Overcoming the Pitfalls of Cytochrome P450 Immobilization through the Use of Fusogenic Liposomes
Abstract
This work describes a new nanotechnology-based immobilization strategy for cytochrome P450s (CYPs), the major class of drug metabolizing enzymes. Immobilization of CYPs on solid supports provides a significant leap forward compared with soluble enzyme assays by enabling the implementation of through-flow microreactors for, for example, determination of time-dependent inhibition. Immobilization of the complex CYP membrane-protein system is however particularly challenging as the preservation of the authentic enzyme kinetic parameters requires the full complexity of the lipid environment. The developed strategy is based on the spontaneous fusion of biotinylated fusogenic liposomes with lipid bilayers to facilitate the gentle biotinylation of human liver microsomes that incorporate all main natural CYP isoforms. The same process is also feasible for the biotinylation of recombinant CYPs expressed in insect cells, same as any membrane-bound enzymes in principle. As a result, CYPs could be immobilized on streptavidin-functionalized surfaces, both those of commercial magnetic beads and customized microfluidic arrays, so that the enzyme kinetic parameters remain unchanged, unlike in previously reported immobilization approaches that often suffer from restricted substrate diffusion to the enzyme's active site and steric hindrances. The specificity and robustness of the functionalization method of customized microfluidic CYP assays are also carefully examined.
Liposomes are widely utilized as nanocarriers in, for example, gene transfection1 and drug delivery,2 where cellular uptake is mainly governed by endocytosis. Certain, tailored, lipid compositions have been found to enable spontaneous bilayer fusion, the propensity of which is mainly promoted by the negative curvature3 of the monolayer as well as by close contact, small size, and unilamellarity of the liposomes.4 Liposome fusion with cell membranes has been demonstrated using customized fusogenic liposomes (FL) comprising of neutral and positively charged lipids,5 and a variety of cell-labeling approaches have been introduced for functionalization of mammalian cells with, for example, fluorescent6 or biotinylated lipids7 or immune cell activating lipopolysaccharides.8 In this work, we introduce a novel use for FLs, which facilitates a universal approach to immobilization of any membrane proteins on solid surfaces via the well-characterized avidin-biotin chemistry (Figure 1a). Here, we focused on the cytochrome P450 (CYP) system, the complexity of which places special demands on immobilization and has puzzled researchers for decades.9 Mammalian CYPs are complex microsomal enzymatic systems consisting of both CYPs themselves as well as several electron-supplying redox partner enzymes, such as cytochrome P450 reductase,10 embedded in a lipid membrane environment which is also essential for their correct function.11 CYPs constitute the majority of drug detoxification pathways,12 and drug–drug interactions mediated by CYPs are thus the primary target of preclinical drug characterization. Human liver microsomes (HLM) are the preferred in vitro matrix, because they show CYP activities much higher than those of intact hepatocytes13 and thus allow for high-throughput drug screening in vitro. By making use of the spontaneous fusion of customized biotinylated FL (b-FL) with subcellular HLM fractions, we were able to biotinylate the HLMs in a natural and gentle manner and thus preserve the authentic enzyme kinetic functions of the membrane-bound CYPs upon immobilization of the biotinylated HLMs on streptavidin-functionalized surfaces, both those of commercial microbeads and of customized microfluidic pillar arrays.
