Aβ38 and Aβ43 do not differentiate between Alzheimer's disease and cerebral amyloid angiopathy
Abstract
Differential diagnosis between Alzheimer's disease (AD) and cerebral amyloid angiopathy (CAA) using cerebrospinal fluid (CSF) biomarkers is challenging. A recent study suggested that the addition of Aβ38 and Aβ43 to a standard AD biomarker panel (Aβ40, Aβ42, t-tau, p-tau) to improve the differential diagnosis. We tested this hypothesis in an independent German cohort of CAA and AD patients and controls using the same analytical techniques. We found excellent discrimination between AD and controls and between CAA and controls, but not between AD and CAA. Adding Aβ38 and Aβ43 to the panel did not improve the discrimination between AD and CAA.
Introduction
Cerebral amyloid angiopathy (CAA) is an age-related disease affecting cerebral vessels by intravascular deposition of amyloid-β (Aβ) peptides. The modified Boston criteria define possible and probable CAA mainly based on radiological criteria while a definitive diagnosis requires neuropathology.1 The frequent overlap between CAA and Alzheimer's disease (AD) complicates the differential diagnosis.2 The distinction is important, especially since microhemorrhages and CAA related inflammation (CAA-ri) are contraindications for new antibody therapies of AD targeting Aβ.3 Standard AD biomarkers Aβ40 and Aβ42, total Tau- (t-tau) and hyperphosphorylated Tau-protein (p-tau) do not differentiate reliably between AD and CAA.4 Recently, de Kort and colleagues reported that the addition of Aβ38 and Aßβ43 to the biomarker panel significantly improves the differentiation.5 We tested this hypothesis in a cohort of well-characterized CAA and AD patients and healthy controls.
Methods and Subjects
Study participants and clinical data
The CAA, AD, and control groups are described in detail elsewhere.4 While CAA and control groups in this study are identical to the ones previously analyzed, insufficient amounts of cerebrospinal fluid (CSF) in 15 AD patients required replacement by 12 newly selected ones. We retrospectively included study participants from the University Medical Center Schleswig-Holstein (Kiel and Lübeck), Germany and the University Hospital Tübingen, Germany. All patients received a diagnostic cranial MRI including gradient-echo T2* or susceptibility-weighted sequences and had CSF samples taken within 3 months of the MRI scan. The CAA group included patients with probable CAA or probable CAA with supporting pathology according to the modified Boston criteria.1 AD was diagnosed using the 2011 National Institute on Aging and the Alzheimer's Association (NIA-AA) criteria.6 AD patients with imaging features of CAA were excluded. The control group consisted of patients without evidence of organic central nervous system disease. We excluded patients from all groups with a concomitant central nervous system disease potentially increasing the level of any of the CSF parameters analyzed with the exception for CAA patients whose surgical treatment of hemorrhage resulted in pathological confirmation of CAA (n = 5). In these cases, we confirmed that the CSF t-tau concentrations were within the typical range of the other CAA patients. The Ethics Committee of the Medical Faculty of the University of Kiel, Lübeck and Tübingen approved this retrospective study (B 255/18, AZ19-108, and 864/2016BO2). The study was conducted following the World Medical Association Declaration of Helsinki. Anonymized data will be shared on request with any qualified investigator.
Cerebrospinal fluid analysis
CSF samples were frozen at −80°C in polypropylene tubes within maximal 48 h at 4°C after sample collection and stored in biobanks under continuous monitoring of freezer temperature. Aβ40, Aβ42, t-tau, and p-tau(181) were measured with a fully automated ECLIA technology based platform (Lumipulse, FujiRebio) in singlicates with intra- and inter-assay variance <4% for Aβ40, Aβ42, p-tau and <7% for t-tau.4 Aβ38 und Aβ43 were measured in duplicates using commercially available ELISA kits (IBL, Japan) with intra- and inter-assay variance of <8.8% for Aβ38 and <6.5% for Aβ43.
Statistical analysis
We used R4.2.1 for analyses and assessed the distribution of variables via histogram analysis, applied t-tests (function: t.test()) to compare approximately normally distributed variables and the Wilcoxon signed-rank test (function: wilcox.test()) for all other variables. Logistic regressions were analyzed using the glm() function and the function predict() to generate the predictors. We performed receiver-operating-characteristic (ROC) analyses with the pROC package and used the Youden-Index to determine the optimal cutoffs and the corresponding diagnostic metrics.7 In case of ties, that is more than one cutoff with identical Youden-Index, we used the cutoff associated with the smallest absolute difference between sensitivity and specificity. We used the function pROC:power.roc.test() to calculate the power curve with a type 1 error rate of 0.05.
