Right-sided vagus nerve stimulation: Worldwide collection and perspectives
Abstract
Objective
Vagus nerve stimulation (VNS) is an established therapy for drug-resistant epilepsy (DRE) and is indicated for implantation on the left vagus nerve-only. In rare cases right-sided VNS may be the only option. With only seven published cases in the literature, data on safety and effectiveness of right-sided VNS is very limited.
Methods
An anonymous 38-item questionnaire was sent to expert surgeons implanting VNS for DRE. The questions covered demographics and clinical characteristics, the reason for right-sided implantation and both neurological and surgical outcomes of right-sided VNS.
Results
The survey captured 38 cases of right-sided VNS (18 females, mean age at surgery of 28.0 ± 16.3 years). Right-sided VNS was performed because of VNS lead deficiency (n = 20), anatomical constraints (n = 8), infection of a left-sided VNS site (n = 9), and presence of a left ventricular shunt (n = 1). Thirty-two patients (84%) had a preoperative cardiac assessment. Three patients presented postoperative cardiac side-effects. Right-sided VNS was stopped at last follow-up in three patients: due to deep infection (n = 1), due to dyspnea (n = 1), and due to sleep apnea syndrome (n = 1). Twenty-one patients (55%) were responders to right-sided VNS and the mean reduction of seizure frequency under right-sided VNS was 56.2 ± 18.8%. Focusing on seizure frequency reduction between right-sided VNS and left-sided VNS: 20 patients experienced similar effectiveness, 1 experienced lesser effectiveness, and 2 patients experienced greater effectiveness with right-sided VNS.
Interpretation
This multicenter case series significantly augments the available literature on right-sided VNS. This suggests comparable effectiveness to left-sided VNS but potentially lower tolerability. Further studies are warranted to better evaluate safety and efficacy of right-sided VNS.
Introduction
Vagus nerve stimulation (VNS) is a neuromodulation therapy indicated as an adjunctive treatment for people suffering from drug-resistant epilepsy who are not candidates for resective surgery.1 VNS therapy was first approved in Europe and the United States in 1994 and 1997, respectively.2 As of now, more than 130,000 patients have been implanted with VNS worldwide (LivaNova data on file). Across the globe regulatory approval for VNS is for implantation on the left cervical vagus nerve-only, based on asymmetrical innervation of the heart by left and right vagus nerves.3, 4 The seminal description of VNS surgical technique published in 1990 recommended left-side implantation, as “stimulation of the right vagus nerve can elicit profound bradycardia.”5 Left branches of the vagus nerve innervate the atrioventricular node while right branches innervate the sinoatrial.6 Since the first human trials, VNS has only been approved on the left side. However the majority of data on cardiac effects of left and right stimulation of vagal fibers stems from animal experiments (mainly dogs) and is not supported by compelling evidence in humans.7, 8
Some drug-resistant epilepsy patients have been implanted with a VNS on the right side in particular situations.9, 10 Reports on outcomes of patients with a right-sided VNS are scarce in the scientific literature. A recent systematic review found out only four articles reporting right-sided VNS with a total of seven patients.11 These patients did not exhibit severe cardiac complications, and in one case, right-sided VNS was reported as being more effective than the left-sided VNS.
Therefore, the aim of this study was to survey VNS surgical experts worldwide on the outcomes of their right-sided VNS patients with a special interest in preoperative cardiac diagnostics and postoperative cardiac complications.
Methods
This study follows the Standards for Quality Improvement Reporting Excellence 2.0 (SQUIRE 2.0) guidelines.12 The local institutional review board approved the study protocol (IRB#1:2024/06; IRB00011789). The requirement to obtain informed consent was waived for this observational retrospective study.
