Effects of tirofiban on large vessel occlusion stroke are modified by etiology and renal function
Abstract
Objective
Renal function can modify the outcomes of large vessel occlusion (LVO) stroke across stroke etiologies in disparate degrees. The presence of renal function deficit can also impair the pharmacokinetics of tirofiban. Hence, this study aimed to investigate the roles of renal function in determining efficacy and safety of intravenous tirofiban before endovascular treatment (EVT) for acute ischemic stroke patients with large vessel occlusion (LVO).
Methods
This study was a post hoc exploratory analysis of the RESCUE-BT trial. The primary outcome was the proportion of patients achieving functional independence (modified Rankin scale 0–2) at 90 days, and the primary safety outcome was the rate of symptomatic intracranial hemorrhage (sICH).
Results
Among 908 individuals with available serum creatinine, decreased estimated glomerular filtration rate (eGFR) status was noted more commonly in patients with cardioembolic stroke (CE), while large artery atherosclerosis (LAA) was predominant in patients with normal renal function. In LAA with normal renal function, tirofiban was associated with higher rates of functional independence at 90 days (41.67% vs 59.80%, p = 0.003). However, for LVO patients with renal dysfunction, tirofiban did not improve functional outcomes for any of the etiologies (LAA, p = 0.876; CE, p = 0.662; others, p = 0.894) and significantly increased the risk of sICH among non-LAA patients (p = 0.020). Mediation analysis showed tirofiban reduced thrombectomy passes (12.27%) and drug/placebo to recanalization time (14.25%) mediated its effects on functional independence.
Conclusion
This present study demonstrated the importance of evaluating renal function before administering intravenous tirofiban among patients with LVO who are planned to undergo EVT.
Introduction
Although endovascular treatment (EVT) has become the first-line therapeutic modality for patients with acute ischemic stroke due to large vessel occlusion (LVO), EVT does not yield favorable outcomes in approximately 30% to 50% of patients even if satisfactory recanalization was achieved.1, 2 Biochemical injury from a hypoxic microenvironment and endothelial damage from mechanical thrombectomy instrumentation could promote platelet activation, adhesion, and aggregation that might result in thromboembolism, reduced reperfusion of cerebral tissue, and futile recanalization after EVT.3 Therefore, a glycoprotein (GP) IIb/IIIa inhibitor with fast-acting and potent antiplatelet effect, tirofiban, was investigated as adjuvant therapy to EVT to further improve outcomes of LVO after EVT.4 Observational studies and a meta-analysis have reported the increased frequency of favorable outcomes after intravenous tirofiban among ischemic stroke patients.5 However, our group recently conducted a randomized clinical trial of RESCUE-BT to investigate the efficacy and safety of intravenous tirofiban administered prior to endovascular thrombectomy among LVO, but the findings failed to support routine preprocedure application of intravenous tirofiban among patients due to potential safety events.6 With the above controversies, more efforts are required to identify a target population of patients who may or may not benefit from intravenous tirofiban.
Renal function deficiency was reported to be an independent risk factor for poor outcomes among ischemic stroke patients.7 Park et al. found an increased frequency of ischemic complications including in-hospital death and poor outcomes among LVO patients with renal insufficiency.8 More recent evidence has suggested that stroke subtypes were differentially distributed across different strata of renal function and modulated the prognostic value of renal function among ischemic stroke patients.9 In addition, tirofiban is also renally cleared and the presence of renal dysfunction might lead to the accumulation of tirofiban in serum, potentially increasing the incidence of bleeding complications.10 The interaction between renal function and outcomes among different subtypes of patients with LVO receiving both intravenous tirofiban and EVT remains largely unknown.
Hence, based on our RESCUE-BT trial, we sought to estimate the role of renal function in determining the efficacy and safety of intravenous tirofiban among patients with LVO receiving EVT. We hypothesized that the presence of renal dysfunction may lead to higher risk of hemorrhagic complications in patients receiving tirofiban. The potential mediators for tirofiban treatment were also identified by a causal mediation analysis in this present research.
Material and Methods
Study design and participants
RESCUE-BT was a double-blind, randomized clinical trial of IV tirofiban plus EVT vs placebo plus EVT for patients presenting with an occlusion of the internal carotid artery (ICA) or middle cerebral artery (MCA) within 24 h of symptom onset.6 The present study is a post hoc analysis of the RESCUE-BT trial with detailed trial protocol and patient eligibility criteria reported previously.6 The study drug was initiated before EVT at a dose of 10 μg/kg intravenous bolus followed by an infusion of 0.15 μg/kg/min for up to 24 h. Among all patients in the RESCUE-BT trial, 908 individuals with serum creatinine available within 24 h before EVT were included in the current analysis (Fig. S1).
