Blood exosome connexins and small RNAs related to demyelinating disease activity
Abstract
Objectives
To assess blood exosome (Ex)-connexin (Cx)43 (encoded by GJA1) and its truncated isoforms in multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD), which show distinct alterations in astroglial Cx43.
Methods
Serum Exs from 48 patients with MS (34 relapsing–remitting, 14 secondary-progressive), 35 with NMOSD, 20 with other inflammatory neurologic diseases (OIND), and 17 healthy controls (HC) were subjected to quantitative Western blotting for Cx43, single-molecule array for neurofilament-L, and quantitative polymerase chain reaction for non-coding RNAs detected by RNA sequencing. Sera from control and astroglia-specific Cx43 inducible conditional knockout (Cx43-icKO) mice with experimental autoimmune encephalomyelitis (EAE) were also tested.
Results
Ex-GJA1-29k was markedly higher in MS than in NMOSD, OIND, and HC; it successively increased at relapse, remission, and secondary progression, and positively correlated with disability scores. Ex-hsa-miR-133b and other hsa-miRs that bind to full-length Cx43 were significantly lower in secondary-progressive MS than in HC, and Ex-hsa-miR-133b was negatively correlated with disability scores. Ex-GJA1-11k expression was lower in NMOSD at relapse than in HC and OIND, and was negatively correlated with disability score worsening and Ex-neurofilament-L levels. NMOSD at relapse had significantly higher expression of small nucleolar RNA (SNORD37, SNORD95, and SNORD97) than HC, and SNORD37 and SNORD95 showed strong negative correlations with disability scores. Control mice showed increased Ex-GJA1-43k and -29k during EAE; this effect was markedly reduced in Cx43-icKO mice with attenuated EAE.
Interpretation
Blood Ex-Cx43-truncated isoforms and small non-coding RNAs, which partially come from brain astroglia, are distinctly dysregulated in MS and NMSOD.
Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system, and neuromyelitis optica spectrum disorder (NMOSD) primarily targets astroglia by anti-aquaporin 4 (AQP4) antibodies. Connexins (Cxs) are transmembrane proteins that form gap junction channels. They have crucial roles in maintaining metabolic homeostasis of the brain.1 We and others have reported widespread glial Cx alterations in MS and NMOSD lesions.2-4 In MS, oligodendroglial Cxs32/47 and astroglial Cx43 are absent in acute plaques, whereas Cx43 is markedly upregulated in chronic plaques, reflecting astrogliosis, despite a persistent loss of Cxs32/47.2-4 Similar glial Cx changes are observed in acute and chronic experimental autoimmune encephalomyelitis (EAE), an animal model of MS.5, 6 In NMOSD, there is early and extensive Cx43 loss in acute lesions, which is partially restored in chronic lesions; persistent oligodendroglial Cx loss follows acute Cx43 loss.4 We revealed that oligodendroglia-specific Cx47 inducible conditional knockout (Cx47-icKO) exacerbates acute and chronic EAE in mice via inflammatory mediator secretion through Cx43 hemichannels.6 By contrast, brain astroglia-specific Cx43-icKO attenuates EAE in mice.7 Brain astroglia are activated prior to EAE onset,8 suggesting their remote proinflammatory effects on spinal cord inflammation involving Cx43.
Cx43 exists on the surface of exosomes (Exs), which can bypass the blood–brain barrier.9 Aside from its channel functions, Cx43 directly binds RNA and is crucial for the selective sorting of microRNA (miR) into Exs.10, 11 Moreover, Cx43 incorporated into Exs facilitates intraluminal miR release into target cells, thereby modulating cell functions.11, 12 Upon cell stress, GJA1, which encodes full-length Cx43, also produces N-terminal-truncated isoforms using internal ribosome entry sites.13, 14 Such truncated isoforms lose channel functions but partially retain RNA-binding sites.10 Furthermore, GJA1-11k localizes in the nucleus and suppresses cell proliferation signals.15, 16 Because Ex-proteins/RNAs protected by lipid bilayers are more stable than free ones, we hypothesized that Cx43 and its truncated isoforms in blood Exs might reflect brain astroglial status and modulate demyelinating diseases. However, no Ex-protein/RNA markers have yet been established in demyelinating diseases because of the limited number of studies of Ex-proteins/RNAs.17-19 Therefore, in the current study, we investigated blood Ex-Cx43, its truncated isoforms, and Cx43-binding non-coding RNAs (ncRNAs)11 in MS and NMOSD, as well as in astroglia-specific Cx43-icKO mouse EAE.7 We aimed to elucidate their roles and biomarker potentials, particularly in progressive MS and acute NMOSD attacks.
Methods
Participants
We enrolled 48 patients with MS, 35 with NMOSD, 20 with other inflammatory neurologic diseases (OIND), and 17 healthy controls (HC) from the Departments of Neurology at Kyushu University Hospital and Fukuoka Central Hospital, with written informed consent (approval numbers 2023-7 and 20-ifh-024, respectively). Diagnoses of MS and NMOSD were based on the 2017 McDonald criteria20 and 2015 NMOSD criteria,21 respectively (Table 1). Patients' medical records were retrospectively reviewed. Disability was scored using Kurtzke's Expanded Disability Status Scale (EDSS).22 Serum samples were collected between July 1, 2014 and December 31, 2023 and stored at −80°C.
