Clinical impact of the triple-layered circular stapler for reducing the anastomotic leakage in rectal cancer surgery: Porcine model and multicenter retrospective cohort analysis
[Correction added on 07 April 2023, after first online publication: The copyright line has been corrected.]
Abstract
Aim
To investigate the impact of the triple-layered circular stapler compared with the double-layered circular stapler on anastomotic leakage after rectal cancer surgery.
Methods
The bursting pressure was compared between porcine ileocolic anastomoses created using a double- or triple-layered stapler. We also retrospectively analyzed the incidence of severe anastomotic leakage in 194 patients who underwent colorectal anastomosis using a double- or triple-layered circular stapler during rectal cancer resection performed in two cancer centers between January 2015 and April 2021.
Results
In the porcine model, the bursting pressure was higher in anastomoses created using the triple-layered stapler than the double-layered stapler (end-to-end anastomosis: 26.4 ± 6.2 mm Hg vs 14.5 ± 4.3 mm Hg, P = .0031; side-to-side anastomosis: 27.7 ± 5.0 mm Hg vs 18.0 ± 2.9 mm Hg, P = .0275). Intersectional leakage occurred in 41% and 83% of anastomoses created using the triple- or double-layered stapler, respectively (P = .0821). In the clinical cohort, the double- and triple-layered stapler was used in 153 and 41 patients, respectively. The incidence of anastomotic leakage was lower for anastomoses created using the triple-layered stapler vs the double-layered stapler (0.0% vs 5.8%, P = .0362). In multivariate analysis, the factors independently associated with a lower incidence of anastomotic leakage were female sex (odds ratio: 0.16, 95% confidence interval: 0.01-0.90, P = .0354) and triple-layered stapler usage (odds ratio: 0.00, 95% confidence interval: 0.00-0.96, P = .0465).
Conclusion
Anastomoses created using a triple-layered circular stapler had high bursting pressure, which might contribute to a lower incidence of anastomotic leakage after rectal cancer surgery.
1 INTRODUCTION
In rectal cancer surgery, the most common major postoperative complication is anastomotic leakage (AL).1, 2 A systematic review and meta-analysis showed that AL after anterior resection for rectal cancer has a negative effect on local recurrence and long-term survival.3, 4 AL is also associated with permanent stoma development, fecal incontinence, and reduced sexual activity, which negatively affect patients' long-term quality of life.5-7 Furthermore, AL greatly increases the medical costs.8 Based on investigations in large patient cohorts, the reported incidence of AL after rectal resection varies from 6.3% to 13.7%.9-12 Some techniques or procedures to prevent AL have been reported, but further improvement is needed.
Recently, the EEA™ Circular Stapler with Tri-Staple™ Technology (tri-EEA) (Medtronic Japan, Tokyo, Japan) was released as the first triple-layered circular stapler, which is expected to decrease the incidence of AL. The tri-EEA has three rows of staples that vary in height, whereas the previously released EEA™ Circular Stapler with DST Series™ Technology (EEA) (Medtronic Japan) deploys two rows of uniform-height staples.13 In the tri-EEA, the staples closest to the lumen have the shortest height to provide the greatest occlusion and barrier to leaks and bleeding. The second and third rows, each incrementally higher, contribute strength to the closure line. To the best of our knowledge, no study has evaluated the impact of the tri-EEA on the anastomotic bursting pressure and incidence of AL in a clinical cohort compared with EEA.
This study was performed to compare the bursting pressure of anastomoses created using the tri-EEA vs the EEA in a porcine model and to evaluate whether the incidence of AL in a multicenter clinical cohort was lower after rectal cancer surgery using the tri-EEA than the EEA.
