP, and 5CzBN–PSP, with additional AIE feature.113 By increasing the number of flexible dendrons, Tang's group not only achieved AIDF characteristics but also realized uniform good uniform film morphology and suitable for solution processing with better solubility for OLED fabrication. More interestingly, the DF lifetimes and PLQYs increased with the number of flexible dendrons. The PLQYs of 5CzBN–SSP, 5CzBN–DSP, and 5CzBN–PSP are 38.0, 45.7, and 69.6%, respectively, and solution-processed nondoped OLEDs using these dendritic molecules as emitters exhibited high EQE values of 7.3, 13.9, and 20.1%, respectively; these values far exceed those of solution-processed OLEDs comprising 5CzBN.114 Among solution-processed nondoped OLEDs based on AIDF dendritic emitters, 5CzBN–PSP has shown a record-high EQE of 20.1% so far.
In order to enhance the electroluminescence efficiency of OLEDs through exciton utilization, Jiang's research group115 developed a new strategy called TADF-sensitized fluorescence (TSF), which involves using a core–dendron system, comprising 2,4,6-tris(3-(6-(9H-carbazol-9-yl)hexyl)-9H-carbazol-9-yl)benzonitrile (3CzBN–Cz) TADF molecules as the host and 1,3,6,8-tetrakis(4-((6-(9H-carbazol-9-yl)hexyl)oxy)phenyl)pyrene (PY–Cz) as the fluorescent emitter dopants. As part of this concept, encapsulation of the host (3CzBN–Cz) and the guest (PY–Cz) with alkyl chain-linked dendrons restricts the intermolecular interactions and increases the intermolecular distance between the emissive cores; consequently, DET from T1 of the host to T1 of the guest was effectively suppressed, whereas fluorescence resonance energy transfer from the host to the guest was efficiently enhanced. As a result, deep-blue nondoped OLED based on 3CzBN–Cz:PY-Cz exhibited a high EQE of 10.16% with stable color purity (FWHM = 65 nm). This work will open up new avenues for the development of highly efficient nondoped OLEDs.
In 2017, Yamamoto, Fujita, and coworkers116 developed three green TADF emitters, G1B, G2B, and G3B, composed of benzophenone as the acceptor, which was attached to three different generations of Cz core as donors; these emitters exhibited AIE enhancement along with isopropyl alcohol resistance, which are beneficial for the fabrication of OLEDs through solution processing. Solution-processed nondoped OLEDs fabricated by depositing TPBi using the spin coating technique and using dendritic molecules G2B and G3B as emitters exhibited high EQE values of 5.7 and 2.9%, respectively. On the other hand, when TPBi was deposited through the vacuum processing, OLEDs comprising G2B and G3B showed a EQEmax values of 4.8% and 3.6%, respectively. Soon afterward, the same research group studied a series of green TADF emitters, namely, tBuG2B, MeG2B, MeOG2B, and PhG2B by substituting alkyl, alkoxy, or aryl groups on dendritic molecule G2B to enhance the OLED performance.117 When tert-butyl and phenyl groups were introduced on terminal positions of G2B, AIE enhancement was realized through suppression of IC in singlet excited states. Meanwhile, in the case of alkoxy group, ACQ character was observed. In addition, subG2B films exhibited moderate to high PLQY values (74, 34, 17, and 41%) and also showed good alcohol resistance. Nondoped OLEDs were fabricated using the substituted G2B molecules as emitters and SPPO13 as the ETL; the SPPO13 emitting layer was deposited via both vacuum deposition and spin coating for comparison. When vacuum deposition was used, the solution-processed nondoped devices comprising tBuG2B, MeG2B, MeOG2B, and PhG2B showed EQE values of 8.9, 7.4, 4.3, and 7.3%, respectively. When spin coating was used, the EQEmax improved to 17.0, 9.0, 6.4, and 8.8% for tBuG2B, MeG2B, MeOG2B, and PhG2B, respectively. Furthermore, the all-soluble OLED based on tBuG2B maintained a high EQE of 13.8% even at a high luminance of 1000 cd m−2.