| Samples | MS | NMOSD | OIND | HC | p-value | |||
|---|---|---|---|---|---|---|---|---|
| MS versus HC | NMOSD versus HC | MS versus NMOSD | OIND versus HC | |||||
| Number of samples | 48 | 35 | 20 | 17 | NS | NS | NS | NS |
| Female | 40 (83.3) | 32 (91.4) | 13 (65) | 11 (64.7) | NS | NS | NS | NS |
| Age at examination, years | 44 [33–51.8] | 50 [38–57] | 49.5 [26.8–53] | 41 [35.5–50] | NS | NS | NS | NS |
| Age at disease onset, years | 28.5 [23.3–38.8] | 40 [30–50] | – | – | – | – | 0.0008 | – |
| Disease duration, years | 11 [4–16] | 6 [1–13] | – | – | – | – | 0.0497 | – |
| AQP4-IgG | – | 27 (77.1) | – | – | – | – | – | – |
| Phase (relapse/remission/progression) | 13 (27.1)/21 (43.8)/14 (29.22) | 15 (42.9) /20 (57.1) | – | – | – | – | – | – |
| EDSS scores | 2.5 [1.5–4.5] | 3 [2–5] | – | – | – | – | NS | – |
| Treatment at time of blood drawing | ||||||||
| None | 18 | 5 | ||||||
| DMF | 13 | – | ||||||
| FTY | 12 | – | ||||||
| IFN-β | 3 | – | ||||||
| PSL | 2 | 27 | ||||||
| AZT | – | 10 | ||||||
| TAC | – | 7 | ||||||
- Values indicate the median [interquartile range] or number (percentage). At the time of blood drawing, 30 patients with MS were under immunotherapy (mostly by dimethyl fumarate or fingolimod) and 18 were untreated. Of the patients with NMOSD, 27 were receiving corticosteroids, 17 were receiving immunosuppressants (either azathioprine or tacrolimus), and 5 were untreated by immunotherapy. NMOSD patients under high efficacy monoclonal antibody therapy were not enrolled in this study.
- AQP4-IgG, aquaporin-4-immunoglobulin G; AZT, azathioprine; DMF, dimethyl fumarate; EDSS, Expanded Disability Status Scale; FTY, fingolimod; HC, healthy control; IFN-β, interferon-beta; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorder; NS, not significant; OIND, other inflammatory neurological disease; PSL, prednisolone; TAC, tacrolimus.
Ex isolation and quantitative Western blot analysis
The ExoQuick™ Exosome Isolation Kit (System Biosciences, Palo Alto, CA, USA) was used to purify Exs from 350 μL serum from each subject according to the manufacturer's instructions. The Ex protein concentration was determined using a Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). Proteins were heated at 95°C for 5 min before equal amounts (20 μg/well) were loaded onto 4%–20% Mini-PROTEAN TGX gels (#4561096; Bio-Rad, Hercules, CA, USA) and electrophoresed using the Mini PROTEAN Tetra system (Bio-Rad). After electrophoresis, separated proteins were transferred onto Immobilon-P Transfer Membranes (IVPH00010; Millipore, Cork, Ireland). The membranes were blocked with EveryBlot Blocking Buffer (#12010020; Bio-Rad) for 5 min at room temperature. Subsequently, membranes were incubated with primary antibodies reacting with the C-terminal portion of Cx43 (1:3000 dilution; ab11370, Abcam, Cambridge, UK) and CD63, an Ex marker (1:1000 dilution; 220803-001, System Biosciences), overnight at 4°C. After washing, membranes were incubated with goat anti-rabbit immunoglobulin G (H + L) horseradish peroxidase-conjugated secondary antibody (1:400,000 dilution for Cx43 and 1:300,000 dilution for CD63) for 1 h at room temperature. Each time, an equal amount of Ex protein from a single HC was used as a control (the GJA1-43k band density from this HC was taken as 1.0) for normalization. Signals were measured using a WSE-6270 LuminoGraph II EM (ATTO, Tokyo, Japan).
Single-molecule array (SIMOA) assay for Ex-proteins
Neurofilament-L (NfL; a neuroaxonal damage marker) and glial fibrillary acidic protein (GFAP; an astroglial damage/reaction marker) in Exs were quantified using the Simoa® Neurology 2-plex B Kit (#103520; Quanterix, Lexington, MA, USA) with a Simoa HD-X (Quanterix) according to the manufacturer's instructions.
Ex-RNA extraction
Serum samples were centrifuged at 3000 × g for 15 min at 4°C. The supernatant was transferred to a new tube and centrifuged again at 12,000 × g for 10 min to remove cell debris. Sera (600 μL) were diluted with an equal volume of phosphate-buffered saline and passed through a 0.45-μm filter (Sartorius, Goettigen, Germany). Exs were lysed using QIAzol lysis reagent (Qiagen, Venlo, Netherlands) before adding Cel-miR-39 spike-in-control (Thermo Fisher Scientific; final concentration 4.65 pM) and 2000 copies of VetMax Xeno internal control (Thermo Fisher Scientific) to each sample. The ExoRNAeasy MIDI Kit (Qiagen) was used to extract a long RNA fraction, which included long intergenic ncRNAs (lincRNAs) followed by a short RNA fraction, which included miR and small nucleolar RNAs (snoRNAs), according to the manufacturer's protocol.
