The uptake of dissolved glucose by juvenile green-lipped mussels (Perna canaliculus)
Abstract
A major expense in the operation of bivalve nurseries is the culture of high-quality live microalgae feeds, and efforts to develop effective alternative feeds have had limited success. Juvenile bivalves are known to be able to absorb dissolved nutrients, but this ability has received minimal attention as a route for supplementary feeding. This study assessed the capacity of juvenile green-lipped mussels (GLMs) (Perna canaliculus) to uptake and assimilate dissolved glucose at five experimental concentrations (i.e. 10 µg mL−1, 100 µg mL−1, 1 mg mL−1, 10 mg mL−1 and 30 mg mL−1) as a supplement to cultured microalgae. Growth and survival of the mussels were measured over 3 weeks. Although all glucose concentrations improved the performance of mussel spat compared to the control, the best performing was a concentration of 1 mg mL−1 of dissolved glucose which enhanced daily spat growth 2.7 times that of the control live microalgal diet without glucose. Survival was high (i.e. >95%) for all treatments except for the highest experimental concentration of glucose, for which a concentration of 30 mg mL−1 resulted in mean mortality of 24%. Mussel spat supplemented with dissolved glucose accumulated up to 30% greater lipid and improved the carbohydrate content per mg g−1 of ash-free dry weight as much as 3.5 times compared to those in the control treatment, indicating that they were in greater nutritional condition. This demonstration that dissolved glucose can be used to fuel growth by GLM spat at concentrations as low as 10 µg mL−1 suggests that other soluble nutrients may also act as a supplemental feed for cultured juvenile molluscs. Dissolved nutrients have the potential to improve the performance of spat in nursery systems while simultaneously reducing the reliance of cultured microalgae as a sole feed input.
1 INTRODUCTION
In the last two decades, global aquaculture production of marine molluscs has grown at an annual rate of 3.5%, and molluscs now represent the second largest animal species group farmed in the ocean (Tacon, 2020; Wijsman et al., 2019). Mussel aquaculture has contributed to this growth significantly, with the annual production of mussels tripling over the same period from 1 million t to over 3 million t (Food and Agriculture Organization [FAO], 2024). However, the increase in mussel production over the last 20 years has occurred primarily in China, with production either stagnating or decreasing in many other major mussel-producing nations, such as the Netherlands, France, New Zealand and Chile (Reguera et al., 2014; FAO, 2019; Avdelas et al., 2021; Kamermans & Capelle, 2019; Skelton & Jeffs, 2022). One of the major reasons for this stagnation is the inefficiencies in the early stages of production, where many juveniles or spat are lost from production (Kamermans & Capelle, 2019; Peteiro et al., 2007; Skelton & Jeffs, 2021).
In New Zealand, the endemic green-lipped mussel (GLM) (Perna canaliculus) or Greenshell when produced in aquaculture forms the basis of the country's most important aquaculture industry, with over 90,000 t of production per annum, worth more than NZ$420 m (Aquaculture New Zealand, 2023; Stenton-Dozey et al., 2021). This level of production currently uses over 350 billion seed mussels a year with the conversion of spat to harvestable mussels typically less than 1%, largely due to extremely high losses of small spat from coastal farms (Jeffs et al., 2018; Skelton & Jeffs, 2022). GLMs are typically seeded out to coastal farms at small sizes, that is, ∼1.0 mm in shell length (SL) due to the rapidly increasing cost of supplying sufficient microalgae to adequately feed spat in nursery systems to a larger size.
To date, substantial research has been targeted at developing alternative feeds capable of replacing live microalgae in bivalve nursery culture (Campanati et al., 2022; Gui et al., 2016b; Nevejan et al., 2007; Skelton et al., 2021). Research has focused on microparticulate (Langdon, 1983), microbound and encapsulated diets (Campanati et al., 2022; Skelton et al., 2021; Willer & Aldridge, 2017), as well as yeast and bacteria (Coutteau et al., 1994; Nell et al., 1994). These alternative feeds have shown some promise in experimental investigations as partial replacements to live microalgae. However, their nutritional value and digestibility are still inferior to cultured microalgae, meaning that commercial operators still rely predominantly on live microalgae (Brown & Blackburn, 2013; Knauer & Southgate, 1999; South et al., 2022).
Consequently, the costs associated with rearing bivalves in nurseries represent a bottleneck preventing New Zealand's GLM industry from transitioning to including an extended nursery phase. Transitioning to a supply model that includes an extended nursery period could help reduce high losses of spat associated with seeding farms with spat at a small and vulnerable size.