The RESCUE-BT trial was registered on the Chinese Clinical Trial Registry (chictr.org.cn, ChiCTR-INR-17014167). The study was approved by the ethics committee of the Xinqiao Hospital, Army Medical University, and all participating centers. Written informed consent was obtained from all patients or their proxy. This study is reported according to the Strengthening of the reporting of observational studies in epidemiology (STROBE) guideline.
Clinical, laboratory, and imaging variables assessment
Demographic variables, vascular risk factors, baseline NIH Stroke Scale (NIHSS) score, workflow measures, and treatment information were prospectively recorded during enrollment.
All imaging data were evaluated based on central evaluation in the RESCUE-BT imaging core laboratory. The location of the target occlusion was assessed based on CT or MR angiography on admission and were classified as internal carotid artery, middle cerebral artery M1 and M2 segment. The extent of ischemic injury was assessed using the Alberta Stroke Program Early CT Score (ASPECTS). According to the TOAST criteria, ischemic stroke in the current study was classified into three subtypes: large artery atherosclerosis (LAA), cardioembolic stroke (CE), and other group (other determined etiology stroke, or undetermined etiology stroke).6 Reperfusion at final angiography was assessed using the expanded Thrombolysis in Cerebral Ischemia score, with grade 2b 50, 2c, or 3 indicating substantial, near-complete, or complete reperfusion, respectively.11 First pass effect was defined as defined as single-pass complete or near-complete reperfusion during EVT for LVO patients.12
The estimated glomerular filtration rate (eGFR) was calculated from the serum creatinine by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.13 The new CKD-EPI equation was specially adjusted for the Asian population.8 Patient groups were divided into patients with normal renal function as defined by eGFR ≥90 mL/min/1.73 m2 and patients with renal insufficiency defined by eGFR <90 mL/min/1.73 m2.
Outcomes definitions
The primary efficacy outcome was the rate of modified Rankin scale (mRS) score 0–2 at 90 days. The mRS was adjudicated by two neurologists who were blinded to treatment allocation through review of the patient's structured video or voice recordings. The score of the European Quality of Life 5-Dimension 5-level scale (EQ-5D-5L) at 90 days was also evaluated to assess the health-related quality of life. In addition to 90-day mRS, the efficacy outcomes were evaluated by the 24 hour/5-7d NIHSS score change from baseline and EQ-5D-5L. The safety outcomes included any intracranial hemorrhage (ICH), and symptomatic ICH (sICH), which was assessed according to the Heidelberg criteria.14
Statistical analysis
Statistical analyses were performed based on the intention-to-treat population. Demographic factors, medical history, and baseline characteristics between groups were compared using the chi-square or Fisher exact test for categorical variables and the Mann–Whitney U test for continuous variables. Missing data are reported in the footnote of the tables. The clinical outcomes were compared between the two treatment arms using binary logistic regression, ordinal logistic regression, and linear regression, as appropriate. Adjusted odds ratio (aOR), common odds ratio (OR), and β coefficient were reported with 95% confidence interval (CI). The results for the efficacy and safety of tirofiban among LVO with different etiologies were also validated in the per-protocol population.
To identify potential mediators of the effect of tirofiban on functional outcomes, we performed cause mediation analysis from the following factors: first pass effect, thrombectomy passes, drug/placebo to recanalization time, reperfusion at final angiogram, and postprocedural reocclusion of the target artery. These variables were selected based on clinical practice and previous reports. All tests were two-sided, and all statistical analyses were performed using R (version 4.2.2, https://www.r-project.org).
Results
Patients' characteristics
Of 948 patients randomized in the RESCUE-BT trial, 908 patients were included in the current study. The median age was 67 (interquartile range [IQR] 57–74) years, with a median NIHSS of 16 (IQR 12–19). The baseline characteristics of the two patient groups categorized according to estimated glomerular filtration rate (eGFR) are outlined in Table 1. Increased median [IQR] age (72 [66–77] vs. 59 [51–67], p < 0.001), pretreatment NIHSS (16 [12–20] vs 15 [10–19], p = 0.001), and systolic blood pressure (146[130–160] vs. 143[125–159] mmHg, p < 0.001) were detected in those with abnormal renal function (eGFR <90 mL/min/1.73 m2). There were 416 large artery atherosclerosis (LAA), 390 cardioembolism (CE) and 102 with other etiologies (other determined etiology stroke, or undetermined etiology stroke). The baseline characteristics according to stroke etiology are reported in Table S1. In both the normal estimated glomerular filtration rate (eGFR) group and renal deficiency group, CE was characterized by an increase in age and NIHSS score, but a decrease in collateral circulation and time from onset to recanalization. Renal impairment contributed to the differential distribution of patterns across stroke subtypes, particularly evident in LAA and CE (Fig. S2).