Next-generation sequencing
RNA samples from four representative patients with secondary-progressive (SP)MS, three with NMOSD at relapse, and four HC were used to construct libraries for small RNA sequencing using the SMARTer smRNA-Seq Kit for Illumina (Takara, Tokyo, Japan) according to the manufacturer's protocol. Cluster amplification and 75-bp single-end sequencing were performed according to the manufacturer's protocol for NovaSeq (Illumina). All read data were checked using FastQC (v0.11.7: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/) and trimmed using Trimmomatic (v0.38: https://github.com/usadellab/Trimmomatic/releases). The trimmed data were processed using RSEM (v1.3.0: https://github.com/deweylab/RSEM), aligned to reference data GRCh38 using bowtie2 (v2.3.4: https://github.com/BenLangmead/bowtie2), and the raw read counts of each gene were obtained. EdgeR (https://bioconductor.org/packages/release/bioc/html/edgeR.html) was then used to obtain normalized counts per million values for each gene.
Quantitative polymerase chain reaction (PCR)
We used real-time PCR to measure the ncRNAs detected by Ex-RNA sequencing for which commercial kits (miR: TaqMan™ MicroRNA Assays, Thermo Fisher Scientific; sno/lincRNA: TaqMan Non-coding Assays, Applied Biosystems, Waltham, MA, USA) were available. First, cDNA was generated from each RNA sample using the TaqMan MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific) for miR or the High-capacity cDNA Reverse Transcription Kit (Thermo Fisher) for sno/lincRNA. It was then subjected to real-time PCR according to the manufacturer's instructions. Additionally, hsa-miR-133b, which was not detected by RNA sequencing but reportedly binds most strongly to Cx43,13 was measured. The ΔΔCt method was used to determine relative expression levels of target ncRNAs normalized to Cel-miR-39 or VetMax Xeno internal control; relative quantification was calculated using 2−ΔΔCt.
Target gene prediction and functional analysis
The miRWalk database (http://mirwalk.uni-hd.de/) was used to predict the common potential target genes of candidate miRs between miRwalk and miRTarBase (https://mirtarbase.cuhk.edu.cn/~miRTarBase/miRTarBase_2022/php/index.php). Target gene functions were analyzed using Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis (https://www.genome.jp/kegg/) and Gene Ontology enrichment analysis (geneontology.org) with the Database for Annotation, Visualization and Integrated Discovery (DAVID) Bioinformatics Resources 6.8 (https://david.ncifcrf.gov/).
Generation of Cx43-icKO mice
All animal experiments were performed according to the guidelines for the proper conduct of animal experiments published by the Science Council of Japan. Ethical approval was granted by the animal care and use committee of Kyushu University (approval number: A25-196). Glutamate aspartate transporter CreERT2;Cx43fl/fl (Cx43-icKO) mice were generated as previously described.7 Cx43-icKO mice were intraperitoneally injected with 1 mg tamoxifen (Sigma-Aldrich, St. Louis, MO, USA) in 100 μL corn oil twice daily for five consecutive days to induce Cx43 knockdown in brain gray matter astroglia. Control (fl/fl) mice were also injected with tamoxifen using the same dose and protocol. All experiments were performed using 8- to 12-week-old female mice.
EAE induction and ex analysis
EAE was induced by immunizing each mouse with 4 mg/mL myelin oligodendrocyte glycoprotein35–55 peptide (S-PEP; Scrum, Tokyo, Japan) emulsified in complete Freund's adjuvant containing 1 mg/mL Mycobacterium tuberculosis H37RA (#7027; Chondrex Inc., Woodinville, WA, USA) at a dose of 200 μg, followed by intraperitoneal injections of 300 ng pertussis toxin (#168–22471; Wako, Osaka, Japan) on Days 0 and 2. Mice were examined daily for signs of EAE. Serum Exs were used for quantitative Western blot analysis, as described above. Moreover, Exs were immunostained with anti-Cx43 primary antibody (1:1000; ab 11370, Abcam) for 2 h at room temperature, washed with phosphate-buffered saline, incubated with Alexa Fluor 488-conjugated secondary antibody (1:1000; Thermo Fisher Scientific) for 1 h at room temperature in the dark, and observed using a BX50 Fluorescence Microscope (Olympus, Tokyo, Japan).
Statistics
Data were compared between groups using Fisher's exact test or a likelihood ratio chi-squared test for categorical data, and the Kruskal–Wallis test followed by the Wilcoxon test with multiple testing for continuous data. Correlations among continuous scales were calculated using Spearman's rank correlation coefficient. All analyses were performed using JMP® Pro version 16.0.0 software (SAS Institute, Cary, NC, USA) and GraphPad Prism software (La Jolla, CA, USA). The significance level was set at p < 0.05.
Results
Patient characteristics
There were no significant differences in sex or age at examination among the HC and patients with MS, NMOSD, and OIND whose samples were used for Western blot analysis (Table 1). Age at disease onset was significantly younger in MS than in NMOSD, and disease duration was marginally longer in MS than in NMOSD. Participants in the SIMOA (Table S1) and RNA assays (Table S2) had essentially the same characteristics as those in the Western blot assay.