Interventions
A 38-item anonymous questionnaire was designed by two senior neurosurgeons (see Table S1). The questionnaire included seven items concerning patients epilepsy description according to the 2017 ILAE classification of seizures and clinical data (sex, age at epilepsy onset, and developmental delay); seven items concerning the first epilepsy surgery of the patients, in particular concerning first left-sided-VNS if applicable; 21 items dealing with right-sided VNS implantation with a special interest for preoperative and postoperative cardiac assessments (performance of a ECG, and/or a 24-h Holter monitor, and/or echocardiography); and three items concerning follow-up. All data were de-identified with no risk for pseudonymization. Additionally, a free comments section was provided at the end of the questionnaire.
An individual e-mail was sent to 133 practitioners identified as VNS experts based on three sources: previous study on VNS teaching, literature review, and attendance to an educational event sponsored by the manufacturer of VNS stimulators. We made sure to select and survey the experts who routinely used VNS. In the absence of a response, a reminder e-mail was sent 2 weeks and another 4 weeks after the initial email in an attempt to increase the response rate. If the experts answered that they had performed one or more cases of right-sided VNS, the dedicated questionnaire was sent by e-mail.
Study of interventions
The answers were aggregated in order to propose general overview of right-sided VNS outcomes and safety. Free comments were analyzed separately and incorporated in the Results section.
Measurements and analysis
Categorical variables were described as number and percentages. Continuous variables were described as mean ± standard deviation.
Results
VNS experts
Figure 1 presents the flowchart of the study and Figure 2 represents the countries of origin of the contributors.
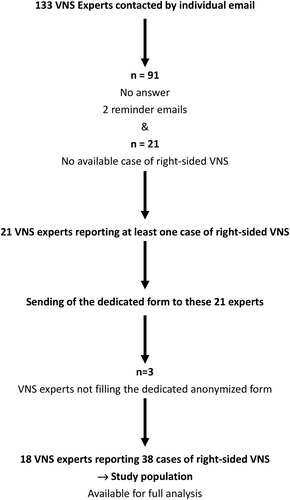
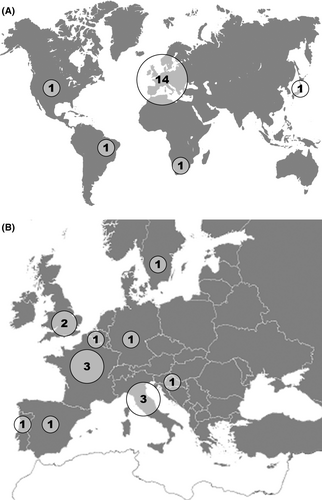
While 133 experts were asked to complete the form, we obtained an answer from 41 (31%). Twenty experts clearly stated that they never saw the need to perform a right-sided VNS. One expert who reported having performed more than 1700 VNS procedures stated that he never encountered case requiring right-sided VNS. Twenty-one experts alleged one or more cases of right-sided VNS: 18 sent fully completed forms. They originated from 13 different countries, mainly from Europe.
Right-sided VNS population
Table 1 presents the population characteristics. Thirty-eight patients (18 females) were included. They presented a mean age at epilepsy onset of 7.0 ± 6.6 years. Developmental delay was present in 25 (66%) cases. According to the 2017 ILAE classification, the epilepsy types of this population were combined generalized and focal in 17 cases, focal in 14 cases, and generalized in 7 cases. The two main epilepsy etiologies were structural etiology in 15 cases, and unknown etiology in 13 cases. In an attempt to be more precise, we proposed different epilepsy types and etiologies: the most frequent epilepsy type was multifocal epilepsy/ symptomatic generalized epilepsy in 15 cases and the epilepsy etiology was unknown in 13 cases. Fifteen (39%) patients had undergone prior resective surgery for their drug-resistant epilepsy. Thirty-four patients (89%) had a previous left-sided VNS. The mean age at first VNS surgery was of 20.4 ± 13.2 years. The duration of follow-up was 4.1 ± 4.0 years (range, 0.25–15).
