Dysphagia and Mortality Risk in Individuals With Primary Progressive Apraxia of Speech
Funding: This work was supported by the National Institute on Deafness and Other Communication Disorders (R01-DC010367, R01-DC012519, R01-DC014942) and the National Institute of Neurological Disorders and Stroke (R01-NS089757).
ABSTRACT
Individuals with primary progressive apraxia of speech (PPAOS) often develop parkinsonism and dysphagia. To evaluate the clinical correlates and impact of dysphagia in this population, we compared enrollment visit data between individuals with (n = 12) versus individuals without (n = 44) dysphagia symptoms. The group with dysphagia had more motor speech symptoms and parkinsonism. Longitudinal analysis revealed that almost everyone developed dysphagia before dying; the average time to death after developing dysphagia was 5.43 years and complications of dysphagia resulted in mortality for 35% of the individuals for whom data were available. These results emphasize the need for dysphagia management and provide useful prognostic estimates.
1 Introduction
Primary progressive apraxia of speech (PPAOS) is a form of frontotemporal lobar degeneration defined by the insidious onset of disturbances in motor speech planning or programming in the absence of other initial difficulties [1-4]. Neuropathologically, PPAOS is typically associated with four-repeat tauopathies, either corticobasal degeneration (CBD) or progressive supranuclear palsy (PSP) pathology [5]. This is likely the reason that many individuals with PPAOS later develop features of atypical parkinsonian conditions, including PSP and corticobasal syndrome [6-10]. Dysphagia, or difficulties with chewing and swallowing, is well-established as a common symptom that can be associated with mortality in individuals with atypical parkinsonian conditions [11-15]. Fatal complications include choking and aspiration pneumonia [16]. Using pathology-defined groups, Müller and colleagues found that 83% of individuals who had PSP pathology and 31% of individuals with CBD had subjective swallowing difficulties, with onset latencies of 42 and 64 months, respectively [11]. Latency to dysphagia was longer than the latency to dysarthria in nearly every participant and was highly correlated with survival time. Similarly, dell'Aquila and colleagues found that early dysphagia symptoms shortened survival time for individuals who presented clinically with PSP [17]. Josephs et al. reported that six of their 13 participants with PPAOS had developed dysphagia (median disease duration of 6.9 years for the full group) and that most of them had also developed dysarthria in the context of a rapidly evolving clinical picture that resembled PSP (note that there is an overlap between the cohort reported by Josephs et al. and the participants included here) [18]. Nevertheless, the relationship between dysphagia and other symptoms (e.g., parkinsonism, dysarthria) and its impact on mortality in PPAOS are still unknown.
2 Methods
2.1 Participants
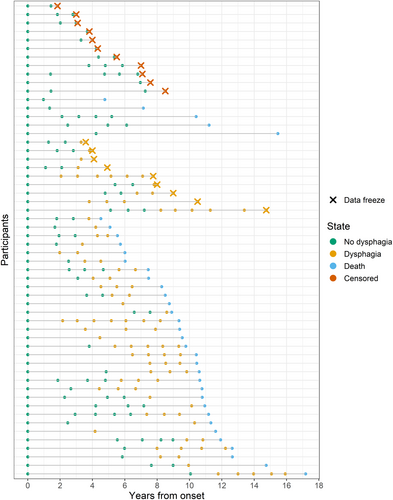
Retrospective data are presented from 56 participants who were recruited by the Neurodegenerative Research Group (NRG) at Mayo Clinic Rochester (see Table 1). Written informed consent was obtained from all participants at each visit in accordance with the Mayo Clinic IRB. Median age at enrollment was 72.6 years (IQR: 14.4 years). All participants had a PPAOS diagnosis at enrollment, meaning that they had unequivocal apraxia of speech (AOS) in the absence of broader language, cognitive, or neurologic symptoms that would meet criteria for another diagnosis. Consensus diagnoses were reached independently for each visit following a comprehensive evaluation by a board-certified neurologist, certified speech-language pathologist, and psychometrist. At each visit, the presence or absence of dysphagia symptoms was also determined by asking the participant or study partner if they had noticed any difficulties with chewing or swallowing. Clinical data for the first visit at which they endorsed dysphagia symptoms is presented in Table S1. Thirty-six (64%) of the participants had died prior to the data freeze (see Figure 1). Cause of death was available for 20 (55%) of these individuals, established by reviewing medical charts and family member reports.