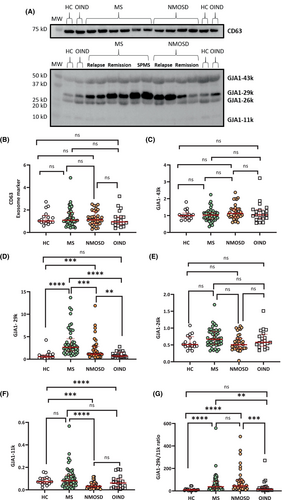
Altered expression of Ex-GJA1-truncated isoforms in MS and NMOSD
Quantitative Western blot analysis revealed no significant differences in CD63 levels among MS, NMOSD, OIND, and HC (Fig. 1A,B). Western blot analysis for Cx43 showed the presence of Cx43 (GJA1-43k) and its three truncated isoforms, GJA1-29k, -26k, and -11k in HC, MS, NMOSD, and OIND (Fig. 1A). Quantitative Western blot analysis showed no significant differences in Ex-GJA1-43k or -26k levels among the four groups; however, Ex-GJA1-29k was markedly higher in MS than in NMOSD, OIND, or HC (MS vs. HC or OIND, p < 0.0001; MS vs. NMOSD, p < 0.001) (Fig. 1C–E). Additionally, NMOSD had higher GJA1-29k expression than HC or OIND (NMOSD vs. HCp <0.001; NMOSD vs. OIND, p < 0.01). Notably, GJA1-11k was lower in NMOSD than in HC (p < 0.01) and MS (p < 0.01) and tended to be lower in NMOSD than in OIND (p = 0.0675) (Fig. 1F), whereas such a decrease was not observed in MS. Consequently, the Ex-GJA1-29k/-11k ratio was significantly higher in MS and NMOSD than in HC and OIND, but did not differ significantly between MS and NMOSD (Fig. 1G).
Successive increase of Ex-GJA1-29k by clinical phase in MS
In MS, Ex-GJA1-29k was higher in all phases compared with HC (relapse vs. HC, p < 0.001; remission vs. HC, p < 0.0001; SPMS vs. HC, p < 0.0001) (Fig. 2A). Ex-GJA1-29k levels successively increased in MS at relapse ≤ MS in remission ≤ SPMS (relapse vs. SPMS, p < 0.05). Other truncated isoforms, including GJA1-11k, showed no significant differences in any phase of MS compared with HC (Fig. 2B). Thus, the GJA1-29k/-11k ratio showed an increasing trend by clinical phase in MS, similar to that of GJA1-29k (Fig. 2C). There was an apparent but non-significant positive correlation between Ex-GJA1-29k and EDSS scores in SPMS only (r = 0.5298, p = 0.0518) (Fig. 2D).
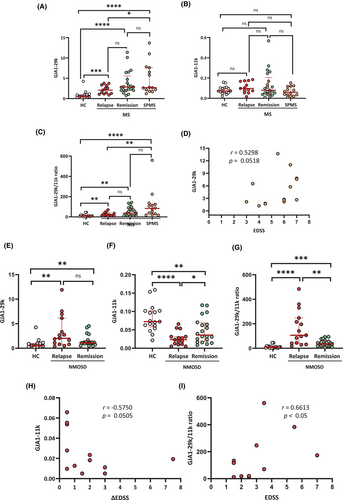
Decreased Ex-GJA1-11k associated with disability worsening in NMOSD
In NMOSD, Ex-GJA1-29k was significantly higher at relapse and in remission compared with HC (relapse or remission vs. HC, p < 0.01) but did not significantly differ between relapse and remission (Fig. 2E). By contrast, GJA1-11k was markedly lower in NMOSD at relapse compared with HC and NMOSD in remission (relapse vs. HC, p < 0.0001; relapse vs. remission, p < 0.05) (Fig. 2F). In remission, GJA-11k was partially recovered but still lower than in HC (p < 0.01). Thus, the GJA1-29k/−11 k ratio was markedly higher at relapse compared with HC and in remission (relapse vs. HC, p < 0.0001; relapse vs. remission, p < 0.01) (Fig. 2G). Even in remission, the GJA1-29k/-11k ratio remained significantly higher than in HC (p < 0.001). At relapse, GJA1-11k demonstrated an apparent but non-significant negative correlation with EDSS score worsening (ΔEDSS scores) (r = −0.5750, p = 0.0505), and the -29k/-11k ratio significantly positively correlated with EDSS scores (r = 0.6613, p < 0.05) (Fig. 2H,I).
Increased Ex-GFAP and -NfL correlate with disability in MS and disability worsening in NMOSD at relapse, respectively
SIMOA assay revealed that Ex-NfL and -GFAP levels correlated with serum NfL and GFAP levels, respectively, in all studied samples (NfL, r = 0.8727, p < 0.0001; GFAP, r = 0.8922, p < 0.0001). Moreover, Ex proportions were significantly greater in GFAP than in NfL (p < 0.0001) (Fig. S1). Compared with HC, Ex-NfL was significantly elevated in NMOSD (p < 0.01) but not in MS (Fig. 3A). By clinical phase, Ex-NfL was elevated at relapse (p < 0.01) but not in other MS phases compared with HC (Fig. 3B). In NMOSD, Ex-NfL was increased at relapse (p < 0.01) but not in remission compared with HC (Fig. 3C). Ex-GFAP was significantly elevated in MS (p < 0.05) but not in NMOSD compared with HC (Fig. 3D). By clinical phase, Ex-GFAP was markedly higher in SPMS than in HC and in MS in remission (both P < 0.01), and was significantly higher in MS at relapse than in HC (p < 0.05) (Fig. 3E). In NMOSD, Ex-GFAP was significantly higher at relapse than in HC (p < 0.01) and in remission (p < 0.05); there was no difference in Ex-GFAP between NMOSD in remission and HC (Fig. 3F). In MS, Ex-GFAP but not Ex-NfL was significantly positively correlated with EDSS scores (r = 0.5073, p < 0.01) (Fig. 3G,J). By contrast, in NMOSD at relapse, Ex-NfL but not Ex-GFAP was significantly positively correlated with ΔEDSS scores (r = 0.6548, p < 0.05) (Fig. 3H,K).