| Parameters | N (%) |
|---|---|
| Sex | |
| Male | 20 (52.6%) |
| Female | 18 (47.4%) |
| Age at epilepsy onset, years (mean ± SD) | 7.0 ± 6.6 |
| Epilepsy type (according to the 2017 ILAE Classification) | |
| Focal | 14 (36.8%) |
| Generalized | 7 (18.4%) |
| Combined generalized and focal | 17 (44.8%) |
| Epilepsy etiology (according to the 2017 ILAE Classification) | |
| Structural | 15 (39.5%) |
| Genetic | 8 (21.1%) |
| Infectious | 1 (2.6%) |
| Metabolic | 1 (2.6%) |
| Unknown | 13 (34.2%) |
| Descriptive epilepsy classification | |
| Focal/eloquent or temporal epilepsy | 8 (21.1%) |
| Multifocal partial epilepsy | 3 (8.0%) |
| Multifocal partial epilepsy/ symptomatic generalized epilepsy | 15 (39.5%) |
| Symptomatic generalized epilepsy | 5 (13.2%) |
| Idiopathic generalized epilepsy | 2 (5.2%) |
| Bilateral temporal lobe epilepsy | 1 (2.6%) |
| Frontal lobe epilepsy | 2 (5.2%) |
| Not available | 2 (5.2%) |
| Descriptive epilepsy etiology | |
| Neuronal migration disorder | 5 (13.2%) |
| Cerebral palsy/static encephalopathy | 2 (5.2%) |
| Lennox–Gastaut syndrome | 10 (26.4%) |
| Traumatic brain injury | 1 (2.6%) |
| Infection | 2 (5.2%) |
| Tumor/cavernoma/ arteriovenous malformation | 1 (2.6%) |
| Genetic/metabolic syndrome | 4 (10.6%) |
| Unknown | 13 (34.2%) |
| Developmental delay | |
| No | 13 (34.2%) |
| Yes | 25 (65.8%) |
| Prior VNS failed intracranial epilepsy surgery | |
| No | 23 (60.5%) |
| Yes | 15 (39.5%) |
| Left-side VNS before right-sided VNS | |
| No | 4 (10.5%) |
| Yes | 34 (89.5%) |
| Age at first VNS surgery, years (mean ± SD) | 20.4 ± 13.2 |
| Duration of follow-up, years (mean ± SD) | 4.1 ± 4.0 |
| At last follow-up, number of antiepileptic drugs | |
| ≤2 | 9 (23.8%) |
| 3 | 11 (28.9%) |
| 4 | 13 (34.2%) |
| ≥5 | 4 (10.5%) |
| Not available | 1 (2.6%) |
- VNS, vagal nerve stimulation.
Rationale for right-sided-VNS and right-sided VNS surgery
Table 2 presents the results of right-sided VNS implantation. Right-sided VNS was implanted after lead breakage of a previous left-sided VNS in 19 cases, due to anatomical constraints in nine cases, after infection of a left-sided VNS in nine cases, and due to the presence of a left ventricular shunt in one case. One case of local anatomical contraindication was an atrio-ventricular canal on which prior surgery had been performed, one case presented significant adhesions with profuse bleeding on manipulation of major vessels during attempted left-sided surgery, and another one was a fixed posture of the neck to the left at time of implant. The six other cases were not further described. Right-sided VNS was performed 8.9 ± 6.1 years after the first left-sided VNS in 34 cases. In four cases, one with a left ventricular shunt and three with local challenging anatomy, a right-sided VNS was implanted in first instance. The surgical procedure used for left-sided-VNS was modified in two instances: in one case, the internal pulse generator was placed more laterally and under the muscle layer due to previous “Twiddler syndrome,” in the other case the helicoidal lead was placed on the right vagus nerve but the internal pulse generator remained in the left pocket. Thirteen (72%) experts surgeons of this panel of 18 experts used optical magnification to position the helical lead around the nerve. No instance of an additional nerve branch which may correspond to a non-recurrent laryngeal nerve was reported.