Results
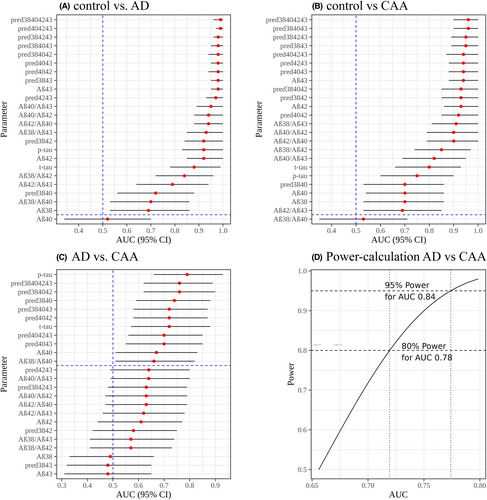
Demographic data and biomarker concentrations of the study populations are summarized in Table 1. Most CSF biomarkers differed significantly between controls and AD or CAA but not between AD and CAA (Table 1). Table 2 and Figure 1C show the results of the ROC analysis for all single markers, marker ratios and predictions of logistic regression analyses with marker combinations (designated, e.g., pred3840 for the predictors Aβ38 and Aβ40) for differentiation between AD and CAA. Figure 1A,B and Tables S1 and S2 show data from the ROC analysis for discriminating CAA from controls and AD from controls. To achieve comparable quantifications, we used the same ratios and combinations as de Kort et al.5, 8 It is apparent that the separation between AD and controls is excellent for most biomarkers including the parameters Aβ42/Aβ40 and p-tau, which are most commonly used in clinical practice (Fig. 1A, Table S1). The same applies to the separation between CAA and controls (Fig. 1B, Table S2). To estimate the areas under the curve (AUCs) which we could reliably expect to detect for the differentiation between AD and CAA with the available sample sizes, we performed a power analysis which showed that we have 95% power to detect clinically relevant AUCs >0.84 (Fig. 1D). Table 2 and Figure 1C show that differentiation between AD and CAA was comparatively poor. As in our previous study4, p-tau achieved the highest AUC (0.79) followed by the combination of Aβ38, Aβ40, Aβ42 (AUC = 0.76), or the combination of all four Aβ species (AUC = 0.76).
| Control (n = 18) | AD (n = 25) | CAA (n = 25) | p-value (Cont/CAA) | p-value (Cont/AD) | p-value (AD/CAA) | Statistical test | |
|---|---|---|---|---|---|---|---|
| Age (mean ± SD) | 72 ± 9 | 70 ± 8 | 75 ± 5 | 0.23 | 0.4 | 0.01 | t-test |
| Sex (F/M) | 8; 10 | 12;13 | 15; 10 | 0.48 | 1 | 0.57 | Chisq |
| Aβ38 (mean ± SD) | 4269 ± 1406 | 3340 ± 1239 | 3556 ± 1878 | 0.16 | 0.03 | 0.63 | t-test |
| Aβ38 (median [IQR]) | 4390 [3438–4793] | 3539 [2287–4064] | 3057 [2485–4077] | 0.03 | 0.03 | 0.94 | Wilcoxon |
| Aβ40 (median [IQR]) | 9353 [7273–11536] | 9119 [6071–11529] | 7131 [5359–7819] | 0.03 | 0.86 | 0.04 | Wilcoxon |
| Aβ42 (median [IQR]) | 776 [547–1063] | 371 [265–460] | 292 [230–362] | 1.90E-06 | 2.10E-07 | 0.2 | Wilcoxon |
| Aβ43 (median [IQR]) | 72 [56–86] | 21 [15–25] | 18 [15–28] | 4.80E-08 | 2.20E-10 | 0.82 | Wilcoxon |
| t-tau (median [IQR]) | 197 [162–248] | 659 [463–851] | 368 [269–522] | 1.10E-03 | 2.17E-05 | 6.60E-03 | Wilcoxon |
| p-tau (median [IQR]) | 30 [25–38] | 115 [77–158] | 49 [37–73] | 6.30E-03 | 4.15E-06 | 3.70E-04 | Wilcoxon |
| Aβ42/Aβ40 (median [IQR]) | 0.097 [0.080–0.102] | 0.041 [0.034–0.048] | 0.046 [0.039–0.062] | 1.10E-05 | 3.03E-08 | 0.11 | Wilcoxon |
| Aβ42/Aβ43 (median [IQR]) | 12 [10–13] | 17 [14–22] | 14 [12–19] | 0.034 | 9.21E-04 | 0.14 | Wilcoxon |
- AD, Alzheimer disease; Aβ, amyloid-β; CAA, cerebral amyloid angiopathy; IQR, interquartile rage; SD, standard deviation.