| No Dysphagia (n = 44) | Dysphagia (n = 12) | p | |
|---|---|---|---|
| Age at AOS onset (Years) | 68 (60, 73) | 72 (62, 76) | 0.412 |
| Females | 23 (52%) | 7 (58%) | 0.963 |
| Education (Years) | 16 (15, 18) | 16 (13, 17) | 0.696 |
| Disease duration at enrollment | 2.8 (1.8, 4.5) | 4.0 (3.0, 4.9) | 0.137 |
| AOS severity (/4) | 1.25 (1.00, 2.00) | 2.00 (1.50, 3.00) | 0.013 |
| AOS subtype | |||
| Phonetic | 14 (32%) | 3 (25%) | 0.404 |
| Prosodic | 23 (52%) | 5 (42%) | |
| Mixed | 7 (16%) | 4 (33%) | |
| Dysarthria | 7 (16%) | 6 (50%) | 0.036 |
| Dysarthria type | |||
| UUMN | 0 (0%) | 1 (17%) | 0.721 |
| Spastic | 4 (57%) | 3 (50%) | |
| Hypokinetic | 1 (14%) | 1 (17%) | |
| Hyperkinetic | 1 (14%) | 0 (%) | |
| Mixed | 1 (14%) | 1 (17%) | |
| WAB aphasia quotient (/100) | 97.6 (96.6, 99.2) | 98.1 (96.5, 98.9) | 0.992 |
| MoCA (/30) | 27 (25, 28) | 28 (26, 29) | 0.179 |
| FAB (/18) | 17.00 (16.00, 17.00) | 17.00 (16.00, 17.25) | 0.562 |
| MDS-UPDRS III (/132) | 6 (4, 12) | 13 (12, 17) | 0.007 |
| WAB praxis (/60) | 58.00 (57.75, 60.00) | 58.50 (57.00, 59.00) | 0.331 |
- Note: Data reflect median (first quartile, third quartile) or n (%). Disease durations were calculated from initial symptom (i.e., AOS) onset, as reported by the participant and care partner, to the time of enrollment. Bolded text indicates significant differences at the p < 0.05 level.
- Abbreviations: ASRS-3 = Apraxia of Speech Rating Scale – Version 3, FAB = Frontal Assessment Battery, MDS-UPDRS III = Movement Disorders Society Sponsored revision of the Unified Parkinson's Disease Rating Scale Part III, MoCA = Montreal Cognitive Assessment, WAB = Western Aphasia Battery – Revised.
2.2 Cross-Sectional Analysis
Enrollment visit data were compared between participants with PPAOS who endorsed dysphagia symptoms (n = 12) and those who did not (n = 44). Motor speech planning and programming was indexed using an AOS rating scale (0 = normal, 4 = severe), whereas motor speech execution was indexed by the proportion of participants with dysarthria [19, 20]. The aphasia quotient from the Western Aphasia Battery—Revised (WAB) is reported as a broad measure of language [21]. The Montreal Assessment of Cognition (MoCA) and Frontal Assessment Battery (FAB) are reported as measures of cognition and frontal functioning, respectively [22, 23]. The motor subtest of the Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS III) was used as an index of parkinsonism and the WAB praxis subtest was used as an index of ideomotor praxis [21, 24]. Wilcoxon rank sum tests and chi-squared tests of independence were used for continuous and categorical variables, respectively.
2.3 Multistate Model
The multistate model included data from all 56 participants, starting at study enrollment and ending at either death or the data freeze for those who were still alive. Participants could hover in one of three states: alive with no dysphagia, alive with dysphagia, and dead (see Figure 1). Once they transitioned out of the alive with no dysphagia state, they could not return, and death was the absorbing state. Transitions could occur during a research visit or based on information retrospectively obtained from the medical charts or family report. Participants who denied dysphagia at their last visit and were still alive at the time of data freeze were censored since their state was unknown (i.e., alive either with or without dysphagia). Those who endorsed dysphagia at their last visit hovered in the alive with dysphagia state until the data freeze. The model was implemented in the R environment (version 4.4.2) using the MSM package [25].
3 Results
3.1 Cross-Sectional Analysis
The results are presented in Table 1. Relative to the participants who denied dysphagia symptoms at enrollment, those who endorsed dysphagia symptoms had significantly more severe AOS, were more likely to have dysarthria, and had higher MDS-UPDRS III scores, indicative of more severe parkinsonism. The two groups did not significantly differ on language, cognitive, or praxis measures.