| Parameters | N (%) |
|---|---|
| Reason to right-sided VNS implantation | |
| Infection of a previous VNS | 9 (23.7%) |
| Local anatomical contraindications | 9 (23.7%) |
| Left ventricular shunt | 1 (2.6%) |
| Lead breakage of a previous VNS | 18 (47.4%) |
| Atrio-ventricular chanel | 1 (2.6%) |
| Delay between first VNS and right-sided VNS, years (mean ± SD) | 8.9 ± 6.1 |
| Preoperative cardiac assessment | |
| No | 6 (15.8%) |
| EKG only | 18 (47.4%) |
| EKG and 24-h Holter monitor | 4 (10.5%) |
| 24-h Holter monitor | 9 (23.7%) |
| Echocardiography | 1 (2.6%) |
| Modification of the surgical procedure for right-sided VNS implantation | |
| No | 36 (94.8%) |
| Yes | 2 (5.2%) |
| Presence of an additional nerve branch which may correspond to a non-recurrent laryngeal nerve | |
| No | 38 (100.0%) |
| Yes | 0 (0.0%) |
| Use of optical magnification to position the helicals around the nerve | |
| No | 17 (44.7%) |
| Yes | 21 (55.3%) |
| Postoperative cardiac assessment | |
| No | 17 (44.7%) |
| EKG only | 16 (42.2%) |
| EKG and 24-h Holter monitor | 1 (2.6%) |
| 24-h Holter monitor | 4 (10.5%) |
| Results of postoperative cardiac assessment | |
| Normal | 19 (90.5%) |
| Pathological | 2 (9.5%) |
| Complication following right-sided VNS | |
| No | 30 (79.0%) |
| Stimulation-induced bradycardia | 1 (2.6%) |
| First-degree atrio-ventricular bloc | 2 (5.2%) |
| Deep infection (removal/revision) | 1 (2.6%) |
| Hiccup | 1 (2.6%) |
| Hoarseness | 3 (8.0%) |
| Reduction in seizure frequency with right-sided VNS | |
| <50% | 10 (26.3%) |
| ≥50% | 21 (55.3%) |
| Not available | 7 (18.4%) |
| Reduction in seizure frequency with right-sided VNS, percentage (mean ± SD) | 56.2 ± 18.8 |
| Right-sided VNS versus left-sided VNS efficacy | |
| Same | 21 (61.8%) |
| Less efficient | 1 (2.9%) |
| More efficient | 2 (5.8%) |
| Not applicable | 10 (29.5%) |
- EKG, electrocardiography; VNS, vagal nerve stimulation.
Right-sided VNS outcomes: Safety and efficacy
Table 2 presents the results of right-sided VNS implantation. Figure 3 presents a patient with delayed cardiological complication after right-sided VNS. A preoperative cardiac assessment was performed in 32 (84%) patients prior to right-sided VNS implantation: in 18 cases, it consisted of an ECG; in 9 cases it was a 24-h Holter monitor; in 1 case of echocardiography, in 4 cases it was both ECG and a 24-h Holter monitor. The case in which echocardiography was performed was in the patient operated on for an atrio-ventricular communication.
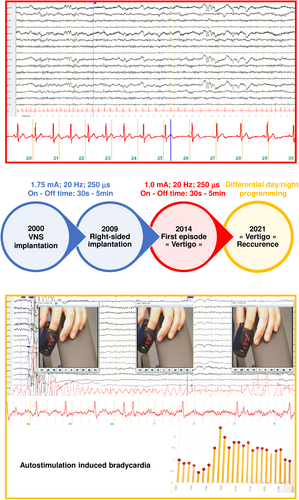
There were no severe complications leading to permanent morbidity in this series of 38 patients. Six patients presented a complication after right-sided VNS. These complications (four acute and two delayed) were transient in all cases. The reported complications were three cases of hoarseness, one case of hiccup, one case of deep infection requiring VNS device removal, and one case of delayed bradycardia induced by the stimulation. The patient having presented the deep infection did not have a new VNS implantation at the end of follow-up. Additionally, two patients did not tolerate side-effects of right-sided VNS and right-sided-VNS was stopped at last follow-up: one patient had a stimulation-related dyspnea and the other was diagnosed with stimulation-related obstructive sleep apnea syndrome.