An approach to supplementary feeding of juvenile mussels that has not been experimentally tested is the use of dissolved nutrients delivered in conjunction with live microalgae. Bivalves are capable of absorbing dissolved nutrients, such as amino acids, carbohydrates, fatty acids and vitamins during larval and adult life stages (Bunde & Fried, 1978; Fitt et al., 1984; Gorham, 1988; Manahan, 1983; Nell & Dunkley, 1984; Nell & Wisely, 1983; Nell et al., 1983). However, it is unknown if dissolved nutrients can improve the performance of mussel spat when used as a supplementary feed with live microalgae. If soluble nutrients can be absorbed and assimilated by mussel spat, there is the potential for this approach to be used for advancing the nursery culture of bivalves.
Supplying GLM spat with supplementary dissolved nutrients presents a potential avenue for reducing the costs of nursery culture. Carbohydrates play a crucial role in bivalve nutrition and are mostly highly soluble in seawater. Dietary sugars are essential for bivalves, and they are a ready source of energy, being used as metabolic intermediates for synthesising other important compounds, with the potential to reduce protein and lipid catabolism and improve growth (Whyte et al., 1989; Zhu et al., 2024). Increasing the proportion of dietary carbohydrates through supplementary dissolved nutrients may be an economical way to meet the energy demands of growing GLM spat while reducing the demand of expensive microalgal feed. Among the different types of carbohydrates found in the live microalgae typically fed to spat, glucose constitutes the primary type of sugar and has a much shorter metabolic pathway in bivalves when compared to other sugars such as mannose, xylose and arabinose (Brown, 1991; Fearman et al., 2009).
The aim of this study was to determine if GLM spat can absorb dissolved glucose from seawater and to assess the potential for dissolved glucose to enhance the performance of mussel spat by supplementing a diet of live microalgae.
2 MATERIALS AND METHODS
2.1 Spat source
Hatchery-reared GLM spat (mean SL of 2 mm ± 0.02 SE) were sourced from a commercial shellfish hatchery (SPATnz Ltd). In the hatchery, the spat were first settled onto fibrous coconut fibre rope, before being separated from the rope, loaded into cool, insulated containers and air-freighted to the laboratory in Auckland. After arriving at the laboratory, the spat were acclimated together in a single tank for 3 days while being fed a mixed microalgal diet consisting of 3 × 105 cells spat−1 day−1 of Diacronema lutheri, Chaetoceros muelleri and Tetraselmis suecica at a ratio of 2:1:1 determined by flow cytometry (Muse Cell Analyzer) cell count performed daily.
2.2 Microalgae culture
Axenic cultures of microalgae were maintained in 1 L Schott bottles of sterilised seawater using Guillard's F/2 media (Guillard & Ryther, 1962) and standard culture protocols (Anderson, 2005), with temperature maintained at 22–23°C and illumination provided by 29 W LED, and they were harvested for feeding to spat, whereas they were in their exponential growth phase (i.e. Helm et al., 2004).
2.3 Glucose uptake experimental design
The rate at which GLM spat absorb dissolved glucose from seawater was determined by comparing the decline in glucose concentrations between two triplicate sets of microplate wells containing spat (treatment) or without spat (control). The control treatment was used to quantify any natural decline in glucose in seawater without mussel spat present, whereas the three replicates in the treatment with spat contained 1 g ww (∼285 individuals) of GLM spat.
The experiment was conducted in 10 mL microplate wells containing 8 mL of filtered and sterilised seawater (UV, 5 µm) with dissolved glucose at a concentration of 1 mg mL−1, corresponding to a quantity of 8 mg of dissolved glucose per replicate. Samples of 200 μL of seawater were taken from every replicate well for glucose analysis at the outset (0 min) and then at 30 min intervals for the 4 h duration of the experiment. Samples were immediately frozen at −80°C for subsequent glucose analysis. After 4 h, the individuals from the spat treatment were rinsed in deionised water to remove external saltwater, drained onto laboratory tissue, placed into 50 mL Eppendorf tubes and frozen at −80°C for subsequent biochemical analysis.
2.4 Feeding experiment design
Each experimental tank was made from a 3 L polyethylene bottle with its base removed. The experimental tanks were inverted on a wooden rack, and to each bottle, 2 L of filtered and sterilised seawater (UV, 5 µm) was added. Each experimental tank was aerated by an air stone inserted into the neck of the bottle and contained a small piece of plastic mesh that floated in suspension, providing a substrate for spat to attach to.
