Prognostic factors for patients with esophageal cancer who achieve pathological complete response in the primary tumor after upfront chemotherapy or chemoradiotherapy
Abstract
Aim
Upfront chemotherapy (uCT) or upfront chemoradiotherapy (uCRT) followed by surgery is generally accepted as the standard treatment for patients with locally advanced esophageal cancer. A substantial proportion of patients achieve a pathological complete response (pCR) of the primary tumor with upfront treatment. This retrospective study aimed to clarify the prognostic factors of patients with esophageal cancer who achieve pCR in the primary tumor after upfront treatment and whether the prognosis of patients with pCR who receive uCT differs from that of patients who receive uCRT.
Methods
This study included 121 patients who achieved pCR of the primary tumor after uCT and 40 patients after uCRT. Univariate and multivariate survival analyses were performed.
Results
Multivariate analysis of overall survival demonstrated that gender, upfront treatment, and pathological lymph node metastasis were independent prognostic factors (p = 0.0086, 0.011, and 0.031, respectively). Multivariate analysis of relapse-free survival demonstrated that gender, cM status, and pathological lymph node metastasis were independent prognostic factors (p = 0.033, 0.014, and 0.0010, respectively). Among patients without pathological lymph node metastasis, the uCT group showed significantly better both overall and relapse-free survival than the uCRT group (p = 0.014 and 0.037, respectively). Recurrence occurred in 24 patients in the uCT group and 9 in the uCRT group. All of local treatment (chemoradiotherapy and surgery) for recurrent lesions was performed in the uCT group.
Conclusions
Male genders and pathological lymph node metastasis are independent poor prognostic factors in patients with esophageal cancer who receive upfront treatment followed by surgery and achieved pCR of the primary tumor.
1 INTRODUCTION
Esophageal cancer is one of the most common malignancies worldwide. It is the sixth leading cause of cancer-related death.1 Neoadjuvant chemotherapy (nCT) or neoadjuvant chemoradiotherapy (nCRT) followed by esophagectomy is generally accepted as the standard treatment for patients with locally advanced esophageal cancer.2-5 A substantial proportion of patients achieves a pathological complete response (pCR) of the primary tumor with upfront treatment. The prognosis of patients with pCR is better compared with that of patients with non-pCR. Several studies have investigated the prognostic factors for patients with pCR after upfront treatment. However, many of these studies included both squamous cell carcinoma and adenocarcinoma or esophageal cancer and Siewert type III esophagogastric cancer.6, 7 It may be difficult to interpret the results obtained from patients with different histological types, tumor locations, and operative procedures.
Upfront chemoradiotherapy (uCRT) is consistently associated with better pathological outcomes and a higher rate of pCR than upfront chemotherapy (uCT). However, this has not translated into improved survival.5, 8, 9 Few studies reported the difference of prognosis of patients with pCR who underwent uCT and that of those who underwent uCRT.10, 11
This retrospective study aimed to clarify the prognostic factors of patients with squamous cell carcinoma of thoracic esophagus who achieve pCR of the primary tumor after uCT or uCRT and to determine whether the prognosis of patients with pCR who receive uCT differs from that of those who receive uCRT.
2 PATIENTS AND METHODS
2.1 Patients
Data were collected from medical records in an esophageal cancer database comprising >3500 consecutive patients who underwent esophagectomy for esophageal cancer between January 2000 and December 2019 at Osaka University Hospital, Osaka International Cancer Institute, Kindai University Hospital, Kansai Rosai Hospital, National Osaka Hospital, and Osaka General Medical Center. Patients who met the following criteria were enrolled in this retrospective study: the primary tumor was located in the thoracic esophagus; clinical tumor-node-metastasis stage was cTany, cNany, or cM0 (including supraclavicular lymph node metastasis); histological type was squamous cell carcinoma; uCT or uCRT followed by subtotal esophagectomy were performed; curative resection (R0) was achieved, and pCR of the primary tumor was achieved. Patients with distant metastases other than supraclavicular lymph node, who received both uCT and uCRT, or who died during the perioperative period were excluded from this study. This study was approved by the Human Ethics Review Committees of Osaka University Hospital, Osaka International Cancer Institute, Kindai University Hospital, Kansai Rosai Hospital, National Osaka Hospital, and Osaka General Medical Center (approved number 16305-3). This study was conducted in accordance with the principles of Declaration of Helsinki.