2 METHODS
2.1 Porcine model
Commercially available small intestine and colon specimens from healthy 6-month-old Japanese domestic pigs weighing 100 to 110 kg were used in this study, which was conducted in full accordance with the principles of the Helsinki Institutional Review Board for animal studies. End-to-end anastomosis using the double-stapling technique (DST) was performed as previously described.14 The colon was closed and cut with a linear stapler using the Signia™ Stapling System (Tri-Staple™ 60-mm purple cartridge; Medtronic Japan). The small intestine was fitted with a 3-0 Polysorb™ (Medtronic Japan) purse-string suture around the open stump. The anvil head of the circular stapler (EEA or tri-EEA) was placed at the edge of the stump, and the anvil shaft was secured. The instrument of the circular stapler was inserted through the open stump of the colon and advanced to the closed stump. The colon wall was penetrated by the center rod of the instrument just beside the colonic closure line. The anvil shaft and the center rod were joined and closed, and the instrument was activated and removed. After anastomosis, it was confirmed that the mucosal layer, muscular layer, and serosa layer were detected without any defect in the entire circumference of both rings that were attached to the instrument.
The side-to-side anastomosis was performed as previously described.14 The anvil head was inserted through the open stump of the small intestine and advanced to the closed stump. The tip of the anvil head was penetrated through the antimesenteric site 2 cm from the closed stump so that the linear staple line and the anvil head did not overlap. The instrument of the circular stapler was inserted into open stump of the colon and advanced to the closed stump. The center rod was brought through the colonic wall 2 cm from the closed stump so that the linear staple line and the circular staple line did not overlap. The anvil shaft and center rod were then joined and closed, and the instrument was activated and removed. The completion of both rings was confirmed as described for the end-to-end anastomosis.
An anastomotic bursting pressure test was performed after the completion of each anastomosis. Both stumps that were not anastomosed were clamped, and a sensor cord was inserted inside the anastomotic site. Air leakage was monitored by placing the specimen in a water-filled basin and observing the water to detect escaped air bubbles. When air leakage began, the bursting pressure was measured using an electronic manometer (PG-100N-102GP; Nidec Copal Electronics, Tokyo, Japan). The leakage site was evaluated to determine whether it was located at the intersection. All experiments were performed in accordance with relevant institutional and national guidelines and regulations for the care and use of animals.
2.2 Patients and specimens
A retrospective multicenter study was performed across two comprehensive cancer centers (Kyushu University and National Hospital Organization Kyushu Cancer Center). Of 536 consecutive patients who underwent surgical resection of rectal malignancies and colorectal anastomosis between January 2015 and April 2021, we analyzed 194 patients who underwent colorectal anastomosis using the EEA or the tri-EEA. We excluded patients in whom other instruments were used for anastomosis, such as the PROXIMATE™ intraluminal stapler (Johnson & Johnson, Tokyo, Japan) or the ECHELON CIRCULAR™ Powered Stapler (Johnson & Johnson) (n = 326), and those who received preoperative chemoradiotherapy (n = 16). The clinicopathological background data were extracted from the database at each hospital. All patients, except those with bowel obstruction, underwent mechanical bowel preparation with magnesium citrate and sennoside on the day before surgery. Surgical procedures were performed or supervised by expert colorectal surgeons qualified by the Endoscopic Surgical Skill Qualification System of Japan,15 each of whom had performed more than 200 laparoscopic colorectal operations.
2.3 Rectal resection and colorectal anastomosis
The inferior mesenteric artery was clipped and cut at the root, and the left colic artery and inferior mesenteric vein were clipped and cut at the same level. The marginal vessels were preserved. Tumor-specific mesorectal excision was performed. After clamping the rectum on the anal side of the tumor, the anal canal was washed thoroughly through the anus. The rectum was cut using the Signia™ Stapling System (Tri-Staple™ 60-mm purple cartridge). After removing the specimen through the abdominal incision, the anastomosis was performed. End-to-end anastomosis was performed using the DST as previously described.16 Side-to-side anastomosis was performed using a previously described method.17 We previously reported that the side-to-side anastomosis was a safe and useful procedure in anastomosis after rectal resection.18 In this study, the type of anastomosis was selected at the surgeon's discretion, and there was no specific indication for each procedure. If the surgeon intended to perform side-to-side anastomosis for very low anterior resection, side-to-end anastomosis was sometimes selected because the stump of the rectum was too short to anastomose to the side wall. Therefore, side-to-end anastomoses were performed with intersection of the circular and linear staple lines of the remnant rectum. After division of the patients according to whether such intersections were present, we included side-to-end anastomosis in the end-to-end anastomosis group. An air leak test was performed after each anastomosis, and it was confirmed that no anastomoses had an air leak. Reinforcement was performed at the surgeon's discretion regardless of the leak test result or the anastomosis type. The reinforcement was not always performed at the intersection; some surgeons performed it only at the non-intersection site. A closed drain was placed behind the anastomotic site in all cases. A transanal drainage tube was placed when considered necessary by the surgeon.