To ensure that feeding quantities were calculated accurately, the number of spat per gram was estimated by counting 10 randomly selected 1 g subsamples of spat. After this, precisely 5 g wet weight of GLM spat (∼2300 individuals) was weighed and added to each tank.
Six glucose concentrations were used, each with three replicates; control – 0 µg mL−1, G10 – 10 µg mL−1, G20 – 100 µ mL−1, G30 – 1 mg mL−1, G40 – 10 mg mL−1, G50 – 30 mg mL−1. The microalgae component of diets consisted of D. lutheri, C. muelleri and T. suecica, and each glucose treatment received the mixed microalgae diet at a rate of 3 × 105 cells mussel−1 day−1 at a ratio of 2:1:1 by cell count daily.
The experiment was conducted over 20 days, and at the outset and every 2 days, 100 randomly selected spat from each tank were placed on a plastic tray with a reference grid and photographed with a digital camera. The SL of spat was subsequently determined using image analyses (ImageJ, NIH).
To prevent bacterial contamination in tanks, spat were only exposed to dissolved glucose for 2 h daily. After 2 h of exposure to the dissolved nutrient, a daily seawater change was conducted by draining the bottles through a 250 µm mesh to retain spat. Once the tanks had been cleaned, the spat were rinsed with clean seawater, then returned to the tanks with fresh seawater, fed their microalgae ration and left to consume the microalgae for the following 22 h.
2.5 Growth and survival
At the end of the feeding experiment, a subsample of 100 randomly selected spat from each replicate tank were inspected under a dissecting microscope to determine the number of alive and dead spat to provide a mean mortality (i.e. alive minus dead) for each experimental tank. Any empty shells from deceased spat were manually removed from samples, and the remaining live spat were photographed for digital size measurement. Spat were then washed in deionised water, drained onto laboratory tissue, placed into 50 mL Eppendorf tubes and frozen at −80°C for subsequent biochemical analyses.
2.6 Ash Free Dry Weight
Once spat were removed from the -80 °C freezer they were freeze dried for 24 h and then weighed to establish their dry weight. Three replicate samples of 100 mg of freeze dried spat from each replicate tank were then used to obtain their ash free dry weight (AFDW). The samples were burnt in a muffle furnace (Nabertherm LT15/11 B410, Germany) at 450 °C for 4 h. The residual ash was then weighed to calculate the proportion of AFDW in relation to total dry weight.
2.7 Calorific content
Three 100 mg subsamples of freeze-dried spat from each replicate tank were used for measuring the calorific content of the spat using a semimicro bomb calorimeter (Parr 6725, Parr Instrument Company). The calorific value per gram of dry mass of spat from each replicate of each treatment was then converted into calorific content per gram of organic tissue using the proportion of AFDW to dry tissue mass of the corresponding spat sample.
2.8 Glucose analysis in seawater
The glucose concentration in seawater solution was determined using the Megazyme d-Glucose Assay Kit (GOPOD Format). The GOPOD reagent buffer was initially prepared by mixing the reagent buffer, p-hydroxybenzoic acid and sodium azide (0.09% w/v) with 1 L of distilled water. Following this, the GOPOD reagent enzymes (glucose oxidase plus peroxides and 4-aminoantipyrine) were dissolved in 20 mL of the GOPOD reagent buffer; this resulting solution served as the glucose determination reagent.
2.9 Protein, lipid and carbohydrate content
To determine the protein content of spat, triplicate subsamples of 50 mg of lyophilised spat from each tank were incubated in 6 mL of 0.1 M NaOH for 16 h at 50°C and then centrifuged at 10,000 rpm at 4°C for 10 min. The protein in each subsample was then quantified by BCA (bicinchoninic acid) method (Smith et al., 1985) using a micro BCA protein assay kit (Thermo Fisher Scientific) and read against bovine serum albumin standard at 562 nm.
The lipid content of the spat was determined for triplicate subsamples, each of 300 mg lyophilised spat for each tank with a modified methanol–chloroform solvent extraction method of Bligh and Dyer (1959). The total lipid extracted from each sample was quantified following the removal of the residual solvent using a stream of nitrogen gas following the method of Wang et al. (2013).
A subsample of 100 mg of spat from each replicate tank was used to determine the total carbohydrate content of spat. The freeze-dried spat were ground in liquid nitrogen and homogenised in 1 mL of distilled water using a homogeniser (Polytron PT 1200). The solutions were centrifuged at 10,000 rpm at 4°C for 10 min. The supernatant was transferred to a 1.5 mL Eppendorf tube and stored at −20°C prior to analysis. The total carbohydrate content of the solutions was determined using the phenol sulphuric acid reagent method (Dubois et al., 1956) and was read against a d-glucose standard curve using a Multiskan SkyHigh Microplate Spectrophotometer at 490 nm (Masuko et al., 2005).