2.2 Upfront treatment
All patients were staged before and after surgery, according to the seventh edition of the Union for International Cancer Control staging system.12 Clinical staging before upfront treatment was based on endoscopy; computed tomography (CT) scan of the neck, chest, and upper abdomen; and 18F-fluorodeoxyglucose positron emission tomography (PET) scan, when possible. Lymph nodes were considered metastasis-positive on the CT scan if they were spherical and >1.0 cm in maximum transverse diameter or if focal major fluorodeoxyglucose uptake was detected on the PET scan. uCT was administered to patients with cT2–4NanyM0, cTanyN1M0, and cTanycNanycM1 (Lym; supraclavicular lymph node). The patients underwent surgery 3–6 weeks after the last day of chemotherapy. uCRT was administered to patients with cT4 or suspected cT4 or those who refused surgical resection. uCRT consisted of cisplatin, 5-fluorouracil (CF), and concurrent radiotherapy (40–60 Gy). In addition to the primary tumor and metastatic lymph nodes, the prophylactic irradiation field was between the supraclavicular and the superior mediastinum in upper thoracic tumors, whereas it was between the superior mediastinum and the cardia in middle and lower thoracic tumors. The patients underwent surgery 4–8 weeks after the last day of radiotherapy.
2.3 Surgical procedure
The standard surgical procedures included transthoracic esophagectomy with mediastinal lymphadenectomy, upper abdominal lymphadenectomy, and the gastric tube reconstruction, and cervical anastomosis. Upper, middle, and lower mediastinal and upper abdominal lymphadenectomy was performed in all patients. Supraclavicular lymphadenectomy was performed in patients with upper thoracic tumors and those with middle or lower thoracic tumors with upper mediastinal or cervical lymph node metastasis. The extent of lymphadenectomy, including around the airway was similar in patients after uCT and uCRT. Jejunal or colonic graft reconstruction was performed in the patients with a history of gastrectomy.
2.4 Evaluation of the histopathological response to upfront treatment
The histopathological response of the primary tumor to upfront treatment was classified according to the Japanese Society for Esophageal Diseases criteria,13 and evaluations were classified into five categories according to the proportion of viable residual tumor cells within the entire cancerous tissue as follows: grade 0, no significant tumor regression; grade 1a, >67% of the entire cancerous tissue comprising residual tumor cells; grade 1b, >33% of the entire cancerous tissue comprising residual tumor cells; grade 2, <33% of the entire cancerous tissue comprising residual tumor cells; and grade 3, no viable residual tumor cells.
2.5 Follow-up evaluation and recurrence pattern
The patients were followed up every 3 months, during the first 2 years after surgery, every 6 months for the next 3 years, then annually. CT scans were performed every 6 months. PET scan was performed, if necessary, to confirm recurrence. The recurrence patterns were divided into the following four types; locoregional, distant lymph nodes, hematogenous, and disseminated. Locoregional recurrence included the local, mediastinal, supraclavicular, and celiac trunk regions.
2.6 Statistical analyses
Associations between two categorical parameters were evaluated using the Mann–Whitney U-test, or chi-squared test, as appropriate. Survival was calculated from the date of surgery to the occurrence of an event or the last recorded follow-up date. A Cox proportional hazards regression model was used to identify prognostic factors for survival. Survival rates were calculated using the Kaplan–Meier method and differences were evaluated using the log-rank test. Two-sided p-values were calculated, and statistical significance was set at p < 0.05. All analyses were performed using the JMP version 13.0 software (SAS Institute, Cary, NC, USA).