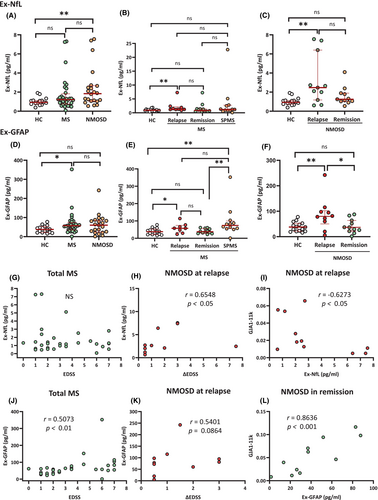
Correlation between Ex-GJA1-truncated isoforms and Ex-NfL/GFAP
Ex-GJA1-11k was negatively correlated with Ex-NfL in NMOSD at relapse (r = −0.6273, p < 0.05) but positively correlated with Ex-GFAP in NMOSD in remission (r = 0.8636, p < 0.001) (Fig. 3I,L). Such correlations were not observed in MS.
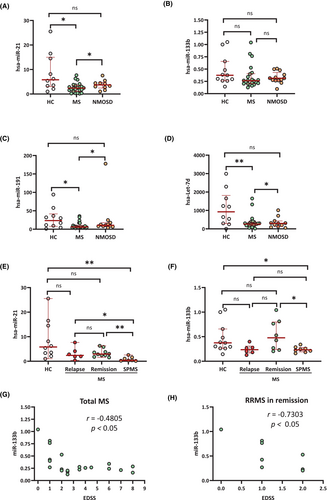
Decreased Ex-Cx43-binding hsa-miRs in SPMS
The ncRNAs detected by Ex-RNA sequencing are shown in Table S3. Among the miRs examined using real-time PCR (Table S4), hsa-miR-21, hsa-miR-191, and hsa-let-7d were significantly lower in MS than in HC and NMOSD; the other hsa-miRs (hsa-miR-199a, hsa-miR-335, and hsa-miR-133b) did not exhibit significant alterations in MS or NMOSD (Fig. 4A–D and Fig. S2A,B). By clinical phase, hsa-miR-21 and hsa-miR-133b were significantly lower in SPMS but not in MS at relapse or in remission compared with HC (Fig. 4E,F). The other hsa-miRs had no significant alterations by clinical phase compared with HC (data not shown). Hsa-miR-133b was significantly negatively correlated with EDSS scores in MS overall (r = −0.4805, p < 0.05) and in MS in remission (r = −0.7303, p < 0.05) (Fig. 4G,H).
Decreased Ex-Cx43-binding hsa-miRs in SPMS target inflammatory pathways
The miRwalk database search predicted the following numbers of target genes in each miR: 27 (including the receptor tyrosine kinase gene) for hsa-miR133b, 157 (including the interleukin-6 family receptor gene) for hsa-let-7d, 65 for hsa-miR-21, and 18 for hsa-miR-191 (Tables S5–8). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of the predicted target genes in each hsa-miR revealed significant enrichment in the following inflammation-related pathways: hsa-miR-133b, Ras signaling pathway and PI3K–Akt signaling pathway; hsa-let-7d, JAK–STAT signaling pathway and P53 signaling pathway; hsa-miR-21, p53 signaling pathway; hsa-miR-191, none (Fig. S3 and Tables S5–S8). Gene ontology analysis also revealed significant enrichment of the predicted target genes in the following inflammation-related biological processes: has-miR-133b, macrophage differentiation, response to hypoxia, and tissue remodeling; hsa-let-7d, regulation of B cells and negative regulation of endothelial cell proliferation; hsa-miR-21, negative regulation of plasminogen activation and negative regulation of fibrinolysis; hsa-miR-191, none (Tables S5–S8).
Increased Ex-sno/lincRNAs in NMOSD at relapse
Of the sno/lincRNAs examined using real-time PCR (Table S4), only SNORD97 was significantly higher in NMOSD than in HC (p < 0.05); all other sno/lincRNAs were not significantly altered in MS or NMOSD compared with HC (Fig. 5A–D and Fig. S2C,E,G). By clinical phase, NMOSD at relapse had significantly higher SNORD37, SNORD95, SNORD97, and Linc01560—but not Linc00501, 00547, or 01482—compared with HC and NMOSD in remission. By contrast, there were no significant alterations in any phase of MS (Fig. 5E–H and Fig. S2D,F,H).
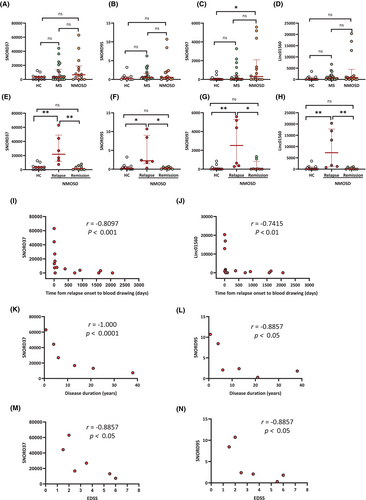
In NMOSD, SNORD37, SNORD95, SNORD97, and Linc01560 were significantly negatively correlated with time from relapse onset to blood drawing (SNORD37, r = −0.8097, p < 0.001; SNORD95, r = −0.5413, p < 0.05; SNORD97, r = −0.5501, p < 0.05; Linc01560, r = −0.7415, p < 0.01) (Fig. 5I,J and Fig. S2I,J). SNORD37 and SNORD95 also showed significant negative correlations with disease duration (SNORD37, r = −1.000, p < 0.0001; SNORD95: r = −0.8857, p < 0.05) (Fig. 5K,L) and EDSS scores (SNORD37, r = −0.8857, p < 0.05; SNORD95, r = −0.8857, p < 0.05) (Fig. 5M,N).