Twenty-one patients benefited from a postoperative cardiac assessment: in 16 cases an ECG; in 4 cases a 24-h Holter monitor, in 1 case both ECG and a 24-h Holter monitor. The postoperative cardiac assessment revealed abnormalities in three cases: two patients with a first-degree atrioventricular block; one patient with delayed bradycardia. The latter case presented a normal postoperative cardiac assessment but developed stimulation-related bradycardia 5 years after the VNS re-implantation at the right side. Reduction of output current resolved the problem without diminishing the antiseizure efficiency of the VNS (Fig. 2). These three patients had activated right-sided VNS stimulation at the end of follow-up.
Twenty-one (55%) patients could be classified as responders to right-sided VNS (>50% reduction in seizure frequency). The mean reduction of seizure frequency was 56.2 ± 18.8% (range, 30–100). Difference in effectiveness of left-sided and right-sided-VNS within the same patient was analyzed in the 24 patients with prior left-sided VNS and sufficient follow-up time: in the remaining 10 patients with a prior left-sided VNS, this comparison was not possible due to a too short interval between left-sided and right-sided VNS and/or missing data. Right-sided VNS was as effective as left-sided-VNS in 21 cases, more effective than left-sided VNS in two cases, and less effective than left-sided VNS in one case.
Latest used parameters of right-sided VNS
Table 3 presented the latest parameters of each patient. In the 35 patients with a rigth-sided VNS still activated at last follow-up, the latest used parameters of rigth-sided VNS were 1.8 ± 0.8 mA (range, 0.25–3); 314.5 ± 111.2 ms (23 patients with 250 ms, 8 patients with 500 ms), 26.5 ± 4.8 Hz (range, 20–30), duty cycle: ON time 29.1 ± 2.7 sec (range, 21–30), OFF time 4.3 ± 1.0 min (range, 1.1–5).
| Patient | Intensity (mA) | Frequency (Hz) | Pulse width (ms) | On time (sec) | Off time (min) |
|---|---|---|---|---|---|
| 1 | – | – | – | – | |
| 2 | 1,5 | 30 | 500 | 30 | 5 |
| 3 | 2 | 30 | 500 | 21 | 3 |
| 4 | 2.25 | 30 | 500 | 30 | 5 |
| 5 | 1.75 | 30 | 500 | 30 | 3 |
| 6 | 2.25 | 20 | 250 | 30 | 1.1 |
| 7 | 1.75 | 20 | 250 | 30 | 5 |
| 8 | 1.5 | 20 | 250 | 30 | 5 |
| 9 | 1.875 | 30 | 250 | 21 | 1.8 |
| 10 | 1.25 | 20 | 250 | 30 | 3 |
| 11 | 0.875 | – | 250 | 30 | 5 |
| 12 | – | – | – | – | |
| 13 | 2.75 | 30 | 500 | 30 | 1.1 |
| 14 | 2.25 | 20 | 250 | 30 | 1.1 |
| 15 | 2.5 | 20 | 250 | 30 | 1.1 |
| 16 | – | – | – | – | |
| 17 | 1.5 | 30 | 250 | 30 | 3 |
| 18 | 1.75 | 30 | 250 | 30 | 5 |
| 19 | 2.25 | 30 | 250 | 30 | 3 |
| 20 | 1 | 30 | 250 | 30 | 5 |
| 21 | 1 | 30 | 250 | 30 | 5 |
| 22 | 2.5 | 30 | 500 | 30 | 3 |
| 23 | 1.75 | 30 | 500 | 30 | 5 |
| 24 | 2 | 30 | 500 | 30 | 5 |
| 25 | 3 | 20 | 250 | 30 | 1.8 |
| 26 | 3 | 20 | 250 | 30 | 1.8 |
| 27 | 0.25 | – | 250 | – | |
| 28 | 1.75 | – | – | – | – |
| 29 | 1.75 | 20 | 250 | 21 | 1.1 |
| 30 | – | – | – | – | – |
| 31 | 1 | 30 | 250 | 30 | 1.8 |
| 32 | 2 | 30 | 250 | 30 | 1.8 |
| 33 | – | – | – | – | – |
| 34 | 1.75 | 25 | 250 | 30 | 5 |
| 35 | 1.75 | 30 | 250 | 30 | 3 |
| 36 | 1.125 | 30 | 250 | 30 | 5 |
| 37 | 1.25 | 30 | 250 | 30 | 5 |
| 38 | 1 | 20 | – | 30 | 5 |
Discussion
This study highlighted worldwide RS-VNS outcomes. The key results are enumerated as follows: (1) although off label, we were able to collect more than quintuple the number of right-sided VNS cases published so far in the literature, with the 38 cases reported in this series, highlights the scarcity of this practice; (2) the reason for switching to the right side was mainly the existence of left-side-VNS lead dysfunction; (3) right-sided VNS was not associated with severe cardiac complications in the vast majority of the cases; (4) however, with three cases of therapy termination tolerability may be lower than left-sided VNS; (5) mean seizure reduction following right-sided VNS (56.2 ± 18.8%) appeared fairly comparable to left-sided VNS; (6) in two cases, right-sided VNS was more efficient than left-sided VNS whereas in one case, right-sided VNS was less efficient than left-sided VNS; (7) right-sided VNS surgery was performed similarly in technique to left-sided VNS surgery.