2.4 Postoperative management
Oral intake started on postoperative day 3, and the transanal tube was removed on the same day. The intra-abdominal drain was removed on postoperative day 4. Postoperative complications that occurred during hospitalization and within 90 days after surgery were extracted and classified using the Clavien-Dindo (CD) system. If patients developed postoperative complications such as fever, abdominal and/or pelvic pain, or elevated inflammatory markers, radiological examinations (computed tomography [CT] and/or transanal enema examination) were performed. AL was diagnosed when a communication between the intra- and extraluminal compartments caused by a defect of the intestinal wall integrity at the anastomosis was detected by CT and/or transanal enema examination.19 In this study, we focused on AL requiring active therapeutic interventional drainage, transanal drainage, or reoperation (CD grade ≥3). The site of leakage was determined by identification of the defect on CT or endoscopy. If AL was detected, reoperation was performed in patients who had no covering stoma, and interventional drainage or transanal drainage was performed in patients who had a covering stoma. We considered reoperation if the infection could not be controlled by drainage. In patients who underwent covering ileostomy, AL was examined not only during hospitalization and within 90 days after surgery but also after stoma closure (however, no patients in this study developed AL after stoma closure).
2.5 Statistical analysis
Continuous variables were analyzed using the Mann-Whitney U-test, while categorical valuables were analyzed with Pearson's χ2 test or Fisher's exact test, as appropriate. A P value of <.05 was considered significant. Statistical analysis was performed using JMP® software version 10.0.2 (SAS Institute).
3 RESULTS
3.1 Impact of the triple-layered circular stapler on anastomotic bursting pressure in a porcine model
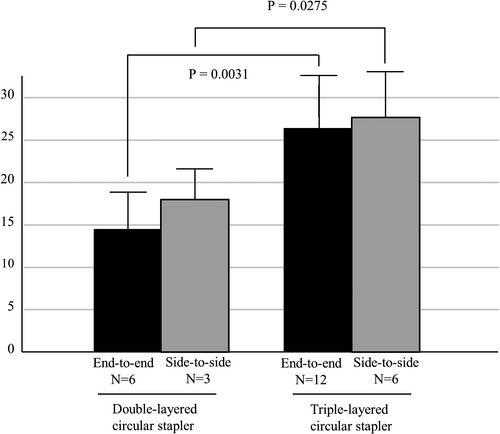
The bursting pressure was measured in a total of 27 anastomoses. End-to-end anastomosis (using the DST) was performed six times using the EEA and 12 times using the tri-EEA. Side-to-side anastomosis was performed three times using the EEA and six times using the tri-EEA. As shown in Figure 1, the bursting pressure was significantly higher in anastomoses created using the tri-EEA than in those created using the EEA, regardless of the type of anastomosis (end-to-end anastomosis: EEA 14.5 ± 4.3 mm Hg vs tri-EEA 26.4 ± 6.2 mm Hg, P =.0031; side-to-side anastomosis: EEA 18.0 ± 2.9 mm Hg vs tri-EEA 27.7 ± 5.0 mm Hg, P =.0275). Among the end-to-end anastomoses, the leakage was located at the intersection site in five of six anastomoses created using the EEA (83%) and in five of 12 anastomoses created using the tri-EEA (41%); however, this difference was not significant (P =.0821).