Blood Ex-GJA1 isoform alterations in EAE
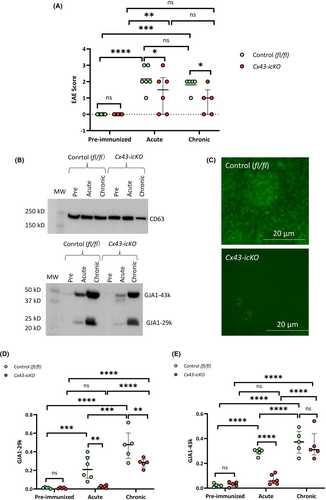
Astroglia-specific Cx43-icKO mice had significantly attenuated EAE in both the acute and chronic phases compared with control mice (Fig. 6A), as previously reported.7 Serum Ex-GJA1-29k progressively increased at acute to chronic phases in control mice (Fig. 6B,D). By contrast, Cx43-icKO mice showed no alterations in Ex-GJA1-29k in the acute phase, but a significant increase in the chronic phase. Furthermore, Ex-GJA1-29k was significantly lower in Cx43-icKO mice than in control mice in the acute and chronic phases, but not in the preimmunized phase. Additionally, GJA1-43k showed similar increases in the acute and chronic phases in control mice (Fig. 6B,E). Cx43-icKO mice had significantly increased GJA1-43k in the chronic but not acute phase, which was further supported by a lack of Cx43 immunostaining in Exs from Cx43-icKO mice with acute EAE (Fig. 6C). Ex-GJA1-43k was significantly lower in Cx43-icKO mice than in control mice at the acute but not chronic phase.
Discussion
In the present study, we revealed marked alterations in Cx43-truncated isoforms and small ncRNAs in peripheral blood Exs from patients with MS and NMOSD. Astrocytes secrete Exs; upon neuroinflammation, this is facilitated via cytokine stimulation.23 Indeed, peripheral blood GFAP-positive Exs are reportedly significantly increased in mice with EAE compared with those without EAE,23 which is consistent with our findings of increased Ex-GFAP in MS and NMOSD at relapse. Such increased Ex-GFAP likely reflects astroglial status under acute inflammatory stress. Notably, SPMS had a greater Ex-GFAP increase than HC and MS in remission, and no NMOSD patients in remission had any Ex-GFAP increase. This large increase of Ex-GFAP in SPMS, which is consistent with the reportedly increased serum GFAP in SPMS,24, 25 is unique to this condition and probably reflects progressive astrogliosis, which is not observed in NMOSD pathology.5 It is also possible that such an increase of Ex-GFAP may relate to the finding that MS shows progression independent of relapse activity, whereas NMOSD rarely shows inter-relapse worsening.26 The positive correlation between EDSS scores and Ex-GFAP but not Ex-NfL suggests that peripheral blood GFAP-positive Exs from astroglia may be associated with progressive disability worsening in MS, particularly at SP.
Of the altered Cx43-truncated isoforms, GJA1-29k – a predominant isoform in peripheral blood Exs from patients with central nervous system demyelinating diseases – commonly increased at relapse in MS and NMOSD. This phenomenon was also observed in acute EAE. By contrast, successive increases of Ex-GJA1-29k from relapse to remission and SP were unique to MS, and were not observed in NMOSD. This is largely consistent with the aforementioned Ex-GFAP changes in MS. Moreover, these features resemble the successive increase in Ex-GJA1-29k from acute to chronic EAE in control mice. The observed common increases in Ex-GJA1-29k during acute MS and NMOSD attacks seem to reflect acutely damaged/stressed astroglia, whereas the further progressive increase in Ex-GJA1-29k that was observed only in SPMS may reflect ongoing disease activity between relapses in MS in contrast to what occurs in NMOSD.2-4
The marked decrease in Ex-GJA1-29k during acute EAE in astrocyte-specific Cx43-icKO mice suggests that Ex-GJA1-29k mostly originates from brain astroglia during acute neuroinflammation. The glutamate aspartate transporter-positive astroglia-specific Cx43-icKO specifically ablates Cx43 in brain gray matter astroglia,27 and attenuates EAE by converting astroglia toward an anti-inflammatory phenotype and suppressing the proinflammatory activation of spinal microglia via remote effects.7 These remote regulatory effects of brain astroglia may be mediated partly though decreased cerebrospinal fluid proinflammatory cytokine/chemokine levels.7 It is also plausible that reactive astroglia-derived Exs augment neuroinflammation.18, 28 Because cellular stress such as hypoxia and oxidation can facilitate Cx43-truncated isoform production and its sorting to Exs,13, 14, 16, 29 GJA1-29k may become more abundant than Cx43 in Exs – particularly in NMOSD at relapse and in SPMS, which respectively involve stress via anti-AQP4 antibody-mediated attacks and relentless, ongoing disease activity. Ex-Cx43 transmits Ex contents, including proteins and miRs, to acceptor cells not only by passage through Cx43 channels but also by fusion and internalization with recipient cells.12, 29 Thus, Exs expressing Cx43 and its truncated isoforms may modulate neuroinflammation by delivering pro- or anti-inflammatory messengers.18, 28 In the chronic EAE phase, the observed significant but relatively mild Ex-GJA-29k decrease in Cx43-icKO mice compared with control mice suggests that spinal cord astroglia – in which Cx43 is not deleted – or other cells such as microglia and T cells that up-regulate Cx43 upon inflammation30, 31 may become a main source of Ex-GJA1-29k. In our study, increased Ex-GJA1-43k was observed in EAE. It is possible that a loss of Cx43-containing Exs in Cx43-icKO mice ameliorates EAE via the decreased delivery of Ex-proinflammatory messengers.18, 27