3 RESULTS
3.1 Patient characteristics
uCT followed by esophagectomy was performed in 1324 patients, of whom 121 patients (9.1%) achieved pCR of the primary tumor. uCRT followed by esophagectomy was performed in 306 patients, of whom 43 patients (14.1%) achieved pCR of the primary tumor. One patient who received uCT and two patients who received uCRT died during the perioperative period. Thus, 161 patients were included in this study analyzed. The demographic and clinicopathological characteristics of these 161 patients are summarized in Table S1. The chemotherapy regimens in the uCT group were as follows; cisplatin, 5-fluorouracil, and docetaxel in 90 patients (DCF therapy)14; cisplatin, 5-fluorouracil, and adriamycin in 22 patients (ACF therapy)15; cisplatin and 5-fluorouracil in 6 patients (CF therapy)16; and others in 2 patients. In the uCRT group, the irradiation doses were <50 Gy in 24 patients and 50 ≤ Gy in 17 patients. All cM1 and pM1 cancers were caused by supraclavicular lymph node metastasis. Pathological lymph node metastasis, including supraclavicular lymph node metastasis, was observed in 47 patients. Postoperative adjuvant therapy was performed in four patients: two received chemotherapy and two received chemoradiotherapy. Figure 1 shows the overall survival (OS) of all patients. The 3- and 5-year OS rates were 83.4% and 78.3%, respectively.
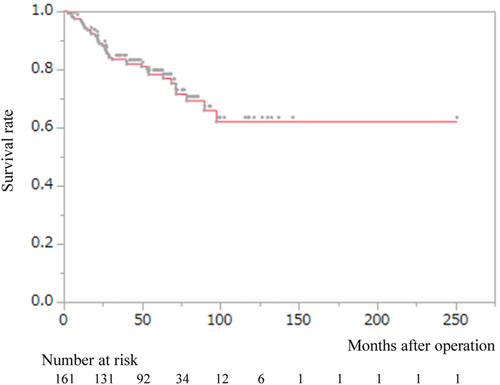
3.2 Univariate and multivariate analyses for overall survival
Table 1 shows the univariate and multivariate analyses for OS. Univariate analysis revealed that pathological lymph node metastasis was significantly associated with OS (p = 0.0047). Multivariate analysis demonstrated that gender, upfront treatment, and pathological lymph node metastasis were independent prognostic factors (p = 0.0086, 0.011, and 0.031, respectively).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| p Value | HR | p Value | ||
| Gender | M vs. F | 0.064 | 3.93 (1.36–16.73) | 0.0086 |
| Age | 70 ≤ vs. < 70 | 0.67 | 1.67 (0.81–3.41) | 0.16 |
| Treatment period | 2000–2009/2010–2019 | 0.61 | 0.47 (0.17–1.17) | 0.11 |
| Tumor location | Ut vs. Mt., Lt | 0.74 | 0.65 (0.24–1.50) | 0.33 |
| cT | T3-4 vs. T1-2 | 0.18 | 1.65 (0.76–3.86) | 0.21 |
| cN | N1 vs. N0 | 0.060 | 2.22 (0.85–4.92) | 0.11 |
| cM | M1 vs. M0 | 0.19 | 2.11 (0.85–4.92) | 0.10 |
| Upfront treatment | Chemoradiotherapy vs. chemotherapy | 0.077 | 2.92 (1.28–6.51) | 0.011 |
| Pathological lymph node metastasis | Positive vs. negative | 0.0047 | 2.14 (1.07–4.23) | 0.031 |
- Abbreviation: HR, hazard ratio.