3.2 Patient characteristics and short-term outcomes
The patient characteristics are summarized in Table 1. The median age was 66 years, 55% were male, and the median body mass index was 21.9 kg/m2. One hundred and eighty-eight patients had rectal cancer, five had neuroendocrine tumors, and one had an endometrial stromal sarcoma. Among the total cohort, the EEA was used in 153 patients (EEA group) and the tri-EEA was used in 41 patients (tri-EEA group). The prevalence of more advanced rectal cancer (stage III-IV) was higher in the tri-EEA group than in the EEA group (20% vs 1%, P <.0001). There were no significant differences between the two groups in other characteristics, including age (P =.6976), sex (P =.8912), body mass index (P =.9763), history of diabetes mellitus (P =.2169), preoperative chemotherapy (P =.3988), tumor location (P =.4058), and maximum tumor diameter (P =.6131).
| Characteristics |
Total N = 194 |
EEA group N = 153 |
tri-EEA group N = 41 |
P value |
|---|---|---|---|---|
| Age (years), median (IQR) | 66 (56.75-71.25) | 66 (56.5-71) | 67 (56.5-73) | .6976 |
| Gender, No.(%) | ||||
| Male | 107 (55) | 84 (55) | 23 (56) | .8912 |
| Female | 87 (45) | 69 (45) | 18 (44) | |
| Body mass index (kg/m2), median (IQR) | 21.9 (19.6-24.7) | 21.8 (19.7-24.5) | 22.1 (19.4-24.9) | .9763 |
| Diabetes mellitus yes, No. (%) | 12 (6) | 11 (7) | 1 (2) | .2169 |
| Preoperative chemotherapy yes, No. (%) | 13 (7) | 9 (6) | 4 (10) | .3988 |
| Location, No. (%) | ||||
| High rectum | 85 (44) | 67 (44) | 18 (44) | .4058 |
| Middle rectum | 83 (43) | 68 (44) | 15 (37) | |
| Low rectum | 26 (13) | 18 (12) | 8 (20) | |
| Pathological stagea, No. (%) | ||||
| I | 58 (31) | 49 (33) | 9 (23) | <.0001 |
| II | 53 (28) | 41 (28) | 12 (30) | |
| III | 68 (37) | 57 (39) | 11 (28) | |
| IV | 9 (5) | 1 (1) | 8 (20) | |
| Maximum tumor diameter (mm), median (IQR) | 35 (25-55) | 35 (25-53) | 40 (29.5-57.5) | .6131 |
| Histologic grade, No. (%) | ||||
| Well/moderately differentiated | 187 (96) | 147 (96) | 40 (98) | .7565 |
| Poorly differentiated | 1 (1) | 1 (1) | 0 (0) | |
| Othersb | 6 (3) | 5 (3) | 1 (2) | |
- Abbreviations: BMI: body mass index; EEA, EEA™ Circular Stapler with DST Series™ Technology; IQR, interquartile range; Tri-EEA, EEA™ Circular Stapler with Tri-Staple™ Technology.
- a Only for rectal cancer patients.
- b Five neuroendocrine tumor and one endometrial stromal sarcoma.
The intraoperative characteristics and short-term outcomes are summarized in Table 2. Compared with the EEA group, the tri-EEA group had a longer operation time (278 minutes vs 227 minutes, P =.0042), lower prevalence of laparoscopic surgery (0% vs 7%, P =.0270), higher prevalence of side-to-side anastomosis (17% vs 6%, P =.0328), and lower prevalence of 25-mm circular stapler usage (0% vs 64%, P <.0001). There were no significant differences between the two groups in intraoperative characteristics, such as the prevalence of low anterior resection (61% in the EEA group vs 51% in the tri-EEA group, P =.2396) and the use of a transanal tube (86% in the EEA group vs 95% in the tri-EEA group, P =.0897). The two groups had similar short-term outcomes, including the postoperative hospital stay (14 days in the EEA group vs 14 days in the tri-EEA group, P =.4757) and incidence of all postoperative complications (CD grade ≥3) (8% in the EEA group vs 2% in the tri-EEA group, P =.1731). The incidence of AL (CD grade ≥3) was significantly lower in the tri-EEA group than in the EEA group (0.0% vs 5.8%, P =.0362).
