Seizures and electrophysiological features in familial cortical myoclonic tremor with epilepsy 1
Funding Information
This study was supported by the Science Technology Department of Zhejiang Province (2019C03017) and the National Natural Science Foundation of China (NSFC) (81571089, 81971208, 81971207, 82071443 and 82201607).
Abstract
Objectives
To investigate and characterize epileptic seizures and electrophysiological features of familial cortical myoclonic tremor with epilepsy (FCMTE) type 1 patients in a large Chinese cohort.
Methods
We systematically evaluated 125 FCMTEtype 1 patients carrying the pentanucleotide (TTTCA) repeat expansion in the SAMD12 gene in China.
Results
Among the 28 probands, epileptic seizures (96.4%, 27/28) were the most common reason for an initial clinic visit. Ninety-seven (77.6%, 97/125) patients had experienced seizures. The seizures onset age was 36.5 ± 9.0 years, which was 6.9 years later than cortical tremors. The seizures were largely rare (<1/year, 58.8%) and occasional (1–6/year, 37.1%). Prolonged prodromes were reported in 57.7% (56/97). Thirty-one patients (24.8%, 31/125) reported photosensitivity history, and 79.5% (31/39) had a photoparoxysmal response. Interictal epileptiform discharges (IEDs) were recorded in 69.1% (56/81) of patients. Thirty-three patients showed generalized IEDs and 72.7% (24/33) were occipitally dominant, while 23 patients presented with focal IEDs with 65.2% (15/23) taking place over the occipital lobe. Overnight EEG of FCMTE patients displayed paradoxical sleep–wake fluctuation, with a higher average IED index of 0.82 ± 0.88/min during wakefulness and a lower IED index of 0.04 ± 0.06/min during non-rapid eye movement sleep stages I–II.
Interpretation
FCMTE type 1 has a benign course of epilepsy and distinct clinical and electrophysiological features. In addition to a positive family history and cortical myoclonus tremor, the seizure prodromes, specific seizure triggers, photosensitivity, distribution of IEDs, and unique fluctuations during sleep–wake cycle are cues for proper genetic testing and an early diagnosis of FCMTE.
Introduction
Familial cortical myoclonic tremor with epilepsy (FCMTE) is an under-recognized disorder, which is characterized by autosomal dominant inheritance, cortical tremors, epileptic seizures, and additional symptoms, depending on the FCMTE subtype.1 This disorder has not been included in the most recent ILAE syndrome classification due to insufficient recognition.2 Since its first report in Japan in the 1970s, different definitions have been used for FCMTE, including benign adult familial myoclonus epilepsy (BAFME), familial adult myoclonic epilepsy (FAME), and autosomal dominant cortical myoclonus and epilepsy (ADCME).3-5 Recently, the intronic pentanucleotide repeat expansions TTTCA and TTTTA were identified in different genes6 (FCMTE1-SAMD12,7, 8 FCMTE2-STARD7,9 FCMTE3-MARCH6,10 FCMTE4-YEATS2,11 FCMTE6-TNRC6A,7 and FCMTE7-RAPGEF27). Phenotypic features of FCMTE, including adolescence or adult onset, myoclonic jerks, and bilateral tonic–clonic seizures (BTCSs), are seen in other epileptic syndromes,12 such as juvenile myoclonic epilepsy (JME) and progressive myoclonus epilepsies (PMEs). Therefore, there is an increased risk of FCMTE misdiagnosis.
In 1990, Ikeda et al.13 characterized the clinical and electrophysiological features of an involuntary tremulous finger movement in FCMTE. This shivering-like finger twitching is associated with giant somatosensory evoked potentials (g-SEPs), enhanced long-loop reflex (C-reflex), and premovement cortical spikes and is considered a unique variant of cortical myoclonus (cortical tremor).13, 14 However, as the main reason for consultation by neurologists, the characteristics of epileptic seizures and EEG features have not been systemically characterized in FCMTE. Inadequate understanding of seizures in FCMTE is the main cause of misdiagnosis in the early stage of this disease.