| All (N = 908) | eGFR<90 mL/min/1.73 m2 (N = 510) | eGFR≥90 mL/min/1.73 m2 (N = 398) | p | |
|---|---|---|---|---|
| Age, years, median [IQR] | 67.00 [57.00, 74.00] | 72.00 [66.00, 77.00] | 59.00 [51.00, 67.00] | <0.001 |
| Men, n (%) | 533 (58.70) | 276 (54.12) | 257 (64.57) | 0.001 |
| Pretreatment NIHSS score, median [IQR] | 16.00 [12.00, 19.00] | 16.00 [12.00, 20.00] | 15.00 [10.00, 19.00] | <0.001 |
| Systolic blood pressure, mmHg, median [IQR] | 145.00 [129.00, 160.00] | 146.00 [130.00, 160.00] | 143.00 [125.25, 159.00] | 0.014 |
| eGFR, mL/min/1.73 m2, median [IQR] | 87.80 [72.06, 97.86] | 75.21 [60.64, 84.33] | 99.29 [94.32, 107.21] | <0.001 |
| Medical history, n (%) | ||||
| Hypertension | 503 (55.40) | 309 (60.59) | 194 (48.74) | <0.001 |
| Coronary heart disease | 156 (17.18) | 106 (20.78) | 50 (12.56) | <0.001 |
| Diabetes mellitus | 200 (22.03) | 117 (22.94) | 83 (20.85) | 0.452 |
| Hyperlipidemia | 131 (14.43) | 65 (12.75) | 66 (16.58) | 0.102 |
| Ischemic stroke | 151 (16.63) | 101 (19.80) | 50 (12.56) | 0.004 |
| Prestroke modified Rankin scale, n (%) | 0.273 | |||
| 0 | 827 (91.08) | 457 (89.61) | 370 (92.96) | |
| 1 | 57 (6.28) | 39 (7.65) | 18 (4.52) | |
| 2 | 21 (2.31) | 12 (2.35) | 9 (2.26) | |
| >2 | 3 (3.30) | 2 (0.39) | 1 (0.25) | |
| Location of occlusion, n (%) | 0.373 | |||
| Terminal internal carotid artery | 185 (20.37) | 112 (21.96) | 73 (18.34) | |
| Middle cerebral artery M1 segment | 587 (64.65) | 321 (62.94) | 266 (66.83) | |
| Middle cerebral artery M2 segment | 136 (14.98) | 77 (15.10) | 59 (14.82) | |
| Baseline ASPECTS, median [IQR] | 8.00 [7.00, 9.00] | 8.00 [7.00, 9.00] | 8.00 [7.00, 9.00] | 0.989 |
| ASITN/SIR score, median, [IQR] | 2.00 [1.00, 3.00] | 2.00 [1.00, 3.00] | 2.00 [1.00, 3.00] | 0.040 |
| Workflow times, min, median (IQR) | ||||
| Onset to drug/placebo | 398.40 [261.08, 625.47] | 371.15 [237.52, 566.42] | 447.50 [302.53, 676.12] | <0.001 |
| Onset to IV study drug | 409.50 [270.00, 630.25] | 376.00 [243.50, 577.00] | 466.50 [309.00, 683.75] | <0.001 |
| Onset to recanalization | 481.00 [325.75, 721.75] | 453.00 [303.00, 660.00] | 538.00 [359.25, 784.50] | <0.001 |
| Procedural characteristics, n (%) | ||||
| Stent-retriever only | 124 (13.66) | 78 (15.29) | 46 (11.56) | 0.104 |
| Local aspiration only | 178 (19.60) | 109 (21.37) | 69 (17.34) | 0.129 |
| Combined stent-retriever and local aspiration | 152 (16.74) | 92 (18.04) | 60 (15.08) | 0.235 |
| Balloon angioplasty | 232 (25.55) | 103 (20.20) | 129 (32.41) | <0.001 |
| Stenting | 126 (13.88) | 61 (11.96) | 65 (16.33) | 0.059 |
- ASITN/SIR, American Society of Intervention and Therapeutic Neuroradiology/Society of Interventional Radiology; ASPECTS, Alberta Stroke, Program Early CT Score; IQR, interquartile range; NIHSS, NIH Stroke Scale.