CYP immobilization on solid support structures has raised considerable interest, since it would facilitate certain mechanistic and relative activity assays,14 such as time-dependent inhibition under continuous flow conditions, as well as more straightforward purification of the reaction products for both production and analytical purposes.15 The ability to manipulate substrate and inhibitor concentrations using flow chemistry additionally speeds up the determination of enzyme/inhibitor kinetics.16 When further combined with the integration and parallelism possibilities of the emerging microarray technology, immobilized enzyme reactors (IMERs) would offer a compelling tool for high-throughput drug metabolism assays.17 However, the commonly applied enzyme immobilization strategies tend to result in diffusion-limited mass transfer (e.g., physical entrapment of enzymes/microsomes18) or steric hindrance due to uncontrolled enzyme orientation (e.g., nonselective covalent bonding via amino acid residues19), which may result in flawed enzyme kinetic determinations and thus impair the in vitro–in vivo correlation.20 Generally, covalent immobilization may also alter the protein surface charge and thus reduce the enzyme activity and/or stability.21 In case of CYPs, this necessitates careful optimization of linkers and other “key molecules” that protect the enzymes' active sites during immobilization.[14]
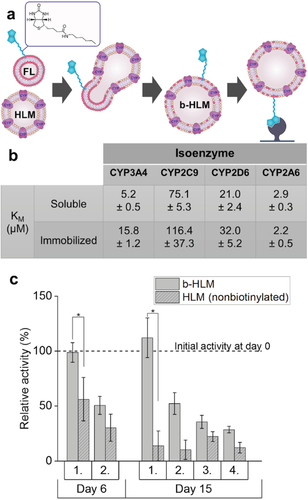
The b-FL composition used in this study was modified from previous works14 and contained both a (spacer-)biotinylated lipid (biotin-cap-1,2-dioleoyl-sn-glycero-3-phophoethanolamine (DOPE), 0.1 m%) and a fluorescent labeled lipid (Lissamine Rhodamine B-DOPE, 0.05 m%) to facilitate not only immobilization of the biotinylated-HLM (b-HLM) but also monitoring of the immobilization efficiency based on fluorescence. Prior to fusion with HLMs (37 °C, 15 min), multilamellar liposomes of the given composition and average hydrodynamic radius of ≈493 ± 87 nm (Figure S1a, Supporting Information) were prepared and extruded through a 100 nm membrane to yield unilamellar b-FLs with an average hydrodynamic radius of 220 ± 25 nm (n = 4 batches) (Figure S1b, Supporting Information). Upon mixing, the opposite net charges of HLM (−44.3 ± 4.9 mV, Figure S2, Supporting Information) and b-FL (69.3 ± 1.8 mV) promoted close contact via electrostatic interactions, facilitating membrane fusion. Successful fusion was confirmed spectroscopically (Figure S3, Supporting Information) according to the protocol previously described by Hoekstra et al.22 As expected, the biotinylation process did not significantly affect the activity of soluble CYP (Figures S4 and S5, Supporting Information), since the membrane fusion is a gentle process and the biotin label is small and locates in the lipid membrane considerably far from the enzyme's active site. Also upon immobilization on streptavidin-functionalized magnetic beads, the enzyme affinities (KM) of four main CYP isoforms were similar to those of the soluble enzymes (Figure 1b and Figure S6, Supporting Information) and the enzyme activities of the immobilized enzymes were preserved over at least 15 d (Figure 1c). Apart from Luciferin-IPA (CYP3A4), the metabolism of the model substrates used followed Michaelis–Menten kinetics in both soluble and immobilized enzyme reactions, as expected. Instead, similar to prior observations made by other CYP3A4 substrates,23 Luciferin-IPA metabolism did not obey the Michaelis–Menten kinetics, but followed a sigmoidal trend in both soluble and immobilized enzyme reactions. These results confirmed that by embedding the biotin in the lipid membrane for anchoring the HLMs on avidin-functionalized surfaces leaves the CYPs' active sites accessible better than by covalent bonding of the CYP enzyme itself (as in, e.g., ref. [19]). Our approach also allows unhampered mass transfer to and from the enzyme's active site (unlike in, e.g., ref. [19]) providing clear advantages over other previously reported immobilization approaches based on enzyme entrapment or covalent bonding. Moreover, no significant loss of CYP activity was observed with immobilized b-HLMs even after two weeks of storing at 4 °C, whereas negative controls prepared by solubilizing nonbiotinylated HLMs on lipid-coated magnetic beads lost half of their activity already in 6 d (Figure 1c). Upon repeated use of the same batch of beads, the CYP activity was typically halved in all cases (Figure 1c), which likely resulted from incomplete recovery of the beads in subsequent incubations. Similar activity loss of CYPs immobilized on magnetic particles was also reported by Schejbal et al.[19]