Between MS and HC, a variety of differentially expressed miRs have been reported,17-19, 32-34 although consistency among studies remains a major issue. However, serum Ex studies are relatively limited.32, 33 One study reported decreased hsa-miR-122-5p, hsa-miR-196b-5p, hsa-miR-301a-3p, and hsa-miR-532-5p in MS at relapse, suggesting disturbed cell-to-cell communications.32 Another study reported the association of nine Ex-hsa-miRs with progressive MS; of these, hsa-miR-337-3p was negatively correlated with EDSS scores, suggesting its protective role.33 Similarly, we observed significant decreases in hsa-miR-21, hsa-miR-133b, hsa-miR-191, and hsa-let-7d in MS or SPMS compared with HC. Although no previous studies have focused on Cx43-binding miRs, hsa-miR-133b and hsa-let-7d are reportedly Cx43-binding miRs.11 Notably, hsa-miR-133b, which is most strongly bound to full-length Cx43,11 negatively correlated with EDSS scores in our investigation, indicating its protective role. The N- and C-terminus domains of Cx43 are reportedly involved in hsa-miR-133b sorting to Exs.11 GJA1-29k loses some of these RNA-binding sites because of N-terminus truncation; this may decrease the sorting of Cx43-binding miRs to Exs. The downregulation of these miRs suggests a loss of the neuroprotective and regulatory mechanisms that help to mitigate inflammation and neuronal damage. Moreover, KEGG pathway analysis indicated the regulatory functions of the potential target genes of these altered miRs in the Ras/PI3–Akt/JAK–STAT signaling pathways, which all play crucial roles in neuroinflammation. Indeed, hsa-miR-133b-positive Ex treatment reportedly improves functional recovery and neurite growth in a stroke model,35 and inhibits nod-like receptor protein (NLRP)3 inflammasome-mediated inflammation.36 Moreover, hsa-miR-191-containing Exs suppress lipopolysaccharide-induced inflammation,37 and hsa-miR-191 overexpression inhibits the NLRP3 inflammasome pathway.38 Hsa-let-7d alleviates neuroinflammation by promoting microglial conversion to an anti-inflammatory phenotype39 and suppresses B-cell activation.40 The Ex-mediated transfer of let-7d from regulatory T cells to T-helper-1 cells also suppresses systemic inflammation.41 Hsa-miR-21 has anti-inflammatory properties by inhibiting nuclear factor-κB and downregulating proinflammatory cytokine production.42, 43 Collectively, these Ex-miRs appear to have protective and anti-inflammatory effects. We therefore speculate that a dysregulated increase of GJA1-29k may induce delivery failures of these miRs, resulting in increased inflammation and the exacerbation of neural damage in SPMS (Fig. 7).
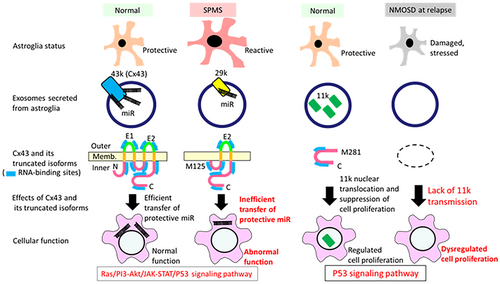
In NMOSD, GJA1-11k (a Cx43 C-terminal tail fragment) was specifically decreased throughout all clinical phases; it was more severely decreased at relapse than in remission. Of note, GJA1-11k was negatively correlated with disability worsening and Ex-NfL levels, which indicates the protective role of GJA1-11k. Nuclear GJA1-11k can inhibit cell proliferation by inducing P53 expression.15, 16 Thus, Ex-GJA1-11k loss may dampen inhibitory signals to glia and immunocytes, thereby leading to severe inflammatory damage in NMOSD (Fig. 7). With NMOSD in remission, GJA1-11k showed a strong positive correlation with Ex-GFAP, which may reflect astroglial recovery; however, it remains to be elucidated whether Ex-GJA1-11 k is mostly derived from brain astroglia or from other immunocytes.
In NMOSD compared with HC, two studies have reported differentially expressed long ncRNAs in peripheral blood mononuclear cells using microarray technology,44, 45 and one study reported differentially expressed Ex-miRs using next-generation sequencing.46 However, the exact roles of these ncRNAs remain elusive. Our study is the first to show increased levels of specific sno/lincRNAs in blood Exs (i.e., SNORD37, SNORD95, SNORD97, and Linc01560) during relapse in NMOSD. The strong negative correlations between these SNORD levels and EDSS scores at relapse suggest that these elevated snoRNAs may attenuate disease severity. SnoRNAs are 60- to 250-nucleotide-long ncRNAs that are required for alternative splicing and the modification of other ncRNAs.47 Conversely, lincRNAs (with more than 200 nucleotides) function as sponges that trap certain miRs,48 thereby regulating gene expression.49 Although the functions of these sno/lincRNAs in neurological diseases remain unknown, they are likely involved in a diverse set of cellular processes such as translational regulation, ribosome biogenesis, apoptosis, immune response, tumorigenesis, and neurodegeneration.50 Thus, these elevated Ex-sno/lincRNAs may limit the extent of neural damage in NMOSD and support recovery. The negative correlations between these sno/lincRNAs and time from relapse onset to blood drawing suggest that these compensatory mechanisms may operate from the very early course of relapse. Additionally, the negative correlations between these sno/lincRNAs and disease duration indicate that these compensatory mechanisms may decrease in functioning as the disease develops, possibly reflecting an exhausted astroglia status.