Interpretation
According to some operators with important VNS experience, it can always be dealt without resorting to right-sided VNS. On the other hand, some teams do not hesitate to implant right-sided VNS after left-sided VNS dysfunction or infection. This may reflect more a surgical habit than an actual need. The cases with a left ventricular shunt or a complex anatomy (atrio-ventricular canal operated on in this series) constitutes the rare instances in which right-sided VNS may be necessary. Another instance is right-sided vocal cord palsy as a postoperative left vocal cord palsy could lead to dreadful consequences. Under these truly exceptional circumstances, a right-sided VNS may turn out to be the only safe option (personal comm from Pr. Gross). Our study may help decision-making when it comes to dealing with lead failure/infection. Given the safety/efficiency profile, the right-sided VNS may at least be discussed in comparison with the inherent technical difficulties of re-do surgery on the left side in special situations. In fact, long-term vocal cord paralysis could be more likely after a VNS revision on the left side: even if most authors report low complication rates of revision surgery, long-term vocal cord paralysis occurred in approximatively 10% of two series.13, 14
Lead breakage is a possibility after VNS implantation. Lead malfunction/fracture complications range from 2.7% to 11.9%.15-19 The older leads presented a higher rate of dysfunction: this rate should logically decrease over the coming years due to the implantation of more robust leads.15, 19
This study challenges the classical view about VNS. VNS for drug-resistant epilepsy has exclusively been approved on the left side because of the alleged greater risk of asystole and bradycardia.6, 20, 21 As mentioned above, this notion mainly stems from one dog model of drug-resistant epilepsy.6 In other animal models (rat or monkey), right-sided VNS seemed to be as effective as left-sided VNS and not associated without increased incidence of cardiac side effects.22, 23 Interestingly, Dr Jacob Zabara, the neurophysiologist at the origin of the modern VNS, wrote: “Bilateral vagal stimulation produces no measurably greater effect than does unilateral stimulation, and right or left vagal stimulation is equally effective in controlling motor seizures (J. Zabara, unpublished observations, 1990).”24 This work described results on 20 dogs.24 In humans, it was shown that left-sided VNS can lead to cardiac adverse events such asystole or bradycardia.25-30
In this international survey, right-sided-VNS did not lead to life-threatening cardiac complication: two patients presented a first-degree atrioventricular block but had the VNS activated during the whole follow-up and one patient had a late onset bradycardia induced by right-sided-VNS, managed with reduction of output current and day/night differentiated stimulation. This series, along with previously reported cases, suggest that right-sided-VNS does not seem to carry a major risk of cardiac complication. This is in line with the works on human VNS anatomy: the greater right vs left cardiac implication is not proven.7, 31-33 Regarding the possible delay in the onset of cardiac side effects, follow-up observation for possible cardiac side effects might be useful over a longer period of several years in both, left-sided and right-sided VNS.