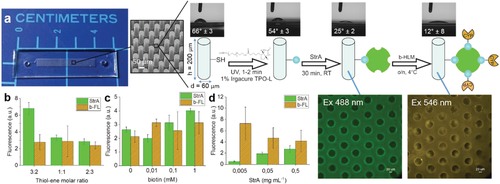
To address the limitations related to handling and packing of magnetic beads, we further developed the concept toward implementation of microfluidic through-flow IMERs. The microfluidic IMERs were fabricated using a custom composition of thiol–enes, which are an emerging class of polymers facilitating low-cost microfabrication via noncleanroom replica molding (Figure S7, Supporting Information) based on UV-induced thiol–ene click chemistry.24 By mixing the thiol or allyl monomers in off-stoichiometric ratios, the thiol–ene surfaces can be readily (bio)functionalized to incorporate excess of either free thiol or allyl functional groups.25 In this work, thiol-rich surfaces were prepared and biotinylated with biotin-PEG4-alkyne via yet another UV-induced thiol–yne reaction, followed by functionalization with fluorescent StrA (ex 488 nm) and eventually with b-HLM. As a preliminary characterization, we determined the water contact angles at each step of the functionalization protocol and observed that the functionalization resulted in clear changes in the surface wettability (Figure 2a). To maximize the surface area, and thus, the amount of immobilized CYP, we implemented a dense micropillar array26 at the reaction zone (Figure 2a). Compared with porous polymer monolith packings, commonly used for enzyme immobilization in capillaries,27 the micropillar arrays are less prone to clogging and reproducibility issues, and thus provide a convenient approach to implementation of well-ordered IMERs by microfabrication means. The robustness of the functionalization protocol was determined with respect to the effects of the bulk monomer (thiol/allyl) ratio and biotin and StrA concentrations on the amounts of immobilized StrA and b-HLM, which were quantitated based on their fluorescence tags (Figure 2b–d). As expected, the amount of bound StrA increased along with increasing amount of biotin, which could be altered by adjusting either the number of free surface thiols (Figure 2b) or the biotin concentration (Figure 2c). The amount of immobilized b-HLM was, however, inversely dependent on the amount of bound StrA (Figure 2d). This likely resulted from more efficient biotin–streptavidin interactions in the absence of steric hindrances caused by excess streptavidin. For further assays, we used 0.1 × 10−3 м biotin and 0.5 mg mL−1 StrA concentrations and a 25 mol% excess of thiol functional groups in the bulk.
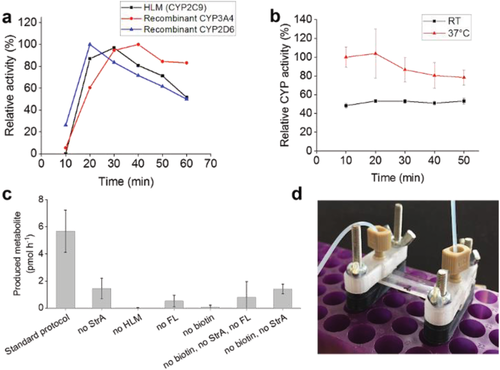
The CYP activities of the IMERs were determined at a flow rate of 5 µL min−1 using temperature-insensitive Luciferin-H (200 × 10−6 м in phosphate buffered saline (PBS)) hydroxylation via CYP2C9 (Figure 3a) and temperature-sensitive coumarin (50 × 10−6 м in 0.1 м Tris buffer) 7-hydroxylation via CYP2A6 (Figure 3b) as the model activities. In both cases, the IMERs maintained their CYP activity for at least 60 min, while the reaction temperature was shown to have an effect on the efficiency of the latter reaction, as expected. The developed method was also shown to be feasible for immobilization of recombinant single CYPs (rCYPs), that is, pure enzyme isoforms expressed in baculovirus-transfected insect cells, which also showed significant CYP activity when immobilized on the IMERs (Figure 3a). This further confirms the universal nature of the developed enzyme immobilization strategy. Since our approach targets the lipid membrane, not the protein itself, it is universally applicable to biotinylation and immobilization of any membrane-bound protein on any avidin-coated surface. Finally, the specificity of the functionalization protocol was determined by omitting each of the functionalization steps at a time and determining the cumulative (60 min) amount of produced metabolite using three parallel IMERs in each condition. Although some traces of nonspecific binding of both StrA and b-HLM were observed in the fluorescence measurements, the activities of the CYP-IMERs prepared using the optimized functionalization protocol were significantly higher than those of any of the negative controls (Figure 3c), suggesting that the nonspecifically adsorbed lipid vesicles leaked out of the IMER upon application of through-flow.