| Alzheimer's disease versus cerebral amyloid angiopathy | ||||
|---|---|---|---|---|
| Parameter | AUC (95% CI) | Youden | Sensitivity | Specificity |
| Aβ38 | 0.49 (0.33–0.66) | 0.16 | 0.16 (0.04–0.32) | 1 (1–1) |
| Aβ40 | 0.67 (0.51–0.83) | 0.44 | 0.76 (0.6–0.92) | 0.68 (0.52–0.84) |
| Aβ42 | 0.61 (0.44–0.77) | 0.32 | 0.84 (0.68–0.96) | 0.48 (0.28–0.68) |
| Aβ43 | 0.48 (0.31–0.65) | 0.12 | 0.52 (0.32–0.72) | 0.6 (0.4–0.8) |
| t-tau | 0.72 (0.57–0.88) | 0.52 | 0.72 (0.52–0.88) | 0.8 (0.64–0.92) |
| p-tau | 0.79 (0.66–0.93) | 0.6 | 0.92 (0.8–1) | 0.68 (0.48–0.84) |
| Aβ42/Aβ40 | 0.63 (0.47–0.79) | 0.36 | 0.36 (0.16–0.56) | 1 (1–1) |
| Aβ38/Aβ40 | 0.66 (0.51–0.82) | 0.36 | 0.56 (0.36–0.76) | 0.8 (0.64–0.96) |
| Aβ38/Aβ42 | 0.57 (0.41–0.73) | 0.2 | 0.56 (0.36–0.76) | 0.64 (0.44–0.8) |
| Aβ38/Aβ43 | 0.57 (0.41–0.74) | 0.2 | 0.24 (0.08–0.4) | 0.96 (0.88–1) |
| Aβ40/Aβ43 | 0.64 (0.49–0.8) | 0.28 | 0.8 (0.64–0.96) | 0.48 (0.28–0.68) |
| Aβ42/Aβ43 | 0.62 (0.46–0.78) | 0.28 | 0.52 (0.32–0.72) | 0.76 (0.6–0.92) |
| pred3840 | 0.74 (0.59–0.88) | 0.52 | 0.92 (0.8–1) | 0.6 (0.4–0.8) |
| pred3842 | 0.58 (0.42–0.75) | 0.24 | 0.32 (0.16–0.52) | 0.92 (0.8–1) |
| pred3843 | 0.48 (0.32–0.65) | 0.16 | 0.48 (0.28–0.68) | 0.68 (0.48–0.84) |
| pred4042 | 0.72 (0.58–0.87) | 0.36 | 0.68 (0.48–0.84) | 0.68 (0.48–0.84) |
| pred4043 | 0.7 (0.55–0.85) | 0.4 | 0.96 (0.88–1) | 0.44 (0.24–0.64) |
| pred4243 | 0.64 (0.49–0.8) | 0.24 | 0.52 (0.32–0.72) | 0.72 (0.52–0.88) |
| pred384042 | 0.76 (0.62–0.9) | 0.48 | 1 (1–1) | 0.48 (0.28–0.68) |
| pred384043 | 0.72 (0.58–0.87) | 0.48 | 0.96 (0.88–1) | 0.52 (0.32–0.72) |
| pred384243 | 0.63 (0.48–0.79) | 0.28 | 0.36 (0.16–0.56) | 0.92 (0.8–1) |
| pred404243 | 0.7 (0.56–0.85) | 0.36 | 0.92 (0.8–1) | 0.44 (0.24–0.64) |
| pred38404243 | 0.76 (0.62–0.89) | 0.48 | 1 (1–1) | 0.48 (0.28–0.68) |
- pred = logistic regression with predictors (Aβ species are abbreviated accordingly: 38 = Aβ38; 40 = Aβ40; 42 = Aβ42; 43 = Aβ43).
- AUC, area under the curve; Aβ, amyloid-β; CI, confidence interval.