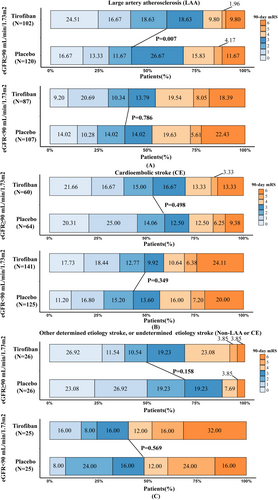
Baseline characteristics and treatment information across different etiologies in both treatment arms are summarized in Tables S2–S4. The baseline characteristics were well balanced for age, baseline NIHSS score, ASPECTS, and eGFR value in both the tirofiban group and placebo group with different renal function across all types of etiologies. There was a decreased rate of balloon angioplasty (61.67% vs 45.10%, p = 0.014) use in the placebo group in LAA patients with normal renal function. There were numerically elevated rates of men in the tirofiban group with other stroke etiology and renal function deficit (40% vs 72%, p = 0.023).
Association between renal impairment and clinical outcomes in LAA patients
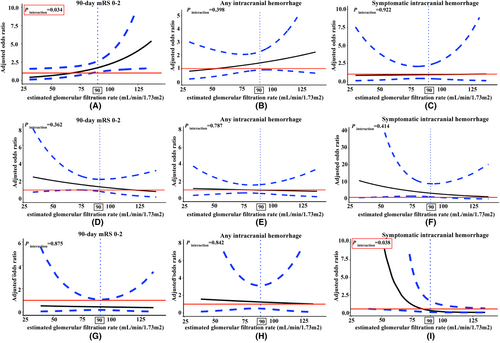
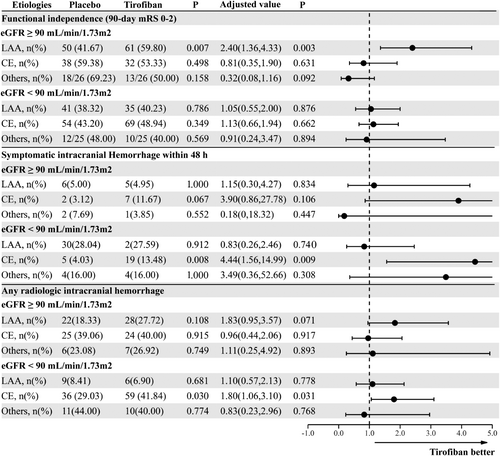
For LAA patients, Figure 1A illustrates the comparisons between treatment modalities to achieve 90-day favorable outcome with eGFR increment as a continuous variable. A significant interaction modulating treatment effect for 90-day mRS 0–2 between the tirofiban and the placebo groups was detected (Fig. 1A, p for interaction = 0.034). A difference was observed when the eGFR was ≥90 mL/min/1.73 m2, with the latter being consistently better than the former. The interaction between treatment and eGFR on any ICH (Fig. 1B, p for interaction = 0.398) or sICH (Fig. 1C, p for interaction = 0.922) was not significant. Based on the threshold value of 90 mL/min/1.73 m2, patients were dichotomized into the normal renal function group and the renal insufficiency group. As shown in Figure 2A and Figure 3, the elevated ratio of functional independence was detected when LAA patients with eGFR higher than 90 mL/min/1.73 m2 were treated with tirofiban (aOR 2.40, 95% CI 1.36–4.33, p = 0.003), while tirofiban was not associated with improved 90-day mRS 0–2 among those with renal insufficiency (aOR 1.05, 95% CI 0.55–2.00, p = 0.876). The probability of any ICH and sICH was similar between the tirofiban and placebo groups across different renal function (Fig. 1). The detailed information for the relationship between renal impairment and clinical outcomes among LAA-caused LVO is illustrated in Table S5. With the differences in the proportion of ballon angioplasty/stenting between placebo and tirofiban group, we detected the association between favorable outcome and tirofiban after integrating the application of ballon angioplasty/stenting into the multivariable analysis (aOR 2.43, 95% CI 1.36–4.44, p = 0.003 Table S5).