In this study, we enrolled a large cohort of FCMTE patients carrying pentanucleotide (TTTCA) repeat expansion within the SAMD12 gene (FCMTE type 1), which is the most common genetic cause of FCMTE in East Asia.6, 8 A series of seizure-related features, including seizure triggers, seizure semiology, photosensitivity, interictal epileptiform discharges (IEDs), as well as correlation between seizures and sleep–wake cycle were characterized. Our study aimed to classify the clinical and electrophysiological features of FCMTE to guide early identification and diagnosis of the disease.
Patients and Methods
Patients
In this observational cohort study, 125 patients were enrolled from 28 families diagnosed with FCMTE at the Second Affiliated Hospital, Zhejiang University School of Medicine between January 2012 and December 2019. The clinical diagnosis of FCMTE was based on previously described criteria, including: (1) autosomal-dominant inheritance, (2) tremulous finger movement, increasing with action and posture, (3) epileptic seizures, (4) cortical origin of the tremulous finger movement demonstrated by electrophysiological studies (i.e., giant SEP, C-reflex, or jerk-locked averaging), and (5) the absence of cognitive decline and other neurological symptoms or prominent progression impairing daily activities in the early stage of disease. “Probable” FCMTE was defined when (1), (5), and two items out of (2), (3), or (4) were satisfied. “Possible” FCMTE was defined when (1), (5), and one item out of (2), (3), or (4) were satisfied.15, 16 The “Probable” and “Possible” FCMTE patients were recommended for further genetic testing. All enrolled patients were confirmed to carry the pathogenic pentanucleotide (TTTCA) repeat expansion in SAMD12 by repeat-primed polymerase chain reaction and long-range polymerase chain reaction as described in our prior study.8
The study protocol conformed to the recommendations of the Declaration of Helsinki and was approved by the Ethics Committee of the Second Affiliated Hospital, Zhejiang University School of Medicine. Written informed consent was obtained from all study participants.
Clinical data
All participants were interviewed by experienced physicians (Z.D.C. and W.L. for movement disorders and D.Y. and W.S. for epilepsy) in person and were subjected to neurological examinations in the hospital or at the home of patient's. Clinical assessments included rating of tremulous myoclonus, photosensitivity phenomenon, prodromes, and trigger evaluation. The organization of the semi-structured interview drew upon to previous studies,17 and contains both structured questions, which could be answered in discrete categories (e.g., “yes,” “no,” or “don't know”), and open-ended questions answered more expansively. Seizure frequency was self-reported as rare (<1/year), occasional (1-6/year), or frequent (>6/year). The self-rating tool for evaluating the severity of cortical tremor consisted of five levels: 0, absence of myoclonus; 1, mild myoclonus without disturbance of daily activity; 2, moderate myoclonus with some disturbance of daily activity; 3, severe myoclonus with clear disturbance of daily activity; and 4, marked myoclonus causing incapacity.18
Electroencephalogram (EEG)
Electroencephalogram recordings were recorded on a 32-channel system according to the international 10/20 system. Overnight video EEG recordings were conducted in the patients who were interviewed at the hospital, and the results were reviewed by two experienced clinical neurophysiologists (X.Q. and Z.J.W.). Different stages, including wakefulness (W), non-rapid eye movement (NREM) stage I, NREM stage II, and rapid eye movement (REM), were defined based upon standard sleep staging criteria.19 Both posterior background activity and frequency of IEDs were calculated. The IED index was defined as the number of IEDs per minute. Portable EEG recordings were conducted for at least 30 minutes at home for patients who were unable to visit the hospital.
Intermittent photic simulation (IPS)
Intermittent photic simulation was performed at the hospital, using a standard procedure following an updated algorithm for visual stimulation, as previously described.20 Because photic simulation can potentially induce increase strong reactions and increase the risk of seizures, all patients were informed of the procedural details and possible adverse effects. Only epileptiform discharges that appeared de novo upon IPS compared with baseline EEG were defined as abnormal. Photoparoxysmal responses (PPRs) were classified into four types according to Waltz's criteria: PPR 1, spikes within the occipital rhythm; PPR 2, parieto-occipital spikes with biphasic slow waves; PPR 3, parieto-occipital spikes and slow waves with spread into the frontal region; and PPR 4, generalized spikes or poly-spikes and waves.21 Photomyoclonic response (PMR) positivity was defined as myoclonus time-locked to the flash, building in intensity as the stimulus continued, and terminating once the stroboscope was switched off.