3.3 Univariate and multivariate analyses for relapse-free survival
Table 2 shows the univariate and multivariate analyses for RFS. Univariate analysis revealed that cM status and pathological lymph node metastasis were significantly associated with OS (p = 0.019 and 0.0009, respectively). Multivariate analysis demonstrated that gender, cM status, and pathological lymph node metastasis were independent prognostic factors (p = 0.033, 0.014, and 0.0010, respectively).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| p Value | HR | p Value | ||
| Gender | M vs. F | 0.29 | 2.42 (1.07–6.56) | 0.033 |
| Age | 70 ≤ vs. < 70 | 0.14 | 1.50 (0.80–2.77) | 0.21 |
| Treatment period | 2000–2009/2010–2019 | 0.62 | 0.26 (0.17–1.35) | 0.63 |
| Tumor location | Ut vs. Mt., Lt | 0.71 | 1.02 (0.45–2.13) | 0.95 |
| cT | T3-4 vs. T1-2 | 0.12 | 1.95 (0.99–4.13) | 0.0528 |
| cN | N1 vs. N0 | 0.25 | 1.32 (0.63–3.11) | 0.48 |
| cM | M1 vs. M0 | 0.019 | 2.71 (1.24–5.57) | 0.014 |
| Upfront treatment | Chemoradiotherapy vs. chemotherapy | 0.31 | 1.91 (0.93–3.76) | 0.077 |
| Pathological lymph node metastasis | Positive vs. negative | 0.0009 | 2.84 (1.54–5.23) | 0.0010 |
- Abbreviation: HR, hazard ratio.
3.4 Comparison of patients with and without pathological lymph node metastasis
Table 3 shows the characteristics of patients based on pathological lymph node status. There were no statistically different factors between the two groups. Multivariate analysis showed no independent risk factors for pathological lymph node metastasis (data not shown).
| Pathological lymph node metastasis | p Value | |||
|---|---|---|---|---|
| No (n = 114) | Yes (n = 47) | |||
| Gender | M/F | 90/24 | 36/11 | 0.39 |
| Age | <70/70≤ | 62/52 | 30/17 | 0.39 |
| Treatment period | 2000–2009/2010–2019 | 25/89 | 5/42 | 0.12 |
| Location | Ut/Mt., Lt | 24/90 | 6/41 | 0.27 |
| cT | T1-2/T3-4 | 39/75 | 14/33 | 0.71 |
| cN | N0/N1 | 29/84 | 7/40 | 0.15 |
| cM | M0/M1 | 101/13 | 37/10 | 0.13 |
| Upfront treatment | Chemotherapy/chemoradiotherapy | 84/30 | 36/11 | 0.84 |
3.5 Comparison between the upfront chemotherapy and upfront chemoradiotherapy groups
Table 4 shows the characteristics of patients based on upfront treatment. Patients in the uCRT group were significantly younger, had more primary tumor located in the upper thoracic area, and had primary tumor with deeper invasion compared with patients in the uCT group. In addition, the rate of patients underwent uCRT in the first period was significantly higher than that of patients underwent uCRT in the second period. Next, we compared the survival by combining upfront treatment and the pathological lymph node status. In patients without pathological lymph node metastasis, the uCT group showed significantly better both OS and RFS than the uCRT group (p = 0.014 and 0.037, respectively; Figure 2A,B). However, in patients with pathological lymph node metastasis there was no significant difference between the two groups both in OS and RFS (Figure 2A,B).
| uCT group (n = 120) | uCRT group (n = 41) | p Value | ||
|---|---|---|---|---|
| Gender | M/F | 98/22 | 32/9 | 0.65 |
| Age | <70/70≤ | 60/60 | 32/9 | 0.0018 |
| Treatment period | 2000–2009/2010–2019 | 14/106 | 16/25 | 0.0003 |
| Tumor location | Ut/Mt., Lt | 16/104 | 14/27 | 0.0051 |
| cT | T1–2/T3–4 | 47/73 | 6/35 | 0.0038 |
| cN | N0/N1 | 27/92 | 9/32 | 1.00 |
| cM | M0/M1 | 102/18 | 26/5 | 0.80 |
| Pathological lymph node metastasis | Negative/positive | 84/36 | 30/11 | 0.84 |
- Abbreviations: uCRT, upfront chemoradiotherapy; uCT, upfront chemotherapy.