2.10 Statistical analyses
A two-way ANOVA was used to compare the mean uptake of dissolved glucose between treatments and among sampling events in the uptake experiment. Where significant interaction or main effects were identified, a pairwise Tukey HSD post hoc test was used to identify differences between individual means.
The mean SL of spat from each treatment in the feeding experiment was calculated for mussel spat subsampled from each experimental tank at 0 day and subsequently every 2 days throughout the experiment. A linear mixed-effects model was fitted to the spat SL measurements with treatment as a fixed effect and with the replicate tank as a random effect and the control as the reference treatment. The regression slope from the mixed-effects model was used to determine the relative 20 days growth of spat for each treatment after 20 days of feeding.
A separate ANOVA was used to compare each biochemical component (i.e. AFDW, lipid, protein, carbohydrate and calorific content) and mortality between treatments in the feeding experiment. All data were checked for normality and homogeneity of variances, and if data defied these parametric assumptions, they were log transformed to ensure they conformed. The percentage mortality data were transformed using an arcsine transformation prior to analysis in the ANOVA. Where significant main effects were identified by an ANOVA, post hoc pairwise comparisons were used to identify differences among individual means.
3 RESULTS
3.1 Glucose uptake experiment
The concentration of dissolved glucose at the beginning of the experiment (i.e. T0) was 1.0 mg mL−1 (±0.01 SE) in the control and also 1.0 mg mL−1 (±0.01 SE) in the spat treatment (p = 0.99) (Figure 1). At the end of the experiment, the glucose concentration had not changed in the control (p = 0.95) but had decreased to 0.91 mg mL−1 (±0.008 SE) in the spat treatment, representing an 8% decline from the starting concentration (p < 0.05). There was an overall significant interaction between the time of sampling and treatment relating to the decrease in glucose concentration over the 4 h period in the spat treatment versus no decrease in the control (F(1, 8) = 37.8, p = 0.02). After 30 min, the mean concentration of glucose in the spat treatment had decreased to 0.07 mg mL−1 (p < 0.05) and continued to decline steadily over the 4 h duration of the experiment. The overall decrease in glucose concentration was estimated to amount to 2.91 µg of glucose uptake per mussel.
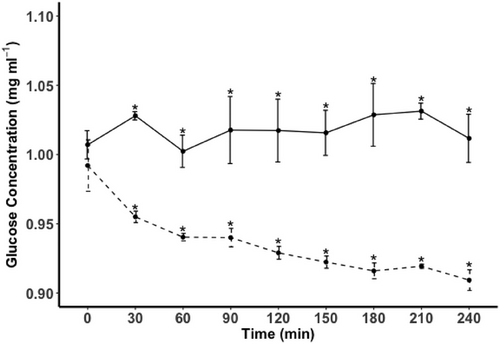
3.2 Ash-free dry weight
The mean percentage ash-free dry weight of the mussels in the spat treatment was 24.3% (±0.9 SE). This enabled an estimate of a mean glucose uptake in the mussel spat of 2.7 mg (±1.0 SE) g−1 of dry organic tissue−1 mass over the 4 h duration of the experiment.
3.3 Feeding experiment
3.3.1 General observations
Spat in the G40 and G50 (i.e. 10 and 30 mg mL−1) treatments excreted significant amounts of mucous within this 2 h exposure to glucose.
3.3.2 Spat growth
There were overall differences in the growth of spat among the six treatments (F(5, 19,786) = 3.3, p = 0.0056). G30 had the highest daily growth, at 25.5 μm d−1 and the control the lowest, with 9.5 μm d−1. The daily growth of spat from all five glucose treatments was greater than those spat in the control (p < 0.001) (Figure 2).