In the 39 reported patients, right-sided VNS appeared as effective as left-sided VNS. Interestingly, in two cases, right-sided VNS proved more effective than left-sided VNS whereas in one case left-sided VNS was more effective. This difference in efficacy was already reported in one case.11 Right-sided VNS and left-sided VNS were equally effective in reducing seizures in a rat model, suggesting that the stimulation of the right vagus nerve should stir as much interest for drug-resistant epilepsy23 as it is left counterpart. The mean last used parameters were 1.8 mA; 26.5 Hz; 314.5 ms; ON Time: 29.1 sec and OFF time: 4.3 min. These parameters were similar to that reported after left-sided VNS.34
In terms of surgical procedure, right-sided VNS was performed as left-sided VNS. The internal pulse generator position was modified in two cases: one case of “Twiddler syndrome” with a subfascial positioning and one case of left-sided internal pulse generator despite a right-sided lead. The right vagus nerve has been described as larger in diameter than the left one but this had not induced a significant change in surgical procedure in this study.7 The inferior laryngeal nerve is known to be non-recurrent on the right side fifty times more often that on the left.35 Taking this into consideration, no particular nerve branch was encountered during right-sided-VNS implantation. Almost 70% of the experts used optical magnification to implant VNS: this result was in line with our previous study dealing with VNS procedure and teaching.36
To date, there has been no report of a patient with bilateral VNS. The safety of right-sided VNS is well-established for heart failure patients.37 For drug-resistant epilepsy, the recommended laterality remains unequivocally the left: this may explain why the VNS responders exhibited significantly greater connectivity on the left hemisphere.38 Noninvasive VNS offers the possibility to test left, right or bilateral VNS. In this way, Peng et al observed different effects of transcutaneous auricular VNS in function of the site of the stimulation (right ear, left ear, or both ears): ipsilesional VNS seemed necessary for rehabilitation of poststroke patients.39 The possibility to adapt the VNS laterality for each patient and/or bilateral VNS could represent strategies to potentiate anti-seizure effects of VNS. Our findings are consistent with a similar efficiency and safety profile of right-sided VNS versus left-sided VNS. The mechanisms of action are still incompletely understood but right-sided VNS may bring into play the right-sided vagal afferent network and lead to a decrease in functional connectivity and to cortical excitability.8
Study limitations
The present study has several limitations. It was retrospective and uncontrolled by nature. Despite being the largest study to date on the topic of right-sided VNS, there was only a small number of questionnaire respondents, so a low number of reported patients. Despite this limitation, it was possible to increase the available data of right-sided VNS four-fold and better characterize his efficiency and safety profile. These patients were already treated for drug-resistant epilepsy and followed up by highly specialized teams: almost all patients had previously been operated for a left-sided VNS leading to a significant inclusion bias in terms of VNS responders. The questionnaire was limited to 38 items, to prevent pseudonymization and to increase response rate.