| Characteristics |
Total N = 194 |
EEA group N = 153 |
tri-EEA group N = 41 |
P value |
|---|---|---|---|---|
| Duration of operation (min), median (IQR) | 237.5 (191.5-301.75) | 227 (186-298) | 278 (220-341) | .0042 |
| Bleeding (g), median (IQR) | 15 (8-30) | 15 (10- 30) | 15 (5.5-33) | .6967 |
| Intraoperative transfusion, yes, No. (%) | 3 (2) | 2(1) | 1(2) | .6218 |
| Procedure, No. (%) | ||||
| Open | 10 (5) | 10 (7) | 0 (0) | .0270 |
| Laparoscopy | 184 (95) | 143 (93) | 41 (100) | |
| Surgery, No. (%) | ||||
| Anterior resection | 79 (41) | 59 (39) | 20 (49) | .2396 |
| Low anterior resection | 115 (59) | 94 (61) | 21 (51) | |
| Covering stoma, yes, No. (%) | 47 (24) | 34 (22) | 13 (32) | .2181 |
| Type of anastomosis, No. (%) | ||||
| End-to-end/Side-to-end | 178 (92) | 144 (94) | 34 (83) | .0328 |
| Side-to-side | 16 (8) | 9 (6) | 7 (17) | |
| Size of circular stapler (mm), No. (%) | ||||
| 25 | 99 (51) | 98 (64) | 0 (0) | <.0001 |
| 28 | 95 (49) | 55 (36) | 41 (100) | |
| Transanal tube, yes, No. (%) | 171 (88) | 132 (86) | 39 (95) | .0897 |
| Postoperative hospital stay (day), median (IQR) | 14 (11-19) | 14 (11-19) | 14 (11.25-24) | .4757 |
| Postoperative complication (CDC≥3), No. (%) | ||||
| None | 181 (93) | 141 (92) | 40 (98) | .1731 |
| Present | 13 (7) | 12 (8) | 1 (2) | |
| Anastomotic leakage (CDC≥3), No. (%) | ||||
| None | 185 (95) | 144 (94) | 41 (100) | .0362 |
| Present | 9 (5) | 9 (6) | 0 (0) | |
- Abbreviations: CDC, Clavien-Dindo Classification; EEA, EEA™ Circular Stapler with DST Series™ Technology; IQR, interquartile range; Tri-EEA, EEA™ Circular Stapler with Tri-Staple™ Technology.
3.3 Risk factors for anastomotic leakage
The results of the univariate and multivariate analyses are described in Table 3. In univariate analysis, the factors significantly correlated with AL were male sex (P =.0247), low anterior resection (P =.0444), and the use of the EEA (P =.0362). In multivariate analysis adjusted for these factors, the use of the tri-EEA for anastomosis was independently associated with the absence of AL (hazard ratio [HR] [95% confidence interval (CI)]; 0.00 [0.00-0.96], P =.0465), while male sex was independently associated with AL (HR [95% CI]; 6.40 [1.12-120.90], P =.0354).