Wang's research group118 also developed two new AIDF emitters, CzTAZPO and sCzTAZPO, based on the asymmetric D–π–A configuration composed of a triazine core as the acceptor, Cz dendritic units as donors, and phenyl spacer as the π-bridge. Additionally, phosphorus oxygen (P=O) groups were introduced to enhance the electron-transporting properties of the target dendritic molecules. Due to their twisted Cz dendritic structures, these emitters simultaneously exhibited high oscillator strengths (f) and small ΔEST of 0.08 eV for CzTAZPO and 0.1 eV for sCzTAZPO, resulting in a high RISC rate and increased fluorescence quantum efficiency. Consequently, nondoped OLEDs employing CzTAZPO as the EML exhibited excellent EL performances with EQEmax and CEmax values of 12.8% and 29.1 cd A−1, respectively; more interestingly, the EQE roll-off was negligible (1.6% at 1000 cd m−2 luminance). Recently, Qi and coworkers119 tailored a series of AIE-active TADF emitters, 3DPAC–BPCTPA, 3DMAC–BPCTPA, and 3PXZ–BPCTPA, based on a star-shaped D–A–D configuration containing phenyl ketone as the acceptor and DMAC, DPAC, or PXZ as the donors. In addition, TPA with propeller structure was introduced as the central core and branched alkyl chains were introduced to increase the free volumes for these dendrimers, which is beneficial to making pinhole-free uniform films during solution-processing. Owing to their highly twisted molecular structures, the surrounding arms showed a nearly orthogonal configuration. Thus, improved PLQYs, small ΔEST values, and a more efficient RISC process in the aggregated state were realized due to the suppression of intermolecular π–π interactions. As a result, solution-processed nondoped and doped OLED based on 3PXZ–BPCTPA exhibited EQEmax values of 12.1 and 17.6%, respectively, which are superior to those of reference nondendritic luminogens of PXZ–BPCTPA.
On the other hand, Wang's research group have published a few reports in TADF–OLEDs based on TSCT polymers with linear and dendritic pattern having AIE characteristics. In these architectures, the donor and acceptor units are spatially close to each other in the dendritic pattern, whereas, in the case of linear pattern, the donor and acceptor are spatially adjacent to each other but physically separated by a nonconjugated polymer backbone. As a result, in these structures, emission occurs due to the intramolecular charge transfer through the space pathway, which is called TSCT emission. For example, in 2019, Wang's group published their first report on TSCT dendrimers, Ac3TRZ3 and TAc3TRZ3, containing circularly arrayed acridan or teracridan as the donors and TRZ as the acceptor in the periphery of a hexaarylbenzene core.120 Because of the spatial separation of the donors and acceptors in the above-mentioned dendrimers (TSCT–HABs), a very small ΔEST of 0.04–0.08 eV and a TADF effect with microsecond-scale lifetimes were realized. In addition, electron interaction could occur through the space pathway to enable efficient emission. Moreover, the nonplanar propeller-shaped structure of the HAB core allowed the dendrimers to exhibit AIE behavior through the RIM mechanism, with emission intensity enhanced by 6–17 fold from the solution to the aggregation state. Consequently, solution processed nondoped OLEDs based on Ac3TRZ3 and TAc3TRZ3 displayed EQEmax values of 3.5 and 3.1%, respectively, whereas doped devices showed much higher device performance with EQEmax values of 11.0 and 14.2%, respectively. Furthermore, the EQEs remained at 6.6 and 10.4% for Ac3TRZ3 and TAc3TRZ3, respectively, at luminance of 1000 cd m−2.
In addition, Wang's group developed four TSCT polymers, P1, P3, P4, and P5, with a nonconjugated polystyrene backbone and acridan and TRZ as the donor and acceptor, respectively; full-color emission (deep blue (455 nm) to red (616 nm)) was realized by tuning charge transfer strength between spatial donor and TRZ acceptor units with different electron-withdrawing abilities. Furthermore, white light emission was also achieved by using two kinds of donor–acceptor pairs to produce blue and yellow emission concurrently from a single polymer, namely, WP.121 For deep-blue emission, one of the phenyl rings on TRZ unit was substituted with a cyclohexane ring to reduce the electron accepting ability, thereby blue-shifting the emission maximum from 486 (P2) to 469 nm (P1). In contrast, for green and red emission, strong electron withdrawing substituents of trifluoromethyl, 4-cyanophenyl, and 4-cyanopyridyl were introduced on TRZ to increase the electron accepting ability, which red-shifted the emission band from 542 nm for P3, 577 nm for P4, and 628 nm for P5. Moreover, TSCT polymers exhibited AIE features along with promising PLQYs of up to 74% in films. Solution-processed nondoped OLEDs based on P1, P3, and P5 showed blue, green, and red emissions with EQEmax values of 7.1, 16.2, and 1.0%, respectively. Furthermore, for white light emission in WP, the acceptor contents were fixed at 5 mol% using simple triazine and 0.4 mol% using 4-cyanophenyl-triazine for balanced blue and yellow emissions, respectively. Thus, the solution-processed nondoped OLED based on WP exhibited white emission with an EQEmax of 14.1% and CIE coordinates of (0.31, 0.42). Therefore, this work had suggested a novel approach for the design of efficient luminescent polymers for full-color emission, including white light with AIDF properties for OLEDs applications.