Discussion
The focus of the present study is the replication of the results by de Kort et. al who achieved excellent separation between AD and CAA by adding Aβ38 and Aβ43 to standard CSF biomarkers for AD.5, 8 As expected, we found an excellent separation between controls and AD. The diagnostic metrics, for example, AUC, sensitivity and specificity using the standard AD biomarkers and the Aβ42/Aβ40 ratio are similar to the ones reported in the literature,9 arguing that we analyzed “typical” AD and control samples. The separation between controls and CAA was also excellent and comparable to previous studies.4, 10 Subsequently, we focused on the separation between AD and CAA. A power analysis showed that our sample of 25 AD and 25 CAA patients was sufficient to reliably detect diagnostically relevant AUC's greater 0.84. However, p-tau was the best diagnostic marker achieving an AUC of 0.79, comparable to the one of 0.75 reported in our previous study4 (Table 1, Fig. 1). Furthermore, the CAA biomarker pattern consisting of (1) a reduction in Aβ42 similar to that seen in Alzheimer's patients, (2) a reduction in Aβ40 (not seen in Alzheimer's), and (3) an increase in t-tau and p-tau levels to levels between controls and Alzheimer's patients has also been found in other studies.10 The reason for this pattern might be that the longer Aβ species (e.g., Aβ42 and Aβ43) dominate in the neuritic plaques of AD, whereas the shorter peptides (e.g., Aβ38 and Aβ40) are predominantly located in the arterial wall in CAA.11
A number of marker combinations including the newly analyzed Aβ38 and Aβ43 yielded diagnostic parameters comparable to p-tau. However, none was better or close to the AUC of 0.96 achieved by the combination of all four Aβ-markers in the study by de Kort et al. (in our study AUC = 0.76) which was also observed for the combinations Aβ38, Aβ42, Aβ43 (in our study AUC = 0.63) and Aβ40, Aβ42, Aβ43 (in our study AUC = 0.7). Studies analyzing Aβ38 and Aβ43 in the differential diagnosis of controls versus AD commonly reported that Aβ38 behaves similar to Aβ40 and Aβ43 to Aβ42, but did not show that Aβ38 or Aβ43 were better markers than Aβ40 and Aβ42.12-14 If the same applies to CAA, it could be one reason for our inability to replicate de Kort's findings, which suggest an additional value of Aβ38 and Aβ43. Neuropathological data show that CAA is present in almost 50% of AD patients, while the overlap is only 14% based on radiological data.2 Additionally, changes in CSF biomarker concentrations can occur years before the clinical manifestation of CAA and AD.15, 16 These data suggest that the diagnostic gold standards, namely the modified Boston criteria for CAA and the NIA-AA criteria for AD do not exclude co-occurrence of manifest or preclinical AD and CAA. We did not exclude patients with dementia from the CAA group since dementia is not an exclusion criterion in the modified Boston criteria but rather a typical clinical manifestation.1 However, the interquartile range of the Montreal-Cognitive-Assessment (MoCA) scores of CAA patients in the publication by de Kort et al. was 22–26 indicating cognitive impairment as well. In addition to this, de Kort found no significant correlation between Aβ levels and MoCA scores.5 Therefore, it is improbable that cognitive impairment is the reason for the different results. Analytical differences were excluded by using the same commercial kits as de Kort et al. However, it should be noted that the ELISA kits for Aβ38 and Aβ43 are for research use only and may not be standardized to the same extent as the diagnostic kits used for the other parameters.
Our study has three weaknesses shared by the study of de Kort et al.5: its retrospective nature, the small samples sizes, and the lack of a pathological gold-standard. In conclusion, the discrimination of CAA and AD based on CSF Aβ- and Tau-parameters remains difficult due to the strong overlap in biomarker concentrations, the frequent co-occurrence of CAA and AD and their partially shared pathogenesis. The discrepancy between the results of our study and those of de Kort et al.5 underlines the need for further studies in this area with larger numbers of patients in order to reach more definitive conclusions.
Acknowledgements
The samples from University Hospital Tübingen were obtained from the Neuro-Biobank of the University of Tübingen, Germany (https://www.hih-tuebingen.de/en/about-us/core-facilities/biobank/). This biobank is supported by the local university, the Hertie Institute and the DZNE. Open Access funding enabled and organized by Projekt DEAL.
Author Contributions
JD: laboratory analysis and writing the manuscript. GK: statistical analysis of the data, preparation of figures and tables as well as writing the manuscript. NGM: clinical analysis of the study patients and writing the manuscript. UJK: radiological evaluation of MRI scans and writing the manuscript. BB, DB, NB, CF, RM, AN, BR, CR, GR, CS, KPW, and CW: recruitment of patients and critical review of the manuscript.
Conflict of Interest
GK received grant for the investigation of autoimmune encephalitis genetics from the German Ministry for Education and Research (BMBF). NGM received support for a patient counseling project from Jazz Pharma, Angelini Pharma, Eisai Pharma, UCB Pharma, LivaNova, and Desitin Pharma, lecture fees from Jazz Pharma and Angelini Pharma and travel grants from Eisai Pharma and Angelini Pharma. NGM is member of advisory board by Angelini Pharma. BR is co-founder and shareholder of AIRAmed. NB received consulting fees from Centogene, Abbvie, Biomarin, Brigebio, Zambon, and payment for honorar lectures from Esteve, Abbott, Abbvie, Merz, and Zambon as well participated on advisory board of Zambon and Biogen. GR received consulting fees from Boehringer-Ingelheim, Daichii-Sankyo, Bristol-Myers Squibb, payments for honorar lectures from Boston Scientific, Bristol-Myers Squibb, AstraZeneca, and Novartis and traveling grants from Bayer, Boehringe-Ingelheim, UCB Pharma, Daiichi Sankyo, and AstraZeneca. The position of BR was partially funded by the Clinician Scientist Program of the Faculty of Medicine of the University of Tübingen (grant #478-0-0) to the Department of Neurodegeneration.