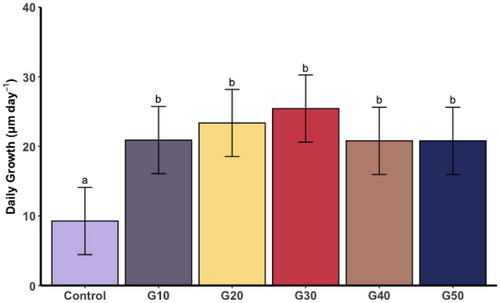
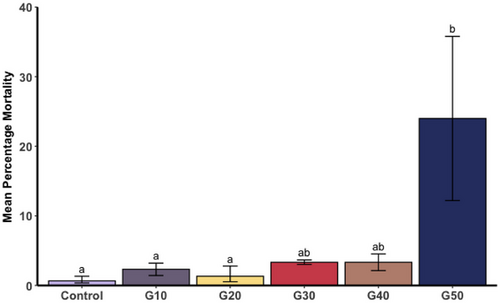
3.3.3 Spat mortality
The mean mortality of mussel spat among the six treatments ranged from 0.67% (±0.7 SE) in the control to 24% (±11.8 SE) in G50 and was different among the treatments (F(5, 12) = 4.9, p = 0.01), with mean mortalities in G50 being higher than the control (p < 0.01), G10 (p = 0.05) and G20 (p = 0.01) (Figure 3).
3.3.4 Proximate analyses
The mean percentage of AFDW of spat ranged from 19.9% (±0.3 SE) in the control to 22.1% (±0.3 SE) in Glucose 4. The mean AFDW of spat was different among treatments (F(5, 47) = 4.28, p = 0.003), with spat in both Glucose 4 and 5 having a higher mean percentage of AFDW than spat in the control (p < 0.01) (Table 1).
| Biochemical parameter | Control | G10 | G20 | G30 | G40 | G50 |
|---|---|---|---|---|---|---|
| Mean percentage AFDW | 19.8 (±0.9)a | 21.3 (±2.0)ab | 21.4 (±0.8)ab | 20.7 (±0.4)ab | 22.1 (±1.2)b | 21.9 (±1.1)b |
| Mean calorific content (calories g−1) | 1420.1 (±202.4) | 1258.5 (±61.9) | 1331.6 (±73.3) | 1321.7 (±239.5) | 1089.9 (±96.4) | 1406.7 (±89.5) |
| Mean lipid content (mg g−1 dry tissue) | 70.3 (±5.1)a | 63.9 (±5.7)a | 65.9 (±10.9)a | 77.8 (±3.8)ab | 83.8 (±11.2)ab | 91.6 (±0.8)b |
| Mean protein content (mg g−1 dry tissue) | 309.5 (±37.2) | 312.5 (±31.2) | 297.4 (±52.2) | 341.0 (±12.5) | 321.6 (±23.5) | 289.2 (±14.2) |
| Mean carbohydrate content (mg g−1 dry tissue) | 25.4 (±2.9)a | 32.2 (±2.0)a | 33.5 (±0.4)a | 30.5 (±2.0)a | 62.6 (±1.4)b | 90.2 (±10.2)c |
- Note: Data were pooled for replicate tanks within each treatment. Means with different letters are different (p < 0.05).
The mean calorific content of spat in the six treatments ranged from 1090 to 1420 calories g−1 of AFDW and did not differ among the six treatments (F(5, 12) = 0.702, p = 0.63) (Table 1).
The mean lipid content of spat in the six treatments ranged from 63 to 91 mg g−1 of AFDW and was different among treatments (F(5, 12) = 6.57, p = 0.004) (Table 1). The mean lipid content of spat from Glucose 5 was greater than for spat from the control, Glucose 1 and Glucose 2.
The mean protein content of spat in the six glucose treatments ranged from 289 to 341 mg g−1 of AFDW with no differences in the mean protein content among the six glucose treatments (F(5, 12) = 0.46, p = 0.46) (Table 1).
The mean carbohydrate content of spat among glucose treatments ranged between 25 and 90 mg g−1 of AFDW and was different among treatments (F(5, 12) = 94.4, p < 0.0001) (Table 1). Spat from Glucose 5 had a mean carbohydrate content of 90 mg g−1 (±10.2 SE) of AFDW, and Glucose 4 had 62.6 mg g−1 (±2.9 SE), which were both higher than the control at 25 mg g−1 (±2.9 SE) (p < 0.001). Additionally, the mean carbohydrate content of spat from Glucose 4 was also higher than that of the control group (p < 0.001).