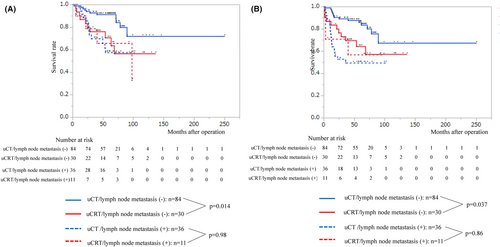
3.6 Recurrence and prognosis
Recurrence occurred in 24 (20%) of 120 patients in uCT group and in 9 (22%) of 41 patients in uCRT group, respectively. Table 5 presents the pattern of first recurrence. There was no difference in the pattern of recurrence between the two groups, although dissemination was slightly higher in the uCRT group (7%) than in the uCRT group (2%, p = 0.11). Table 6 shows treatments for recurrence. Chemoradiotherapy for recurrence was performed in six patients, all of whom recurred after uCT. Resection of recurrent lesions was performed in five patients (brain in four and lymph node in one), all of whom recurred after uCT. Twenty-four (20%) patients died in the uCT group, and 14 (34%) patients died in the uCRT group (p = 0.88). Death due to esophageal cancer was observed 15 (13%) patients in the uCT group and in 8 (20%) patients in the uCRT group, respectively (p = 0.30). Death due to other disease was observed in 9 (7%) patients in the uCT group and in 6 (15%) patients in the uCRT group (p = 0.21).
| uCT (n = 120) | uCRT (n = 41) | p Value | |
|---|---|---|---|
| Overall | 24 (20%) | 9 (22%) | 0.82 |
| Locoregional | 10 (8%) | 2 (5%) | 0.73 |
| Distant lymph node | 7 (6%) | 4 (10%) | 0.47 |
| Hematogenous | 14 (12%) | 4 (10%) | 1.0 |
| Dissemination | 2 (2%) | 3 (7%) | 0.11 |
- Abbreviations: uCRT, upfront chemoradiotherapy; uCT, upfront chemotherapy.
| (a) | |||
|---|---|---|---|
| Upfront treatment | Recurrence | Treatment for recurrence (+)/(−) | Treatment methods CT/CRT/resection |
| uCT (n = 120) | 24 (20%) | 21/3 | 10/6/5 |
| uCRT (n = 41) | 9 (22%) | 4/5 | 4/0/0 |
| (b) | |||
|---|---|---|---|
| Pathological lymph node metastasis: | Recurrence | Treatment for recurrence (+)/(−) | Treatment methods CT/CRT/resection |
| Negative (n = 114) | 13 (11%) | 9/4 | 5/0/4 |
| Positive (n = 47) | 20 (43%) | 16/4 | 9/6/1 |
- Abbreviations: CRT, chemoradiotherapy; CT, chemotherapy; uCRT, upfront chemoradiotherapy; uCT, upfront chemotherapy.
4 DISCUSSION
This study demonstrated that male gender and pathological lymph node metastasis were independent poor prognostic factors for both OS and RFS in patients with esophageal cancer who received uCT or uCRT followed by surgery and achieved pCR in the primary tumor. In addition, uCRT and cM1 were independent poor prognostic factors for OS and RFS, respectively.
Schroeder et al.6 reported that nodal metastatic disease was an independent prognostic factor in 201 patients with esophageal cancer who achieved pCR in the primary tumor after nCT or nCRT followed by esophagectomy. Okamura et al.11 reported that residual nodal disease was an independent risk factor influencing both OS and RFS in 300 patients with esophageal cancer who achieved pCR in the primary tumor after nCT or nCRT. Our results were consistent with these previous studies. To improve the prognosis of patients with pCR of the primary tumor but with pathological lymph node metastasis, appropriate adjuvant treatment should be considered. Several investigators have reported that postoperative adjuvant chemotherapy may improve outcomes in patients receiving neoadjuvant therapy and undergoing surgery.17, 18 Recently, the Check Mate 577 trial showed that adjuvant nivolumab therapy improved disease-free survival in patients with esophageal cancer who had received nCRT and had residual pathological disease.19 Although it remains unknown whether the outcomes in patients after nCT can be improved by the adjuvant nivolumab therapy, adjuvant nivolumab therapy is a promising treatment strategy for patients who achieved a pCR in the primary tumor but with pathological lymph node metastasis.