Association between renal impairment and clinical outcomes in non-LAA patients
Among non-LAA patients with renal insufficiency, tirofiban was associated with an elevated frequency of sICH (Table 2, aOR 2.86, 95% CI 1.21–7.30, p = 0.020). More specifically, for CE patients, though nonsignificant interaction among the eGFR and 90-day mRS 0–2 (p for interaction = 0.362, Fig. 1D), any ICH (p for interaction = 0.787, Fig. 1E) and sICH (p for interaction = 0.414, Fig. 1F), the obvious trend of sICH (aOR 4.44, 95% CI 1.56–14.99, p = 0.009, Fig. 3) and any ICH (aOR 1.80, 95% CI 1.06–3.10, p = 0.031, Fig. 3) were observed among the decreased eGFR group after tirofiban. In multivariable analysis, tirofiban failed to improve the frequency of 90-day mRS 0–2 among CE patients with normal eGFR (aOR 0.81, 95% CI 0.35–1.90, p = 0.631) and eGFR deficits (aOR 1.13, 95% CI 0.66–1.94, p = 0.662). The detailed effects of tirofiban on outcomes among CE patients are shown in Table S6.
| eGFR ≥90 mL/min/1.73 m2 | ||||||
|---|---|---|---|---|---|---|
| All patients (n = 176) | Placebo (n = 90) | Tirofiban (n = 86) | p | Adjusted valuea | pa | |
| Efficacy outcomes | ||||||
| Modified Rankin scale score at 90 days, n/N (%) | ||||||
| Modified Rankin scale score at 90 days, median (IQR) | 2.00 [1.00, 4.00] | 2.00 [1.00, 3.00] | 2.00 [1.00, 4.00] | 0.368 | 1.29 (0.76, 2.22) | 0.345 |
| 0 to 1 or return to premorbid score | 79 (44.89) | 44 (48.89) | 35 (40.70) | 0.275 | 0.68 (0.35, 1.31) | 0.255 |
| 0 to 2 | 101 (57.39) | 56 (62.22) | 45 (52.33) | 0.184 | 0.63 (0.32, 1.24) | 0.183 |
| NIHSS score change from baseline, median [IQR] | ||||||
| 24 h after drug/placebo | −3.00 [−8.00, 0.00] | −3.00 [−8.00, 0.00] | −2.50 [−8.75, 0.00] | 0.793 | −0.18 (−2.68, 2.32) | 0.887 |
| 5–7 days after drug/placebo or at early discharge | −6.00 [−13.00, −1.00] | −7.00 [−13.75, −2.00] | −5.50 [−12.00, −1.00] | 0.225 | 1.23 (−1.83, 4.29) | 0.427 |
| EQ-5D-5L score at 90 days, median [IQR] | 0.85 [0.31, 1.00] | 0.92 [0.46, 0.99] | 0.78 [0.27, 1.00] | 0.412 | −0.06 (−0.16, 0.04) | 0.207 |
| Safety outcome, n/N (%) | ||||||
| Mortality at 90 days | 16 (9.09) | 7 (7.78) | 9 (10.47) | 0.535 | 1.31 (0.45, 3.94) | 0.614 |
| Symptomatic intracranial Hemorrhage within 48 h | 12 (6.82) | 4 (4.44) | 8 (9.30) | 0.201 | 2.33 (0.66, 9.6) | 0.204 |
| Any radiologic intracranial hemorrhage | 62 (35.23) | 31 (34.44) | 31 (36.05) | 0.824 | 1 (0.52, 1.94) | 0.991 |
| eGFR < 90 mL/min/1.73 m2 | ||||||
|---|---|---|---|---|---|---|
| All patients (n = 316) | Placebo (n = 150) | Tirofiban (n = 166) | p | Adjusted valuea | pa | |
| Efficacy outcomes | ||||||
| Modified Rankin scale score at 90 days, n/N (%) | ||||||
| Modified Rankin scale score at 90 days, median (IQR) | 3.00 [1.00, 5.00] | 3.00 [1.00, 4.75] | 3.00 [1.00, 5.75] | 0.726 | 1.05 (0.70, 1.57) | 0.816 |
| 0 to 1 or return to premorbid score | 104 (32.91) | 45 (30.00) | 59 (35.54) | 0.295 | 1.17 (0.69, 2) | 0.566 |
| 0 to 2 | 145 (45.89) | 66 (44.00) | 79 (47.59) | 0.522 | 1.08 (0.66, 1.78) | 0.753 |
| NIHSS score change from baseline, median [IQR] | ||||||
| 24 h after drug/placebo | −3.00 [−7.00, 2.00] | −2.00 [−7.75, 2.00] | −3.00 [−7.00, 2.00] | 0.995 | 0.24 (−1.78, 2.27) | 0.812 |
| 5–7 days after drug/placebo or at early discharge | −6.00 [−11.00, 1.00] | −7.00 [−12.00, 1.00] | −6.00 [−11.00, 0.00] | 0.491 | 0.8 (−1.77, 3.38) | 0.540 |
| EQ-5D-5L score at 90 days, median [IQR] | 0.62 [0.06, 0.96] | 0.62 [0.11, 0.94] | 0.62 [0.01, 0.96] | 0.481 | −0.01 (−0.09, 0.07) | 0.873 |
| Safety outcome, n/N (%) | ||||||
| Mortality at 90 days | 71 (22.47) | 29(19.33) | 42(25.30) | 0.204 | 1.51 (0.86, 2.69) | 0.154 |