On the basis of these results, the developed immobilization method, which is based on natural membrane fusion between biotinylated fusogenic liposomes and the desired lipid membrane incorporating the enzymes of interest, provides a universal tool for studying membrane-bound enzymes on solid surfaces. Furthermore, the thiol–ene click chemistry-based microchip fabrication and functionalization protocol, specifically used in this study, further provides a robust tool for implementation of through-flow IMERs for drug metabolism research. Efficient immobilization of the CYP system combined with the advantages of the microarray technology pave the way to novel methodologies, such as mechanistic drug inhibition studies. The concept developed herein is particularly suitable for applications where unaltered enzyme kinetics are of paramount importance, such as the preclinical drug screening and prediction of the in vivo drug response on the basis of in vitro enzyme kinetic results.
Experimental Section
Liposome Preparation and Fusion with Human Liver Microsomes or Recombinant Cytochrome P450: b-FL were prepared using a lipid composition modified from Csiszár et al.6 and Hersch et al.7 by mixing stock solutions (in chloroform) of DOPE (10 mg mL−1), DOTAP (10 mg mL−1), biotin-cap-DOPE (10 mg mL−1), and Lissamine Rhodamine B-DOPE (1 mg mL−1) in a lipid mass ratio of 1:1:0.1:0.05, respectively. After mixing, the bulk solvent was evaporated under a stream of nitrogen. To remove any solvent residues, the lipid mixture was kept in vacuum for 2 h. Next, the dry lipid film was solvated in PBS to yield a total lipid concentration of 2 mg mL−1 and vortexed for 1 h at room temperature. To prepare unilamellar vesicles, the multilamellar liposome mixture was passed through a polycarbonate membrane (pore size = 100 nm) 51 times using a benchtop extruder (Avanti Polar Lipids, Alabaster, AL). The size distribution of multilamellar and unilamellar b-FLs (Figure S1, Supporting Information) was determined by dynamic light scattering (DLS) using Zetasizer APS and the size distribution of HLM and the zeta potential of b-FLs and HLM (Figure S2, Supporting Information) was determined using Zetasizer Nano ZS (both devices from Malvern, Worcestershire, UK). Before measurements, the HLM stock (20 mg mL−1 total protein) was diluted to a concentration of 0.125 mg mL−1 and the b-FL stock to a concentration of 0.002 mg mL−1 with deionized water. The b-FL dispersion was stored at ≤4 °C until use. For HLM immobilization, equal volumes of the b-FL dispersion (2 mg mL−1 total lipid) and the HLM stock solution (20 mg mL−1 total protein) were mixed and incubated at 37 °C for 15 min before applying the biotinylated HLM (b-HLM) vesicles onto the streptavidin-functionalized magnetic beads or microchips. The rCYP isoenzymes were biotinylated in a similar manner by mixing the b-FL stock with an equal volume of the rCYP3A4, rCYP2D6, or rCYP2C9 stock (each 1000 nmol L−1) followed by incubation at 37 °C for 15 min.