4 DISCUSSION
4.1 Nutrient uptake
It has been known for some time that bivalves can uptake dissolved molecules, such as amino acids and sugars from seawater using the ctenidia on the gills (Bamford & Gingles, 1974; Jørgensen, 1990; Manahan, 1983; Pequignat, 1973; Welborn & Manahan, 1990). Despite this, the potential for providing dissolved nutrients for supplementing the feeding of bivalves in culture, especially juveniles, has been largely overlooked. The results from the nutrient uptake experiment demonstrate that green-lipped spat steadily take up dissolved glucose from seawater with a concentration of 1 mg mL−1 at a rate of 0.62 mg h−1 g−1 of dry tissue mass over a 4 h period. This is similar to the rate of uptake (i.e. 1.4 mg h−1 g−1 of wet tissue mass over 4 h) of glucose measured in an adult Sydney rock oyster from dissolved glucose at a concentration of 7 µg mL−1 (Nell et al., 1983). The uptake of dissolved glucose from the seawater by the oysters was thought to involve metabolically active mechanisms of uptake, due to the low concentration of the dissolved glucose. However, when dissolved nutrients are present in seawater at higher concentrations, bivalves are thought to utilise passive uptake, that is, diffusion (Bamford & Gingles, 1974; Fankboner & De Burgh, 1978; Nell et al., 1983; Swift et al., 1975; Welborn & Manahan, 1990). Accordingly, it could be inferred that the higher concentration of dissolved glucose used in this experiment (i.e. 1 mg mL−1) facilitated the uptake of the glucose by the mussel spat via passive diffusion (D'Sa & Kim, 2017). However, this would seem inconsistent with the rate of glucose uptake by GLM spat which appeared to be higher in the first 30 min of exposure but thereafter remained consistent over the remainder of the 4 h of exposure, despite the declining concentration of the dissolved glucose. Regardless of the mechanism of uptake, the continuing uptake of dissolved glucose over 4 h suggests that longer periods of exposure would continue to provide supplemental glucose nutrition to GLM spat.
At the concentration of 1 mg mL−1 of dissolved glucose in seawater, the mean uptake of glucose g−1 of mussel spat dry tissue over 4 h (i.e. 2.7 mg) would have contributed a total of 45 J (10.8 cal) of additional energy to the spat (Maclean et al., 2003). The GLM spat in the glucose feeding experiment had a mean total energy content of 1305 cal g−1 of dry tissue, so that the additional 10.8 cal g−1 of dry tissue would equate to around 0.8% of the mean total energy content of the spat. Despite this being a small contribution, bivalves can efficiently utilise carbohydrates for energy, as compared to lipids and proteins (Whyte et al., 1990). Carbohydrates also typically constitute the smallest proximate component in GLM spat, accounting for less than 30 mg g−1 of dry tissue mass (Sim-Smith & Jeffs, 2011; Skelton et al., 2024; Supono et al., 2020, 2022). Therefore, although the uptake of dissolved glucose may only account for a small percentage of total energy, daily contributions from dissolved glucose could contribute significantly to the total carbohydrate and energy supply for GLM spat. Furthermore, carbohydrates typically constitute the smallest biochemical component of microalgae species that are cultured as bivalve feed, constituting as low as 5% of the organic material in some species (Brown et al., 1989). Given the importance of dietary carbohydrates in meeting energy demands and as a metabolic intermediate for the catabolism of lipids and proteins (Whyte et al., 1989), the uptake of dissolved glucose may significantly contribute to the overall energy requirements of GLM spat, particularly if cultured microalgae species are low in sugar content.
4.2 Feeding experiment
The greater daily increase in GLM spat SLs indicated that adding dissolved glucose to mixed microalgae boosted the growth of the spat. The diet with the highest daily growth, G30, recorded growth of 25.5 μm d−1, which was 2.7 times greater than that of spat fed the microalgae control diet. The significant difference in daily growth of spat in the glucose treatments indicates that glucose and, therefore, carbohydrates play a vital role in energy production for GLM spat. It is widely recognised that lipids, particularly neutral lipids, are the principal energy reserves in bivalve larvae (Aarab et al., 2013; Holland & Spencer, 1973; Matias et al., 2011; Millar & Scott, 1967; Pernet et al., 2003; Whyte et al., 1992). However, once larvae settle and become juveniles, it appears that they switch to utilising carbohydrates as primary source of catabolic energy (Albentosa et al., 2007; Enright et al., 1986; Supono et al., 2021; Supono et al., 2022; Whyte et al., 1989), possibly associated with the marked differences in the feeding biology of larvae and juveniles (Gui et al., 2016a). Previous studies also reinforce this, as in starvation experiments, carbohydrates were used as an energy reserve, whereas proteins and lipids were conserved (Sim-Smith & Jeffs, 2011; Supono et al., 2022). This result highlights that carbohydrates are also important in the nutritional requirements in juvenile GLM for supporting growth. It is likely that glucose is easily assimilated into the critic acid cycle, meaning that diets containing higher sugar content allow amino acids derived from protein to be spared for tissue formation, contributing to increased growth (Berg et al., 2007). The presence of dissolved glucose in seawater may have also increased the filtration rates of mussel spat, as has been demonstrated by increased pumping rates of the oyster (Crassostrea virginica) when exposed to dissolved carbohydrates (Collier et al., 1953). Increased filtration would have promoted heightened feeding activity, allowing spat to capture microalgae with higher efficiency, contributing to greater microalgae consumption and higher growth.