It would be interesting to determine whether there is a difference in the prognosis between patients with pCR in the primary tumor after uCT plus surgery and those after uCRT plus surgery. Our study revealed that uCRT was an independent poor prognostic factor for OS, but not for RFS. However, the interpretation of this result requires caution. As shown in Table 4, the characteristics of patients, such as age, treatment period, tumor location, and CT, who underwent uCT and those of patients who underwent uCRT were different. These differences in patients' characteristics may have influenced the results. Our results differ from those of a previous report by Okamura et al.,11 who found no significant differences in either OS or RFS in patients with esophageal squamous cell cancer who achieved pCR in the primary tumor. One possible reason for this discrepancy is that the patients analyzed were different. Our study included patients with cT4b tumors, whereas Okamura et al. analyzed resectable tumors. After recurrence, all of local treatment (chemoradiotherapy and surgery) for recurrent lesions was performed in the uCT group as shown in Table 6. This may have contributed to the improved OS in the uCT group. An interesting finding of our study was that in patients without pathological lymph node metastasis, the uCT group showed significantly better both OS and RFS than the uCRT group (p = 0.014, 0.037, respectively, Figure 2A,B). This result is in line with that of a previous study by Lartigue, et al., who analyzed patients with esophageal adenocarcinoma who achieved pCR in both primary tumor and lymph nodes after nCT or nCRT,10 which revealed that the OS of patients who had undergone nCT plus surgery tended to be better than that of patients who had undergone nCRT plus surgery and that the RFS of patients who had undergone nCT plus surgery was significantly better than that of patients who had undergone nCRT plus surgery. In our study, the systemic effects of the chemotherapy used in the uCRT regimens (CF) may have been lower than those of the uCT regimens (ACF and DCF). Pathological lymph node status best reflects systemic micrometastases in patients with esophageal cancer who have received uCT or uCRT followed by surgery.20-22 However, the significance of pathological lymph node status may be different in patients after uCT than in those after uCRT. Chemotherapy is a systemic treatment, whereas radiotherapy is a local treatment. Many of the excised lymph nodes were irradiated in patients who received uCRT. The pathological status of lymph nodes in the radiation field may differ from that of those outside the radiation field. The pathological lymph node status of resected specimens after uCT may be better reflect the systemic micrometastases than that after uCRT.
The results of our study and previous study by Lartigue et al.10 may serve as a warning against esophageal preservation strategy after uCRT or uCT.23, 24 Although Noordman et al. reported that after nCRT for esophageal cancer, clinical response evaluation with endoscopic ultrasonography, bite-on-bite biopsies, and fine-needle aspiration of suspicious lymph nodes combined with PET scans were adequate for the detection of locoregional residual disease, it may be extremely difficult to assess the pathological lymph node status without surgical resection. Patients who respond well to upfront treatment are more likely to be cured by surgery. Worsening of the disease in such cases during follow-up without surgery is an undesirable outcome. Establishing a reliable method for evaluating residual disease both in primary tumors and potentially metastatic lymph nodes is desirable.