Conclusion and future perspectives
This retrospective case series suggests that right-sided VNS may be safe and effective but potentially less tolerable than left-sided VNS. Right-sided VNS implantation may potentially be a useful approach when left-sided VNS is not possible. Prospective studies with clear inclusion criteria would have to be performed to properly investigate the effectiveness and safety profile of right-sided VNS for drug-resistant epilepsy.
Author Contributions
Amelia Alvarez-Sala: investigation, writing – original draft, and writing – review and editing. Sami Barrit: investigation, writing – original draft, and writing – review and editing. François Caire: investigation, writing – original draft, and writing – review and editing. Romain Carron: conceptualization, methodology, validation; investigation, writing – original draft, writing – review and editing, supervision, project administration, and validation. Ramesh Chelvarajah: validation, visualization, investigation, writing – original draft, and writing – review and editing. Alessandro De Benedictis: investigation, writing – original draft, and writing – review and editing. Philippe Domenech: investigation, writing – original draft, and writing – review and editing. riëm el tahry: investigation, writing – original draft, and writing – review and editing. Riccardo Fornaro: investigation, writing – original draft, and writing – review and editing. Raphael Gaillard: investigation, writing – original draft, and writing – review and editing. Bertrand Godet: investigation, writing – original draft, and writing – review and editing. Marc Guenot: investigation, writing – original draft, and writing – review and editing. Masaki Iwasaki: investigation, writing – original draft, and writing – review and editing. Elena Jiltsova: investigation, writing – original draft, and writing – review and editing. Jason Labuschagne: investigation, writing – original draft, and writing – review and editing. Keiya Lijima: investigation, writing – original draft, and writing – review and editing. Shailendra Magdum: investigation, writing – original draft, and writing – review and editing. Alessandro Moiraghi: investigation, writing – original draft, and writing – review and editing. Goran mrak: investigation, writing – original draft, and writing – review and editing. Marcus Neale: investigation, writing – original draft, and writing – review and editing. Johan Pallud: supervision, project administration, investigation, writing – original draft, and writing – review and editing. Guillaume Penchet: investigation, writing –original draft, and writing – review and editing. Željka Petelin Gadže: investigation, writing – original draft, and writing – review and editing. Alexandre Rainha Campos: investigation, writing – original draft, and writing – review and editing. Herbert Rooijakkers: investigation, writing – original draft, and writing – review and editing. Cristina V. Torres: investigation, writing – original draft, and writing – review and editing. Arjune Sen: validation, visualization, investigation, writing – original draft, and writing – review and editing. Fernando Vale: investigation, writing – original draft, and writing – review and editing. Berthold Voges: visualization, investigation, writing – original draft, and writing – review and editing. Tatiana Von Hertwig Fernandes De Oliveira: investigation, writing – original draft, and writing – review and editing; Marc Zanello: conceptualization, methodology, validation, investigation, writing – original draft, writing – review and editing, and visualization.
Acknowledgement
No funding.
Conflict of Interest
Dr Marc Zanello reports a relationship with LivaNova PLC that includes travel reimbursement. Dr Romain Carron reports a relationship with LivaNova PLC that includes speaking, lecture fees, and travel reimbursement. Pr Philippe Domenech reports a relationship with LivaNova PLC that includes speaking, lecture fees, and travel reimbursement. Pr Ramesh Chelvarajah reports a relationship with LivaNova PLC that includes consultancy, speaking, lecture fees, and travel reimbursement. Pr Fernando Vale reports a relationship with LivaNova PLC that includes speaking /lecturing. Dr Berthold Voges reports a relationship with LivaNova PLC that includes speaking, lecture fees, and travel reimbursement. This study did not involve any patient. The authors did not receive any financial support from LivaNova or from any other source.
Funding Information
The authors would like to thank: Mrs Maxine DIBUE, Mr Massimiliano BOFFINI, Mrs Rachel DELMAS, Mrs Celine FETTET, Mrs Maeva GINOUX.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