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex | ||||
| Male vs Female | 6.95 (1.24-130.22) | .0247 | 6.40 (1.12-120.90) | .0354 |
| Age | ||||
| ≤65 year vs >65 years | 0.86 (0.21-3.36) | .8297 | ||
| BMI | ||||
| ≤25 kg/m2 vs >25 kg/m2 | 0.57 (0.14-2.79) | .4543 | ||
| Location | ||||
| High rectum vs middle/low rectum | 0.35 (0.05-1.50) | .1659 | ||
| Pathological Stagea | ||||
| I-II vs III-IV | 0.55 (0.13-2.13) | .3769 | ||
| Maximum tumor diameter | ||||
| ≤50 mm vs >50 mm | 0.40 (0.08-1.86) | .2371 | ||
| Surgery | ||||
| Anterior resection vs low anterior resection | 0.17 (0.01-0.96) | .0444 | 4.68 (0.11-1.24) | .0911 |
| Covering stoma | ||||
| No vs yes | 0.62 (0.16-3.05) | .5281 | ||
| Type of anastomosis | ||||
| Side-to-side vs end-to-end/side-to-end | 0.00 (0.00-5.44) | .2076 | ||
| Size of circular stapler | ||||
| 25 vs 28 | 0.47 (0.10-1.85) | .2869 | ||
| Stapler, No. (%) | ||||
| Tri-EEA vs EEA | 0.00 (0.00-0.86) | .0362 | 0.00 (0.00-0.96) | .0465 |
- Abbreviations: BMI, body mass index; CI, confidence interval; EEA, EEA™ Circular Stapler with DST Series™ Technology; HR, hazard ratio; Tri-EEA, EEA™ Circular Stapler with Tri-Staple™ Technology.
- a Only for rectal cancer.
3.4 Details of patients who developed anastomotic leakage
The details of the nine patients with AL are summarized in Table 4. Eight of nine patients were male, and eight had undergone low anterior resection. Three patients had a covering stoma after low anterior resection. All nine patients had a transanal tube. The anastomosis had been performed in an end-to-end fashion with the EEA in all nine patients. The leak was located at the intersection site in six patients and a site other than the intersection in three patients.
| No. | Sex | Age | BMI | Location | Preoperative chemotherapy |
Pathological Stage |
Surgery |
Covering stoma |
Type of anastomosis |
Transanal tube |
EEA/tri-EEA | Site of leakage |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 24 | 14.5 | Middle rectum | None | IIIB | LAR | None | End-to-end | Present | EEA | Not intersection |
| 2 | M | 87 | 20.5 | Middle rectum | None | IIIB | LAR | None | End-to-end | Present | EEA | Intersection |
| 3 | M | 64 | 29.0 | Middle rectum | None | IIIA | LAR | Present | End-to-end | Present | EEA | Intersection |
| 4 | M | 62 | 21.6 | Upper rectum | None | IIIB | LAR | None | End-to-end | Present | EEA | Intersection |
| 5 | M | 81 | 24.4 | Middle rectum | None | II | LAR | Present | End-to-end | Present | EEA | Intersection |
| 6 | M | 61 | 28.5 | Middle rectum | None | II | LAR | None | End-to-end | Present | EEA | Intersection |
| 7 | M | 75 | 20.9 | Lower rectum | None | I | LAR | None | End-to-end | Present | EEA | Not intersection |
| 8 | M | 67 | 27.1 | Middle rectum | None | II | LAR | Present | End-to-end | Present | EEA | Not intersection |
| 9 | M | 69 | 19.6 | Upper rectum | None | IIIA | AR | None | End-to-end | Present | EEA | Intersection |
- Abbreviations: AR, anterior resection; BMI, body mass index; EEA, EEA™ Circular Stapler with DST Series™ Technology; F, female; LAR, low anterior resection; M, male; Tri-EEA, EEA™ Circular Stapler with Tri-Staple™ Technology.
4 DISCUSSION
In this porcine model study and the retrospective clinical cohort analysis, we evaluated the impact of the tri-EEA on the anastomotic strength compared with the previously used EEA. In the porcine model, the anastomoses created using the tri-EEA had significantly higher bursting pressure than those created using the EEA. Furthermore, in the clinical cohort, the patients in whom the tri-EEA was used had a significantly lower incidence of AL than those in whom the EEA was used. Use of the tri-EEA was independently associated with the absence of AL. The present study is the first to demonstrate that triple-layered anastomosis was superior to double-layered anastomosis in patients who underwent rectal resection for rectal malignancies.