Furthermore, another three TSCT polymers, PH, PF and PTF, were developed using acridan as the donor and triphenylboranes as acceptor unit with different substituents of hydrogen, fluorine, and trifluoromethyl.122 By increasing the electron withdrawing capability of the substituent on the triarylborane acceptors, from hydrogen to trifluoromethy, the charge transfer strength between the donor and acceptor could be enhanced, thereby red-shifting the emission band from 429 nm for PH-X polymer, 443 nm for PF-X polymer, and 483 nm for PTF-X polymer in solid state films and leading to improved PLQYs of 26–53%. Moreover, the resulting TSCT polymers exhibited small ΔEST values smaller than 0.1 eV, DF with microsecond-scale lifetimes (0.19–0.98 μs), and AIE with up to a 33-folds increase in emission intensity upon aggregation. For the solution-processed nondoped OLED based on PTF, an EQE of up to 7.0% could be achieved by maintained the acceptor contents at 20 mol%. Soon afterward, three kinds of TSCT blue polymers, PBO–TB, PBO–H, and PBO–F, were synthesized, comprising a nonconjugated polystyrene backbone, acridan donor, and oxygen-bridged triphenylborane having different substituents of tert-butyl, hydrogen, or fluorine as the acceptor.123 Due to the different acceptor strengths, deep-blue to sky-blue emission and improved PLQYs of up to 70% in solid-state film were realized, as well, AIE with up to a 27-fold increase in emission intensity upon aggregation was observed. Therefore, solution-processed nondoped OLED based on PBO-F polymer exhibited excellent EL performances with an EQEmax of 15.0%, and a blue emission with CIE coordinates of (0.16, 0.27).
Other research groups have also carried out research on highly efficient AIDF polymers for OLED applications and achieved significant performances with good thermal stability. For example, Yan's group first reported on the development of solution-processed single white-light-emitting polymers with both TADF and AEE characteristics. The designed copolymers, P1, P2, and P3, consist of yellow AIDF PTZ–DBTO2 as an emitter and DBT unit as the blue fluorescence emitter as a host leads to a white-light-emitting copolymer.124 The resulting copolymers exhibited TADF and AIE characteristics. More interestingly, P3 showed varied emission upon aggregation with THF/water mixture because of its dual emission, namely from sky-blue through white to yellowish green. Furthermore, doped OLEDs of P1 and P2 showed yellow emission with CIE coordinates of (0.46, 0.43) and (0.45, 0.40). Interestingly, the solution-processed doped OLED of P3 exhibited two-color warm-white-light-emission with high color rending indexes (CRI) of 77, and excellent EL performances with CEmax of 23.0 cd A−1, PEmax of 32.8 lm W−1, and EQEmax of 10.4% with CIE coordinates of (0.37, 0.38).
On the other hand, cyclopentadiene (CP)-based polymers also showed high PLQY and good EL performance in fluorescent OLEDs because of the high degree of conjugation resulting from multiple linkage sites. For example, Xie, Li, and coworkers125 synthesized CP based A4 + B2 type hyperbranched polymers, CP1, CP2, and CP3, by conducting Suzuki−Miyaura coupling reaction between tetrabromo (A4)-substituted CP and diboronate ester (B2) of TPE (CP1), 9-phenylcarbazole (CP2), and fluorene (CP3). The PLQY of CP1 was 50.1%, which is higher than those of CP2 and CP3, 26.5 and 43.8% respectively in neat films. Furthermore, doped OLEDs were fabricated with different host materials, namely, mCP and bipolar TADF host material, CzAcSF, with CP1, CP2, and CP3 as dopants. The CP1-based device exhibited excellent EL performance with an EQEmax of up to 6.36% when mCP was used as the host. More importantly, the EQE further increased to 9.74% when mCP was replaced with CzAcSF. This work had suggested that device fabrication using a TADF host and fluorescent polymers as emitters provides an alternative approach for developing high-performance OLEDs. In addition, Tang's group126 explored two TPE-based AIE polymers, pTPE–DPA–Cz and pTPE–DPA–Flu, by incorporating the AIE unit of TPE–DPA in the main chains. The resulting polymers exhibited PL efficiencies of up to 63.3% in doped thin films. Furthermore, polymer light-emitting devices (PLED) were constructed using these AIE polymers as EMLs through a solution process. The doped PLED device of pTPE–DPA–Flu (5%) with a CBP host exhibited an EQEmax of 3.26%, whereas nondoped PLED device of pTPE–DPA–Cz showed a maximum CE of up to 3.69 cd A−1, an EQEmax of 1.46%, and small efficiency roll-offs. The relevant data from the aforementioned representative reports of AIDF dendrimers and macromolecules are summarized in Table 9, and their corresponding chemical structures are shown in Figure 10.