The results of this experiment also show that supplying excessive quantities of dissolved glucose to GLM spat can negatively impact their performance. Increasing the concentration of dissolved glucose above 1 mg mL−1 (i.e. G40 and G50) resulted in reduced growth (i.e. lower than G10, G20 and G30) and spat mortality increased markedly for G50. Hence, increasing glucose concentrations above 1 mg mL−1 does not appear to offer any additional benefits for the mussel spat. Further, the discrepancy in spat growth between G40 and G50 relative to the other glucose treatments could also be attributed to the production of excess mucous from the mussels. Bivalves are known to release mucous when the concentrations of suspended matter exceed the capacity for ingestion (Jørgensen, 1990). Mucous is produced by GLM spat as a means of initiating dispersal by drifting on buoyant mucous threads (Buchanan & Babcock, 1997; Jørgensen, 1990; Martel & Chia, 1991), and one of the triggers for this behaviour is undesirable environmental conditions (Sanjayasari & Jeffs, 2019). It is possible that concentrations of glucose above 1 mg mL−1 physiologically stressed spat, initiating the release of large amounts of mucous. The metabolic cost of mucous production in bivalves is significant, accounting for up to 15% of total energetic expenditure (Davies & Hawkins, 1998). Given that mucous excretion is energetically expensive for mussel spat, this may explain the decreased growth in G40 and G50 treatments.
The two highest levels of glucose supplementation (i.e. G40 and G50) led to spat accumulating a higher proportion of organic tissue compared to other glucose treatments. This may be in part due to the carbohydrate content of spat in both G40 and G50 being higher than all other treatments, including the control, suggesting that it was supplied in excess to metabolic needs and enabled spat to accumulate more organic tissue. Despite the difference in carbohydrate content among glucose treatments, the carbohydrate content of spat in all glucose treatments was substantially higher than hatchery-reared GLM spat of the same size documented by Supono et al. (2022), where carbohydrate content accounted for only 18 mg g−1 of ash-free dry weight. This suggests that supplying dissolved glucose to GLM spat can not only improve the growth of spat but can also improve the amount of stored carbohydrates over a period as short as 3 weeks. Given that GLMs are known to use stored glycogen as a metabolic reserve (Supono et al., 2021), those spat with higher carbohydrate content prior to seeding may also experience improved future growth, particularly during periods of low food availability. Therefore, depending on the specific aims of nursery culture, it may be valuable to supply concentrations of glucose that support maximum growth initially and then increase the concentration prior to seeding out to boost the amount of stored glycogen in spat.
Protein was the dominant biochemical component of spat across the six glucose treatments, relative to lipids and carbohydrates. However, there was no difference in the total protein content of GLM spat among the glucose treatments, indicating that the supplementary dissolved glucose did not increase in protein synthesis in mussel spat. In other studies that have reported the protein content of GLM mussel spat of this size, protein content has been reported to range anywhere from 100 to 400 mg g−1 of AFDW (Skelton et al., 2024; Supono et al., 2022). However, hatchery-reared GLM spat which are in superior nutritional condition compared to those which have been wild-caught typically contain a total protein content at the higher end of this range (Skelton et al., 2024). The proportional protein content of spat from this experiment was lower than measured previously in hatchery-reared GLM spat of a comparable size (Supono et al., 2022), indicating that supplementary glucose did not have any sparing effect on protein and that it is likely the microalgal diet was deficient in protein, which may have constrained the observed growth of the spat.
The mean lipid content of spat tended to increase with the increasing glucose concentration, but only the lipid content of spat in G50 was significantly higher than all other treatments. This trend may be due to metabolic sparing of lipid, through the alternative use of the supplied glucose, and/or lipogenesis resulting from an excess supply of carbohydrates (Gabbot, 1975). This phenomenon aligns with findings by Zhu et al. (2024), who reported the sparing of protein in juvenile clams (Sinonovacula constricta) due to excess dietary carbohydrates.