| Symptomatic intracranial Hemorrhage within 48 h | 32 (10.16) | 9 (6.04) | 23 (13.86) | 0.022 | 2.86 (1.21, 7.30) | 0.020 |
| Any radiologic intracranial hemorrhage | 116 (36.83) | 47 (31.54) | 69 (41.57) | 0.066 | 1.58 (0.98, 2.58) | 0.063 |
- a Multivariable regression analysis adjusted for age, baseline NIH Stroke Scale, baseline Alberta Stroke Program Early CT Score, occlusion site and onset to drug/placebo time.
For patients with stroke caused by the other etiologies, a significant interaction between eGFR and sICH was detected (Fig. 1I, p for interaction = 0.038), while p for interaction between the eGFR and 90-day mRS 0–2 (p for interaction = 0.875, Fig. 1G), and any ICH (p for interaction = 0.842, Fig. 1H) was nonsignificant. None of the efficacy and safety outcomes were significant between the tirofiban and placebo group across different renal function (p > 0.05, Table S7).
Mediation analysis for LAA patients
The proportion of first pass effect in the tirofiban group was numerically higher than that of the placebo group (21.57% vs 8.33%, p = 0.005), which did not achieve statistical significance for 90-day mRS 0–2 (aOR 1.56; 95% CI 0.95–2.57; p = 0.08). There was no difference in the reperfusion at final angiogram, or postprocedural reocclusion of the target artery between both arms, though these variables were independently associated with functional independence at 90 days (Table S8). The number of thrombectomy passes (1.00[IQR, 1.00–2.00] vs 1.00[IQR, 0.25–2.00]; β = −0.50; 95% CI −0.86 to −0.13; p = 0.007) and drug/placebo to recanalization time (1.63[IQR, 1.00, 2.49] vs 1.27 [IQR, 0.71, 1.84]; β = −0.41; 95% CI −0.69 to −0.12; p = 0.005) were lower in the tirofiban group than in the placebo group and also acted as independent predictors of functional independence (No. of passes: aOR 0.84; 95% CI 0.73–0.96; p = 0.011; drug/placebo to recanalization time: aOR 0.77; 95% CI 0.65–0.91; p = 0.003), these two factors were analyzed as potential mediators of the effect of tirofiban on functional independence. As shown in Figure S3, mediation analyses indicated a partial mediation effect of thrombectomy pass and drug/placebo to recanalization time. Treatment reduced passes of thrombectomy accounted for 12.27% (95% CI 1.04%–37.00%) and drug/placebo to recanalization time 14.25% (95% CI 1.00%–57.00%) of the beneficial effect of tirofiban on functional independence.
Sensitivity analysis
In line with previous findings, tirofiban was associated with a higher rate of 90-day functional independence (65.75% vs 41.67%, aOR 2.98, 95% CI 1.48–6.17, p = 0.003) only in those with LAA and normal renal function in the per-protocol (PP) population (Table S9). Among non-LAA patients with renal insufficiency, tirofiban was associated with an elevated frequency of sICH (PP population: Table S10, aOR 3.35, 95% CI 1.17–11.18, p = 0.032). Specially, tirofiban increased the frequency of sICH in CE patients with renal function deficiency (Table S11, aOR 7.56, 95% CI 1.18–52.57, p = 0.014). No increased rate of sICH or ICH was detected among all subtypes of stroke with eGFR higher than 90 mL/min/1.73 m2. We also found that tirofiban failed to increase the rate of functional independence at 90 days among LAA patients even with minor renal function deficiency (60 < eGFR < 90 mL/min/1.73 m2, 40.24% vs. 42.86%, aOR 1.04, 95% CI 0.50–2.20, p = 0.911; Table S12). We further explored the impacts of tirofiban on outcomes by additionally adjusting for types of EVT modality including stent-retriever, thrombectomy with aspiration, combined technique with stent-retriever and aspiration in Table S13. The identical conclusion as before were obtained.