3.2 Multistate Model
Of the 36 participants who died, 31 had documented evidence of dysphagia prior to dying (see Figure 1). The remaining five were lost to follow-up several years before death; therefore, it is unclear if they developed dysphagia later in their disease course. For this reason, the transition intensity between the alive without dysphagia state and death approached zero. The mean sojourn time in the no dysphagia state was 5.98 years (before transitioning to the dysphagia or death states; 95% CI: 4.45–8.05 years) and the mean sojourn time in the dysphagia state was 5.43 years (before transitioning to the death state; 95% CI: 3.89–7.60 years). Model estimates for the cumulative probability of transitioning from and to each state can be found in Figure 2.

3.3 Dysphagia Management and Cause of Death
Nearly all participants with dysphagia had modified diets (e.g., thickened liquids, pureed or soft foods, and smaller bites). Enteral nutrition was proposed to many of the participants and families, but only six of them had documented evidence of pursuing that option. None of the six survived longer than 8 months after placement of their percutaneous endoscopic gastrostomy (PEG) tube. Seven of the 20 participants for whom the cause of death data were available (35%) died as a direct result of complications from their dysphagia (aspiration pneumonia for six participants and asphyxiation for one). Other causes included complications from falls and loss of appetite, resulting in malnutrition and brain death.
4 Discussion
Although the initial difficulties experienced by individuals with PPAOS are limited to motor speech planning and programming, they experience a broad range of symptoms as the disease progresses. Before dying, 31/36 (86%) of this cohort endorsed dysphagia; the remaining five may have developed dysphagia but were lost to follow-up. The cross-sectional comparison between participants who enrolled in the research study with dysphagia symptoms versus without dysphagia symptoms demonstrated that swallow function is associated with motor speech function. Based on previous research, we expected that almost all participants who endorsed dysphagia symptoms would have dysarthria, whereas only 60% of them did [11, 13]. Across participants, dysphagia complaints included both the oral (e.g., slow, inefficient chewing) and pharyngeal (e.g., coughing on liquids or secretions) phases in addition to gorging (e.g., eating or drinking too much and/or too quickly). The latter may have contributed to the endorsement of dysphagia symptoms in participants who did not have neuromuscular features that would have also caused dysarthria, but the nature of dysphagia warrants further investigation. AOS severity and motor subtest scores on the MDS-UPDRS also differed between groups, which could both be indicators of a slight difference in disease severity between groups at enrollment.
The multistate model has the benefit of drawing on the full longitudinal dataset to inform prognostication over time. Model estimates suggest that the average time of being dysphagia-free after initial symptom onset is 5.98 years, and the average time of living with dysphagia is 5.43 years. This model does not establish causality. However, the cause of death data suggest that complications from dysphagia contributed to mortality for 35% of the participants for whom those data were available. Most, if not all, of them were making diet modifications, but only six had documentation of receiving enteral nutrition. Survival past feeding tube placement was never longer than 8 months. These results have direct implications for clinical decision-making, education, and prognostication.
For the first time, these results underline the importance of monitoring for dysphagia in individuals with PPAOS, but a few limitations warrant consideration. For one, the timelines are limited to data from annual visits and retrospective chart review, which limits precision, but these data are well-suited for a multistate model. As with any longitudinal study, information about early symptom development (e.g., prior to diagnosis and study enrollment) is especially sparse and the presented timelines rely on estimates of symptom onset. Only indexing dysphagia symptoms by self-report could also be considered a limitation, but this has the benefit of being easily translated to the clinic and implemented by any member of the care team. Future research would benefit from the addition of instrumental measures of swallowing because the extent to which subjective reports accurately reflect objective findings is unclear [26-29]. Instrumental studies would also allow for trials of dysphagia management strategies that might mitigate the risk of fatal complications.
Author Contributions
G.M.: study design, data collection, statistical analysis, writing of the first draft, editing of the manuscript; N.T.T.P.: statistical analysis, editing of the manuscript; S.M.B.: data collection, editing of the manuscript; H.M.C.: data collection, editing of the manuscript; J.R.D.: data collection, editing of the manuscript; J.L.W.: editing of the manuscript; H.B.: statistical analysis, editing of the manuscript; K.A.J.: editing of the manuscript; R.L.U.: study design, data collection, statistical analysis, editing of the manuscript.
Acknowledgments
The authors are grateful to the participants and their families for making this research possible. Funding was provided by the National Institutes of Health grant numbers R01-DC014942, R01-NS089757, R01-DC012519, and R01-DC010367. The funding agency had no involvement in the study design, in collecting, analyzing, and interpreting the data, in writing the report, or in the decision to submit the article for publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The dataset and analysis code used for the current study are available from the corresponding author upon reasonable request.