There are several limitations to the present study. First, relatively few patients with MS and NMOSD were enrolled, partly because of the rarity of these diseases in Japan. In particular, the number of relapsed patients was limited because of the recent availability of highly efficacious drugs for both diseases. Second, Western blot analysis, SIMOA, and PCR studies were not done for all enrolled patients with MS and NMOSD because of limited sample availability. Thus, the current findings should be regarded as preliminary. Third, although patients under highly efficacious monoclonal antibody therapy were excluded, the effects of other disease-modifying drugs were not evaluated because of the small numbers of participants.
In conclusion, our study revealed that blood Ex-Cx43-truncated isoforms and ncRNAs, which are believed to arise primarily from brain astroglia during neuroinflammation, were distinctly dysregulated in MS and NMSOD. Increased Ex-GJA1-29k and decreased Ex-hsa-miR-133b in SPMS were positively and negatively correlated with disability scores, respectively, whereas decreased GJA1-11k and increased SNORD37 and SNORD95 were negatively correlated with worsening disability at NMOSD relapse. These distinct changes in Ex-Cx43-truncated isoforms and ncRNAs between MS and NMOSD may reflect astroglial status in each disease.
Acknowledgments
This study was supported by grants from the Japan Agency for Medical Research and Development (AMED), Japan (Grant Nos. 21ek0109547h0001, 22ek0109547h0002, 23ek0109547h0003, 23ek0109626h0001, 23ek010962h0002, and 24wm0625504s01001) and from Japan Society for the Promotion of Science KAKENHI (Grant Nos. JP22H02985, JP22K07351, JP23K18266, JP23K14761, JP23K24246, JP24K02371, JP24K10666, and JP24K18710), Japan. We thank Cell Innovator JP (http://www.cell-innovator.com/jp) for the RNA sequencing and data analysis, and Bronwen Gardner, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Conflicts of Interest
G.M. reports grants from JSPS KAKENHI (Grant Nos. JP20K22910 and JP24K18710). J.K. reports research funds from Sumitomo Pharma, Daiichi Sankyo, Mitsubishi Tanabe Pharma, and Yamasa Corporation; and consultancy fees, speaking fees, and/or honoraria from Novartis Pharma, Argenx, Biogen Japan, Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., Alexion Pharma, and Kyushu University. Y.N. reports grants from JSPS KAKENHI (Grant Nos. JP21K07467 and JP24K10666) and speaking fees and/or honoraria from Novartis Pharma, Biogen Japan, Takeda Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., and Chugai-Igakusha. M.W. reports a grant from JSPS KAKENHI (Grant No. JP22K07351) and payment or honoraria for lectures, speakers' bureaus, manuscript writing, and/or participation on an advisory board from Novartis Pharma, Biogen Japan, Mitsubishi Tanabe Pharma, Alexion Pharma, Chugai Pharmaceutical, Argenx, UCB Japan, Viatris, and Daiichi Sankyo. A.S. reports a grant from JSPS KAKENHI (Grant No. JP23K14761). X.Z. reports grants from JSPS KAKENHI (Grant Nos. JP21K15703 and JP23K14783). K.M. reports a grant from JSPS KAKENHI (Grant No. JP23K06965) and speaker honoraria from Novartis Pharma, Biogen Japan, Alexion, Chugai Pharmaceutical, and Tanabe Mitsubishi. R.Y. reports honoraria from Teijin Pharma, Ono Pharmaceutical, Takeda Pharmaceutical, Eisai, Novartis, Nihon Pharmaceutical, and CSL Behring. N.I. reports grants from JSPS KAKENHI (Grant No. JP24K02371), AMED Japan (Grant No. 24wm0625504s01001), the Health and Labour Sciences Research Grant on Intractable Diseases (Neuroimmunological Diseases) from the Ministry of Health, Labour and Welfare of Japan (23FC1009), Sumitomo Pharma, Daiichi Sankyo Co. Ltd., Mitsubishi Tanabe Pharma, Novartis Pharma, Biogen Japan, Yamasa Corporation, Kyowa Kirin Co., Ltd., and Nippon Boehringer Ingelheim Co., Ltd.; and honoraria from Alexion Pharma, Novartis Pharma, Argenx, Mitsubishi Tanabe Pharma, Biogen Japan, the Takeda Pharmaceutical Co. Ltd., UCB Japan, Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., Teijin Healthcare Co. Ltd., Amgen Inc., and Eisai Co. Ltd. T.I. reports a grant from JSPS KAKENHI (Grant No. JP22K07499) and speaking fees and/or honoraria from Tsumura & Co., NANZANDO Co. Ltd., CSZ Co. Ltd., and KOWA Co. Ltd. E.O.T., S.N., and H.Y. report that they have nothing to declare.
Author Contributions
G.M. and J.K. conceived the experiments. All authors contributed to the experimental design. J.K., Y.N., M.W., A.S., K.M., and N.I. collected the clinical data. G.M., J.K., E.O.T., S.N., X.Z., K.M., R.Y., and T.I. performed the experiments. G.M., J.K., and M.W. analyzed the results. K.M., R.Y., H.Y., and J.K. provided technical advice for the analyses. G.M., J.K., Y.N., M.W., R.Y., N.I., and T.I. were involved in the interpretation of the results and drafted the manuscript. All authors reviewed the manuscript.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