Our study revealed that the male gender was independently associated with worse OS and RFS. We analyzed patients with esophageal cancer who did not achieve a pathological complete response in the primary tumor after upfront chemotherapy or chemoradiotherapy (952 male and 159 female). Univariate analysis revealed that female gender was significantly associated with better OS (p = 0.044). Multivariate analysis including gender, age, tumor location, cT, cN, cM, and pathological lymph node status revealed that pathological lymph node metastasis was significantly associated with poor OS, and female gender marginally associated with better OS (HR 0.78, 95% CI 0.59–1.01) (data not shown). Several investigators have reported gender differences in the prognosis of patients with esophageal cancer. A population-based nationwide cohort study in Sweden revealed that women who underwent esophagectomy for esophageal squamous cell carcinoma had a better prognosis than men.25 From a large pooled analysis using four prospective randomized trials (nCT vs. surgery alone, perioperative chemotherapy vs. surgery alone, nCT vs. nCT, and perioperative chemotherapy vs. perioperative chemotherapy combined with molecularly targeted drugs), significant improvements in OS and disease-specific survival (DSS) were observed in females compared with males.26 Our results are consistent with those of these studies. In the study by Athauda et al., the prognostic effect of surgery was more pronounced in patients who received nCT, suggesting a predictive effect of exposure to chemotherapy. Certainly, females experienced significantly more grade III non-hematological toxicities (nausea, vomiting, and diarrhea), and tended to have a better histologic response to chemotherapy than males.26 A number of systemic anticancer therapies also display a toxicity–response relationship, whereby higher rates of toxicity are associated with better responses to therapy.15, 27, 28 As the recommended doses of cytotoxic drugs have been determined through phase I and II studies which have a male preponderance, female patients may be treated with doses above those that will be effective, but at the cost of increased toxicities.
Our study has several limitations. First, the number of patients analyzed in this study was relatively limited. Second, this was a retrospective study including patients from January 2000 to December 2019 and the indication criteria for upfront treatment were varied. Surgical invasiveness as well as irradiation techniques in particular differ between recent years and 20 years ago. Characteristics of patients, such as age, treatment period, tumor location, and cT, differed between uCT group and uCRT group, as shown in Table 4. These differences may have influenced the results. When we analyzed only patients with esophagectomy during the second period (2010–2019), male gender, uCRT, and pathological lymph node metastasis were independent poor prognostic factors for OS (p = 0,0014, 0.035, 0.0031, respectively, data not shown). Male gender, cM1, and pathological lymph node metastasis were independent poor prognostic factors for RFS (p = 0.029, 0,050, <0.0001, respectively, data not shown). Third, we lacked data that may have affected the prognosis, such as comorbidities, adverse events of upfront treatment, or postoperative complications.
In conclusion, male gender and pathological lymph node metastasis are independent poor prognostic factors in patients with esophageal cancer who undergo upfront treatment followed by surgery and achieved a pCR in the primary tumor.
AUTHOR CONTRIBUTIONS
Masaaki Motoori: Conceptualization; formal analysis; methodology; writing – original draft. Koji Tanaka: Data curation; investigation. Hiroshi Miyata: Project administration; writing – review and editing. Makoto Yamasaki: Project administration; supervision. Osamu Shiraishi: Data curation; funding acquisition. Atsushi Takeno: Data curation; writing – review and editing. Tomoki Makino: Methodology; project administration. Keijiro Sugimura: Conceptualization; data curation; methodology. Takushi Yasuda: Conceptualization; project administration; writing – review and editing. Yuichiro Doki: Conceptualization; project administration; writing – review and editing.
ACKNOWLEDGMENTS
We would like to thank Drs. Kotaro Yamashita, Kota Momose (Osaka University), Takeshi Kanemura, Takahito Sugase, Norihiro Matsuura (Osaka International Cancer Institute), Yutaka Kimura (Kindai University Nara Hospital), Motohiro Hirao (National Hospital Organization Osaka National Hospital), Kazumasa Fujitani (Osaka General Medical Center), and Masahiko Yano (Kyowakai Hospital) for data acquisition and advice on preparing this manuscript. We would like to thank Editage (www.editage.jp) for English language editing.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
Y. Doki is an Editorial Board member of the Annals of Gastroenterological Surgery. All remaining authors declare no conflicts of interest.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: This study was approved by the local ethics committees of each participating institution (Approval No. 16305–3) and was in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1964 and later versions.
Informed Consent: N/A.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.
DISCLOSURE OF ANY COMMERCIAL INTEREST
None.