Previous studies have shown that the intrarectal pressure measured using high-resolution manometry is 80 mm Hg during simulated defecation and up to 200 mm Hg during squeezing.20, 21 In addition, the peak intrarectal pressure is higher in males than in females, which might contribute to male sex being a reported risk factor for AL.11, 21, 22 Although these intrarectal pressures are much higher than the anastomotic bursting pressure in our porcine model, the anastomotic bursting pressure in patients who undergo low anterior resection reportedly becomes much higher at 3-6 months after surgery.23 Because high intrarectal pressure during defecation can lead to AL, we usually advise patients to maintain soft stool and avoid excess force during defecation for at least 1-2 months after surgery. This gap between the intrarectal pressure and the anastomotic bursting pressure is effectively bridged by the placement of a transanal tube and reinforcing sutures, especially in patients with risk factors such as a short distance from the anastomosis to the anus, male sex, and advanced tumor stage.24, 25 Our study suggests that the higher anastomotic bursting pressure resulting from the use of the tri-EEA may also be effective in preventing AL.
Other than anastomotic strength and intrarectal pressure, it has been reported that the blood flow and tension at the anastomotic site are important factors related to AL.26-29 A recently proposed technology with which to evaluate the blood flow is indocyanine green fluorescence-guided visualization, and the clinical significance of this method has been reported.29, 30 Some studies have shown that high ligation of the inferior mesenteric artery is associated with a higher risk of AL, although this finding is controversial.31, 32 We do not routinely use indocyanine green fluorescence-guided visualization, and we clip the inferior mesenteric artery at the root, unless the patient has severe arteriosclerosis on CT, because low ligation sometimes leads to tension at the anastomotic site. Selective mobilization of the splenic flexure is an established method for reducing the tension at the anastomotic site.26, 27 We usually perform splenic flexure mobilization when there seems to be tension at the anastomotic site. As a result, our overall incidence of AL was 4.6% (9/194), which is lower than that reported in previous studies with large patient cohorts.9-12 However, some previous single-institutional retrospective studies have reported very low incidences of AL22, 33; these institutions would have undertaken their own preventative measures to achieve these low incidences. Because AL is caused by multiple factors, every conceivable precautionary measure should be undertaken to reduce the incidence of AL.
End-to-end anastomosis (using the DST) is often used for anastomosis after rectal resection. Endoscopic evaluation of patients with AL after anastomosis using the DST has shown that AL often occurs at the intersection of the anastomosis lines of the circular stapler and linear stapler.14 Our porcine model study also showed that five of six anastomoses created with the EEA were ruptured at the intersection. In our clinical cohort study, the intersection was ruptured in six of nine patients with AL, and the EEA had been used in all nine of these patients. This finding indicates the presence of a structural weakness at the intersection of anastomoses created with the EEA via the DST. In contrast, less than half of the anastomoses created with the tri-EEA in our porcine model were ruptured at the intersection. Although the difference in the incidence of rupture at the intersection between the EEA and tri-EEA groups was not significant, our results might suggest that the tri-EEA reduces the vulnerability of the intersection. In our porcine model, the anastomoses created with the tri-EEA had a significantly higher bursting pressure than those created with the EEA, even in side-to-side anastomoses that have no intersection. This implies that the use of triple-layered stapler leads to higher bursting pressure at the circular anastomosis line than the use of the double-layered stapler. Side-to-side anastomosis reportedly produces a superior bursting pressure than that produced by end-to-end anastomosis.18 Although it is possible that the use of the side-to-side anastomosis technique affected the incidence of AL in the present study, the univariate and multivariate analyses did not prove that side-to-side anastomosis had a significant impact on the development of AL. Finally, we observed no AL in any patients in the tri-EEA group. This suggests that AL after colorectal anastomosis with the tri-EEA might be rare in appropriately managed cases.