Despite the proportionally higher carbohydrate content of the GLM spat at the highest concentrations of glucose supplementation (i.e. G40 and G50), as well as the higher lipid content of spat in G50, there was no difference in the total energy content (calorific content) of GLM spat among all six experimental glucose treatments. The lack of difference in the calorific content of spat in the glucose treatments compared to the control indicates that the assimilated glucose is quickly catabolised to support higher growth. However, given that spat in G40 and G50 contained greater lipid content, it would be expected that spat would exhibit higher overall energy content, as lipids provide more than double the energy per unit mass than proteins and carbohydrates (Krebs, 1964). Consequently, ash-free dry weight appears to be a poor metric for nutritional condition in GLM spat, with an absence of any relationship between these two variables for more than 30 samples of wild-caught and hatchery-reared GLM spat (Skelton et al. (2024). This may be due in part to changes in the dimensions of the shells of spat, (i.e. mainly mineral content) in relation to the dry tissue mass (i.e. organic content) contained within the shells of individuals, a relationship which is not linear, especially for spat around the size range used in the current experiment (Sanjayasari, 2021). Therefore, although spat in all treatments exposed to dissolved glucose had similar AFDW, those spat with significantly greater SL would be expected to have accumulated proportionately greater overall total organic tissue mass and corresponding proximate components.
Investigation into the biochemical composition of mussel spat from this experiment has demonstrated that the delivery of dissolved glucose as a supplementary nutrient to live microalgae can improve the carbohydrate content of hatchery-reared GLM spat over 3 weeks. Hence, this method may be a cost-effective approach for rapidly improving the nutritional condition of wild-caught spat prior to seeding out on coastal farms and potentially improve the retention of these juvenile mussels during early grow-out stages. Between 65% and 80% of the spat used by New Zealand's GLM industry are wild-sourced from Te Oneroa-a-Tōhē (Ninety Mile Beach), and this results in inconsistency in the nutritional/proximate biochemical condition of spat that are supplied to mussel farms across the country (Skelton et al., 2024; Supono et al., 2022). These wild-caught GLM spat are likely to have depleted glycogen stores, as during harvesting and transport spat endure extended periods without food and anaerobic stress (De Zwaan & Wijsman, 1976; Sim-Smith & Jeffs, 2011; Supono et al., 2022). When GLM spat have depleted glycogen stores, their ability to produce byssus threads and remain attached to settlement substrate is impaired (Babarro et al., 2008), and they have a greater propensity to detach from initial settlement substrate (Supono et al., 2021). Improving the carbohydrate content of GLM spat should be one of the key objectives of nursery culture, as greater glycogen stores will make spat more resilient to periods of food scarcity. The results presented suggest that exposing spat to dissolved glucose could provide a convenient conduit for improving the amount of stored energy in spat during land-based nursery culture.
5 CONCLUSIONS
In the early stages of longline aquaculture, both the size and nutritional condition of GLM spat are important factors that impact the magnitude of spat losses (Carton et al., 2007; Delorme et al., 2020; Supono et al., 2021). Our study has demonstrated that supplying dissolved glucose as a supplement to microalgae at concentrations between 10 µg mL−1 and 1 mg mL−1 can be used to provide spat with additional nutrition which is rapidly utilised for supporting growth. These findings can be applied in nursery culture for reducing feeding costs and potentially for recovering nutritionally depleted wild spat prior to seeding out on coastal farms. Moreover, soluble nutrients have typically been overlooked as supplementary feed in bivalve nursery culture, and this finding highlights opportunities to experimentally test other types of dissolved feeds. Future studies could also explore the utilisation of cheaper carbohydrates, like sucrose, to manage the expenses associated with incorporating additional feeds in shellfish nurseries.
AUTHOR CONTRIBUTIONS
Andy Jordan: Conceptualisation; data curation; methodology; project administration; writing—original draft; writing—review and editing. Andrew Jeffs: Conceptualisation; methodology; project administration; resources; validation; writing—review and editing. Brad Skelton: Methodology; validation; writing—review and editing. Maria Mugica: Investigation; methodology; resources.
ACKNOWLEDGEMENTS
We are grateful to SPATnz Ltd for providing the mussel spat for this research. This research was supported by the Marine Farming Association, and the Ministry for Primary Industries through the Sustainable Food and Fibre Futures fund SFFF-23044.
CONFLICT OF INTEREST STATEMENT
The authors have no potential conflicts of interest for this research.
ETHICS STATEMENT
The invertebrate animals used in this study (i.e. juvenile mussels) are exempt from the provisions of New Zealand's Animal Welfare Act 1999 which controls the use of animals in research.
Open Research
DATA AVAILABILITY STATEMENT
The data used in this study will be made available upon request.