Discussion
This post hoc analysis of the RESCUE-BT randomized trial explored the role of renal function in determining outcomes of LVO stroke patients who received both of preoperative intravenous tirofiban and EVT. Our analysis showed that (1) tirofiban increased the risk of sICH among non-LAA patients with renal insufficiency while the use of intravenous tirofiban in stroke patients with normal renal function was not associated with increased risk of hemorrhagic complications across different LVO etiologies; (2)tirofiban only improved 90-day mRS 0–2 outcomes in LVO caused by LAA with normal renal function rather than all LAA patients; (3) a lower number of thrombectomy passes and the shortened drug/placebo to recanalization time could be mediators in the benefit of tirofiban. The present study highlights the potential importance of evaluating renal function before administering intravenous tirofiban and further clarified the LVO patients who could benefit from tirofiban.
For the safety outcomes, intravenous tirofiban use was not associated with increase in intracranial hemorrhage among LVO patients with normal renal function receiving EVT. This might be related to biopharmaceutical factors. Pharmacologically, tirofiban is cleared by the kidney, and thus, the blood concentration of tirofiban depends on the renal function.15 Showing a relationship with blood concentration of tirofiban, the incidence of hemorrhage was also reported to be increased in acute coronary syndrome patients with declining renal function after intravenous tirofiban.10 In line with previous articles, we found that renal function impairment may augment the risk of intracranial hemorrhage, particularly in CE patients.10 Biologically, the kidney is a capillary-rich organ and renal function is closely related to the structural integrity of these capillaries. Renal function was reported to be a surrogate assessment for cerebral microcirculatory status, which is difficult to assess.16 In addition, we observed an increased proportion of patients with ischemic stroke in the medical history among LVO patients with renal insufficiency and CE that can be interpreted as a marker for previous cerebral damage and frailty of brain tissue, which indicated the impaired ability of acute stroke patients to cope with the injury, even if treated by tirofiban.17
For the efficacy outcomes, our research highlighted that in contrast to our prior studies, tirofiban improved outcomes among LAA patients with normal renal function rather than all LAA patients.18 Clot composition with different stroke etiologies might explain our findings.19 CE clots are rich in red cells, whereas LAA-related occlusions consist mainly of platelets which are referred to as white thrombi.19 Tirofiban might be beneficial to LAA patients by preventing further platelet aggregation. In addition, the persisting atherosclerotic lesion has an irregular and disrupted surface exposed to rapidly flowing blood, precipitating platelet activation and reocclusion that may be responsive to tirofiban.20 Our recent RESCUE-BT2 trial demonstrated that tirofiban was effective among stroke patients with no evidence of complete occlusion of large or medium-sized vessels.21 Though present study focused on LVO patients underwent EVT, we found that tirofiban could promote outcomes among LVO with atherosclerotic lesion and most patients with small infarctions in RESCUTE-BT2 were presumed to be atherosclerotic.21 Therefore, tirofiban could be an adjuvant strategy to EVT among LAA patients by preventing platelet aggregation. More importantly, our results illustrated that LAA patients with normal renal function benefited from intravenous tirofiban. As mentioned above, antiplatelet drugs may have a ceiling effect in improving on outcomes in patients with pre-existing structural damage and frail brain tissue.22 The incremental risk of hemorrhage from pre-existing structural damage and frail brain tissue may also offset the potential benefits of the tirofiban.23
The mediation analysis illustrated partial mediation effects of decreased numbers of thrombectomy pass and shortened drug/placebo to recanalization time with tirofiban among LVO. The decreased numbers of thrombectomy pass by tirofiban might lie in two aspects. Firstly, during EVT, tirofiban may help to prevent platelet aggregation, thrombus extension, and even promote thrombolysis before thrombectomy, thus facilitating thrombus debulking.11 Secondly, tirofiban could inhibit fibrinogen-dependent platelet aggregation and prevent mural thrombus and/or reocclusion, which may be helpful for the neurointerventionalist to distinguish atherosclerotic stenosis and directly resort to balloon dilatation or stent implantation, so as to avert additional thrombectomy passes.24 The decreased numbers of thrombectomy pass were tightly related to drug/placebo to recanalization time, which reduced the duration of ischemia and hypoxia exposure and alleviated tissue impairment.25