The operation time was longer in the tri-EEA than in the EEA group. However, in the multivariate analysis adjusted for the factors significantly correlated with the operation time in the univariate analysis, a longer operation time (>300 minutes) was significantly associated with the tumor location (Rb vs RS/Ra; HR, 2.38; 95% CI, 1.01-5.67; P =.0498), but not male sex (P =.2316) or the device used for the anastomosis (P =.4050) (data not shown). This analysis showed no direct relationship between the device used and the operation time.
Our study has some limitations. First, the animal experiments on anastomosis strength were not intracorporeal, and were performed using the colon and small intestine of pigs. We chose to perform extracorporeal experiment so not to be influenced by other factors than the devise (the tri-EEA or the EEA), such as the preparation status, the blood flow, or the wall thickness of the intestine. Regarding the wall thickness, the rectum usually lacks serosa and has thick muscle layers. Therefore, our animal model did not specifically reflect the actual situation in clinical practice. However, the aim of our animal study was to compare the strength of anastomoses created using the tri-EEA vs the EEA, and we successfully proved the superiority of anastomoses created using the tri-EEA. Second, the sample size was relatively small. Although our multicenter study analyzed about 200 patients with rectal cancer, only nine patients had AL. Third, our study is subject to the selection bias inherent in observational retrospective studies. Neoadjuvant chemoradiotherapy is used to reduce the incidence of local recurrence, but it may cause tissue edema, fibrosis, and even necrosis.34 There was a meta-analysis examining the influence of the neoadjuvant chemoradiotherapy to AL, and the authors concluded that neoadjuvant radiotherapy did not increase the incidence of AL.35 However, the P value was marginal (Odds ratio = 1.24, 95%CI; 0.97-1.58, P =.08), and we thought it was still controversial. So, we excluded the patients who underwent neoadjuvant chemoradiotherapy. The impact of the tri-EEA on the anastomosis strength in patients who undergo neoadjuvant chemoradiation therapy warrants further investigation. As shown in Tables 1 and 2, the tri-EEA group had higher prevalence of more advanced rectal cancer, laparoscopic surgery, side-to-side anastomosis, and use of the 28-mm circular stapler. Although advanced tumor stage is reportedly a risk factor for AL,11 the tri-EEA group had a lower incidence of AL. In this study, nine patients had stage IV rectal cancer (Table 1). All of these patients underwent total mesorectal excision with D3 lymph node dissection, and we do not believe that it affected the outcome. Because only 10 patients underwent open surgery, and because all patients who had AL underwent laparoscopic surgery, we could not statistically analyze the impact of laparoscopic surgery on our results. The incidence of AL was associated with the use of the tri-EEA, even when open surgery was excluded (0.0% in the tri-EEA group vs 6.3% in the EEA group, P =.0307). We used the tri-EEA with a circular stapler size of 28 mm, while the EEA was used with 25-, 28-, and 31-mm circular staplers; therefore, the size of the circular stapler was 28 mm in all patients in the tri-EEA group. None of these factors were risk factors for AL in our univariate and multivariate analyses. Further prospective studies are necessary to confirm the clinical significance of anastomosis using the tri-EEA.
5 CONCLUSIONS
This animal model and multicenter retrospective study revealed that anastomoses created with the tri-EEA had higher bursting pressure than those created with the EEA, and that anastomoses created with the tri-EEA were associated with a lower incidence of AL. Our study indicated that the use of the tri-EEA leads to safer rectal surgery, suggesting that the indications for covering stomas might need to be reevaluated.
AUTHOR CONTRIBUTIONS
Each person listed as an author or coauthor meets all four criteria listed in the Author Guideline of Annals of Gastroenterological Surgery.
DISCLOSURE
Conflict of Interest: The authors declare no conflicts of interest for this article.
The protocol for this research project has been approved by a suitably constituted ethics committee of each institution (committee of Kyushu University, Approval No. 2019-209; committee of National Hospital Organization Kyushu Cancer Center, Approval No. 2019-67), and it conforms to the provisions of the Declaration of Helsinki. Informed consent was obtained from each patient.
Our experimental porcine model was established by purchasing commercially available small intestine and colon specimens; it was not performed as a live animal experiment.