Enzyme Immobilization on Streptavidin-Functionalized Magnetic Beads: Desired amount of streptavidin prefunctionalized, superparamagnetic beads (Dynabeads M-280, 10 mg mL−1, Life Technologies) were pretreated according to the supplier's protocol (https://tools.thermofisher.com/content/sfs/manuals/MAN0014017_Dynabeads_M280_Streptavidin_UG.pdf). Next, the beads were divided in 25 µL aliquots (corresponding to 0.25 mg of beads) of which the supernatant was separated and discarded. The b-HLM vesicles (15 µL) were immediately added onto the beads and the suspension was incubated on a tube rotator at room temperature for 30 min, after which the supernatant was discarded and the beads were washed 4–5 times with PBS (50 µL) using magnetic separation. After functionalization, the beads were stored in PBS (50 µL) at ≤4 °C until use. In addition to b-HLM vesicles, the pretreated beads were also functionalized with biotinylated DOPE or DPPE lipids and then with bare HLM vesicles (negative control), as described in the Supporting Information.
Microchip Fabrication and Biofunctionalization: The IMERs were implemented on micropillar arrays that were fabricated in-house and sequentially functionalized with biotin and streptavidin. Briefly, the micropillar arrays were fabricated from thiol–ene polymers following a previously developed protocol.26 The microreactor design comprised a 30-mm-long and 4-mm-wide microchannel featuring an array of ≈13 600 micropillars (Ø 60 µm) (Figure 2a). The channel height was 200 µm and the total chip volume ≈25 µL (including the connecting channels between inlet/outlet and excluding the space occupied by the micropillars). The chip fabrication protocol included four steps: (i) SU-8 master fabrication in cleanroom conditions, (ii) casting of a polydimethylsiloxane (PDMS) mold using the SU-8 master as a template, (iii) UV replica molding of the microchannel and the cover layers out of thiol–ene polymers using the PDMS mold, and (iv) bonding of the two thiol–ene layers by lamination (Figure S6, Supporting Information). The cleanroom fabrication of the SU-8 masters (step i) is described in the Supporting Information. The PDMS molds (step ii) were prepared by mixing the base elastomer and the curing agent in a ratio of 10:1 or 9:1 (w/w), degassing in vacuum for 30 min, and pouring the mixture onto the SU-8 master prior to curing by heat (80 °C for 3 h or 65 °C overnight). The thiol–ene layers (step iii) were prepared by mixing tetrathiol and triallyl monomers in a molar ratio of 3:2 (with respect to free thiol and allyl functional groups), which resulted in thiol-rich (25 mol% excess of thiols) bulk composition. In addition, stoichiometric compositions (tetrathiol and triallyl monomers mixed in a molar ratio resulting in equal amounts free thiol and allyl functional groups) were used as controls during the validation of the biofunctionalization process. Next, the monomer mixture was poured onto the PDMS mold, degassed in vacuum, and cured under UV for 5 min (Dymax 5000-EC Series UV flood exposure lamp, nominal power 225 mW cm−2, Dymax Corporation, Torrington, CT). Both microchannel and cover layers (with inlet/outlet holes) were prepared in the same manner. The two, preheated (70 °C) layers were laminated together and exposed to UV for an additional 2 min (step iv). The average amount of free surface thiols was determined by titration with Ellman's reagent and was, for instance, 133 ± 13 nm−2 when 50 mol% excess of thiol functional groups was used in the bulk composition. Finally, the thiol–ene surface was functionalized with streptavidin according to a protocol adopted from Lafleur et al.[25] Briefly, free surface thiols were functionalized with biotinylated PEG4-alkyne (0.1 × 10−3 м in ethylene glycol with 1% Igracure TPO-L as the photoinitiator) using photopolymerization under UV (λ = 365 nm) for 1–2 min (Figure 2a). Next, the microreactor was thoroughly rinsed sequentially with methanol and deionized water (≥5 mL each) and filled with Alexa Fluor Streptavidin (0.5 mg mL−1, in PBS). After incubation at room temperature for 30–45 min, the microreactor was again thoroughly rinsed with PBS (≥5 mL) and filled with the b-HLM vesicles or biotinylated rCYPs. Finally, after incubation at ≤4 °C overnight (b-HLM) or at room temperature for 30 min (biotinylated rCYP), the microreactor was thoroughly rinsed with PBS (≥5 mL) and used immediately. For validation of the surface biofunctionalization process, controls were prepared using varying biotin (0.01–1 × 10−3 м) and streptavidin (0.005–0.5 mg mL−1) concentrations and characterized in terms of the efficiency of streptavidin and b-HLM immobilization by fluorescence (for details, see the Supporting Information). The success of the surface modifications was also monitored based on changes of the water contact angle (for details, see the Supporting Information) compared to that of native thiol–ene surface (Figure 2a).