Several limitations are noted in the current study. First, the dose of tirofiban in this study was administered similar to studies of acute myocardial infarction.26 Patients with severe renal insufficiency (eGFR <30 mL/min/1.73 m2, serum creatinine >220 umol/L or 2.5 mg/dl) were excluded from RESCUE-BT trial. Our findings suggest that the current dosage of tirofiban may have been inappropriate in LVO patients with mild-to-moderate renal function impairment, and further dose exploration studies may be needed to determine the optimal dosage among these patients.23 Second, enrolled patients were recruited in China, and these findings may not be generalizable to a western population. Third, we applied the newly developed CKD-EPI equation in 2021 to estimate the GFR, instead of measuring creatinine clearance directly. The lack of repeated measurements of renal function also could limit the accurate evaluation of the clinical effects of renal function impairment on stroke outcomes.13 Though a minor degree of inaccuracy might be introduced into the study, this accurate but preoperatively surrogate assessment of creatinine clearance might be more operational, due to the importance of shortening onset to recanalization on promoting outcomes among LVO patients.27 As we did not measure tirofiban levels in our study, we do not know whether the hemorrhagic effect of tirofiban in patients with other stroke etiology and renal insufficiency is related to cumulative levels of tirofiban or other comorbidities related to patients with renal disease. Last, the simultaneous stratification by etiology and renal function led to the limited sample size in several groups during data analysis. Hence, our findings are considered exploratory and further research is recommended to confirm our findings.
Conclusion
This post hoc analysis of the RESCUE-BT trial demonstrated the important role of evaluating renal function before starting intravenous tirofiban among patients with LVO. Preoperative normal renal function was associated with the safety of intravenous tirofiban across all etiologies of LVO and its efficacy among LAA-caused LVO. The reduced number of thrombectomy passes and shortened drug/placebo to recanalization time might act as potential mediators of the beneficial effect of tirofiban. Our findings need to be confirmed in further randomized trials.
Author Contributions
Wenjie Zi had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Chang Liu, Fengli Li, Liyuan Chen, Jiacheng Huang. Acquisition, analysis, or interpretation of data: Chang Liu, Fengli Li, Liyuan Chen, Jiacheng Huang, Hongfei Sang, Thanh N. Nguyen, FRCPC, Jeffrey L. Saver, Mohamad Abdalkader, Weiling Kong, Jie Yang, Changwei Guo, Chen Gong, Liping Huang, Yanzhu Pan, Xinxin Wang, Yangmei Chen, Zhongming Qiu, Wenjie Zi. Drafting of the manuscript: Chang Liu, Fengli Li, Liyuan Chen, Jiacheng Huang. Wenjie Zi. Critical revision of the manuscript for important intellectual content: Thanh N. Nguyen, FRCPC, Jeffrey L. Saver, Mohamad Abdalkader, Yangmei Chen, Zhongming Qiu, Wenjie Zi. Statistical analysis: Chang Liu, Liyuan Chen. Administrative, technical, or material support: Chang Liu, Yangmei Chen, Zhongming Qiu, Wenjie Zi. Supervision: Wenjie Zi.
Acknowledgments
We thank all study participants for their contribution to the study and the research staff at all the participating hospitals.
Funding Information
This work was supported by National Natural Science Foundation of China (Nos. 82001264 and 82071323), Chongqing Technology Innovation and Application Development Project (No. 2022TIAD-KPX0017) and The First Batch of Key Disciplines on Public Health in Chongqing.
Conflict of Interest
Dr Saver reported receiving contracted hourly payments for service on clinical trial steering committees advising on rigorous trial design and conduct from Medtronic, Cerenovus, NeuroVasc, Boehringer Ingelheim (prevention only); stock options for service on Clinical Trial Steering Committees advising on rigorous trail design and conduct from Rapid Medical; and contracted hourly payments for service on data safety monitoring committee advising on rigorous trial design, safety, and conduct from MIVI outside the submitted work.
Ethics Approval
This study involved human participants and was approved by the Xinqiao Hospital Ethics Committee, ID: 201900301. Participants gave written informed consent prior to participating in the study.
Open Research
Data Availability Statement
Anonymized data will be shared to qualified investigators whose proposal of data use has been approved by the corresponding author and the RESCUE BT investigators.