4 CONCLUSIONS AND OUTLOOK
Since Tang et al. introduced the phenomenon of AIE to scientific community, wonderful progress has been made in various fields, ranging from molecular design and mechanism to material applications based on the development of high-performance nondoped electroluminescent devices. In particular, recent cutting-edge research indicates that great progress has been made in enhancing the stability and efficiency of pure organic nondoped OLEDs toward small- to medium-sized molecules to macromolecules having AIE feature. Therefore, in this review, we systematically analyzed and summarized the molecular design strategies, properties and applications of AIE/AIDF luminogens in nondoped OLEDs. AIDF luminogens are especially important because of their distinctive advantage of harvesting both single and triplet excitons for light emission and effectively suppress ACQ and bimolecular exciton annihilation. Ultimately, AIDF luminogens can serve as promising materials for nondoped OLEDs with excellent electroluminescent performances and negligible efficiency roll-offs. More importantly, D–A-based AIDF molecules enable highly efficient tuning the emission color, from the desired color to full color emission, by varying the strengths of donors and acceptors.
However, these results are not sufficient for commercial applications, and further research is required toward robust AIDF luminogens with high solid-state PL efficiencies, high exciton utilization efficiencies, and negligible efficiency roll-offs to improve the stability and EL performance of nondoped OLEDs. Therefore, continuous efforts have been made to design novel AIDF materials along with computational analysis to improve the understanding of structure–property relationship and the photophysical process of AIDF phenomenon with mechanism. Finally, we hope this review will provide useful insights in the design of new luminogens with AIE features for nondoped and doped OLEDs.
ACKNOWLEDGMENTS
The authors acknowledge the financial support from the National Research Foundation of Korea (NRF2022R1A2B5B02001454, NRF2019R1A2C2002647, and NRF2019R1A6A1A11044070) and LG Display Co. Limited (Q1830291).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Biographies

Jinhyo Hwang received her PhD degree in organic chemistry from Korea University, in 2022 under the supervision of Prof. Dong Hoon Choi. She received her BS degree from Seoul Women's University, Seoul, Korea. Her main interests are the design and synthesis of organic semiconducting materials for TADF OLED and optoelectronic devices.

Peethani Nagaraju received his PhD degree in chemistry from Indian Institute of Chemical Technology (IICT), India in 2018 under the supervision of Dr. V. Jayathirtha Rao. He is currently working as a postdoctoral research fellow in Prof. Dong Hoon Choi's group at Korea University, South Korea. His research interests are design and synthesis of novel organic luminescent materials for OLED applications.

Min Ju Cho is currently a research professor in the Research Institute for Natural Sciences in Korea University, Korea. He received his PhD in chemistry from Korea University under the supervision of Prof. Dong Hoon Choi in 2009. He was a postdoctoral fellow (2009–2012) under supervision of Prof. Paras N. Prasad at the Institute for Lasers, Photonics, and Biophotonics, University at Buffalo, State University of New York. He has published about 200 papers in Progress in Polymer Science, Advanced Materials, ACS Nano, and so on. His research focuses on organic semiconducting materials for electronic and optoelectronic device applications.

Dong Hoon Choi was born in Korea in 1960. He received his PhD in macromolecular science and engineering from the University of Michigan in Ann Arbor, Michigan, USA, in 1991. After working as a postdoctoral researcher at The State University of New York, Buffalo, USA, he joined the Korea Institute of Science and Technology in 1992 as a senior scientist in the Functional Polymer Design Laboratory. In 1995, he became a professor in the Department of Environmental Applied Chemistry at Kyunghee University and a research associate at the University of Washington, WA, USA. He was appointed Professor of chemistry at Korea University in 2005 and is currently a fellow at the Korean Academy of Science and Technology. He is the Director of the Brain Korea21 program and the Director of the Priority Research Center supported by the Ministry of Education of the Republic of Korea. His main research interests are the synthesis and application of organic semiconducting materials exhibiting exciton recombination and dissociation phenomena. He has published more than 400 scientific papers and has 60 domestic and international patents.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available at https://doi.org/10.1002/agt2.199.