CYP Incubations with Enzymes Immobilized on Magnetic Beads: The CYPs immobilized on magnetic beads were incubated in Eppendorf tubes similar to soluble enzymes (see the Supporting Information) with the following exceptions: Instead of the specified amount of HLM (see Table S1, Supporting Information), 0.25 mg of magnetic beads incorporating the immobilized CYPs were added to each reaction and preincubated with the substrate prior to addition of NADPH. The reactions were stopped by separating the beads from the supernatant using an external magnet. After reaction, the beads were washed twice with PBS buffer (50 µL) and stored (in PBS buffer) at ≤4 °C until further use. The supernatants (100 µL) were either acidified for fluorescence detection by adding 10 µL of 4 м perchloric acid (CYP2A6) or allowed to react with the Luciferin detection reagent (100 µL) for luminescence detection (all other CYP isoforms).
CYP Incubations on Immobilized Enzyme Microreactors: The CYP activity of the IMERs was assessed by infusing the substrate solution containing NADPH (0.1 × 10−3 м, in the specified buffer) through the IMER at a flow rate of 5 µL min−1 and collecting fractions of 50 or 100 µL of the outcome for further (off-line) analysis. The collected fractions were either acidified or allowed to react with the luminescence detection reagent as previously specified. For reactions conducted at physiological temperatures, the IMERs were heated using a resistive heater (R = 0.5 Ω, P = 0.6–0.8 W) connected to an external power supply. The temperature on the top surface of the IMER was measured with a thermocouple and adjusted to 35 °C (corresponding to roughly two degrees higher temperature inside the microchannel on the basis of a prior publication28) using a proportional–integral–derivative (PID) controller connected to the circuit.
Statistical Analysis: All results are presented as mean ± standard deviation from repeated experiments, the number of which is indicated in the context of each dataset. Enzyme kinetic parameters were calculated with Origin 2018 software using the Hill function with value of n fixed as 1 (for isoenzymes obeying Michaelis–Menten kinetics) or with a floating value of n (sigmoidal kinetics). Best fit model for CYP enzyme kinetics was chosen by using Akaike information criteria. Significant differences in stability between b-HLM and nonbiotinylated HLM (Figure 1c) were determined with Student's t-test. Significant differences between the standard protocol and negative controls (Figure 3c) were determined using one-way ANOVA followed by Dunnett's test. Statistical analysis was performed with Origin 2018 or SPSS Version 25.
Acknowledgements
The research leading to these results received funding from the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013)/ERC Grant Agreement No. 311705 (CUMTAS). This work was also financially supported by the Academy of Finland (Grant Nos. 304400, 309608, and 297360), the University of Helsinki Research Funds, and the DPDR doctoral programme, University of Helsinki. The authors thank the Electron Microscopy Unit of the Institute of Biotechnology, University of Helsinki, for providing access to the scanning electron microscope, and the Micronova Nanofabrication Centre, Aalto University, for providing access to the cleanroom facilities.
Conflict of Interest
The authors declare no conflict of interest.