Statistical analysis
A single-level variance component model was used to analyze tremor severity as well as potential contributing factors, including sex, age at tremor onset, and duration of tremor. Comparisons between the number and frequency of epileptiform discharges during each sleep stage were conducted using Wilcoxon signed rank tests. P < 0.05 was considered statistically significant.
Results
Demographics
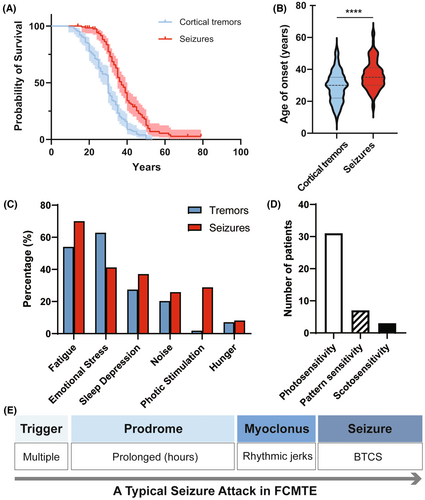
A total of 125 symptomatic carriers of the SAMD12 pentanucleotide (TTTCA) repeat expansion from 28 FCMTE pedigrees were enrolled. These subjects were from 11 different Chinese provinces, representing the Eastern, Center, and Northeast regions of the country (Figure S1). All patients (68 males) presented with cortical tremors with or without epileptic seizures (Table 1). All patients were normal or had nonspecific abnormalities upon brain MRI. Among the 28 probands, the reason for their initial neurologist visit was most often epileptic seizures (n = 27) and rarely cortical tremors (n = 1). The mean age at the time of interview was 48.7 ± 15.5 years, ranging from 14 to 81 years. The mean onset age of cortical tremors was 29.3 ± 9.4 years (median: 30; range: 9–53), with >95% penetrance by 43 years and full penetrance by 53 years (Fig. 1A, Table S1). Ninety-seven patients (77.6%, 97/125) had previously experienced seizures. The mean onset age of seizures was 36.5 ± 9.0 years (median: 35; range: 16–63), with a penetrance of >90% by 51 years and >95% by 62 years (Fig. 1A, Table S2). Notably, >95% patients experienced seizures after the age of 24.
| Number of patients | n = 125 |
|---|---|
| Male/female | 68/57 |
| Age (years) | 48.7 ± 15.5 |
| Cortical tremor (n = 125, 100%) | |
| Mean age of onset (years) | 29.3 ± 9.4 |
| Mean duration (years) | 19.4 ± 12.6 |
| Tremor self-rating (n = 79) Level 1/level 2/level 3/level 4 | 38/39/2/0 |
| Tremor self-rating with course (preceding 20 years, n = 45) | P < 0.01 |
| Seizures (n = 97, 77.6%) | |
| Mean age at seizure onset (years) | 36.5 ± 9.0 |
| Mean seizure duration (years) | 16.3 ± 12.4 |
| Seizure frequency | |
| Rare | 57 (58.8%) |
| Occasional | 36 (37.1%) |
| Frequent | 4 (4.1%) |
| Photosensitivity phenomenon (n = 31, 24.8%) | |
| ASMs treatment | 64 (66.0%) |
| Monotherapy | 48 |
| Multidrug | 16 |
| Unmedicated | 33 |
- ASMs, anti-seizure medicines.
Epileptic seizures
Semi-structured interviews revealed that 97 patients had a history of epileptic seizures. All seizures presented as BTCSs, often preceded by myoclonic jerks. Six patients (6.2%, 6/97) experienced eye or head deviation preceding BTCSs. The seizures had an average onset age much later than cortical tremors, with a mean interval of 6.9 ± 8.4 years (P < 0.001, Fig. 1B). Chronologically, the seizures occurred at a significantly later age than cortical the tremors in 69.1% (67/97) of patients, at a similar time in 27.8% (27/97) of patients, and earlier than cortical tremors in 3.1% (3/97) of patients. The frequency of BTCSs was rare (<1/year) in 58.8% (57/97) of patients, occasional (1–6/year) in 37.1% (36/97) of patients, and frequent (>6/year) in 4.1% (4/97) of patients (Table 1). Self-rating demonstrated that cortical tremors progressively worsened over the course of the disease (P < 0.01, Table 1), while the frequency of BTCSs did not. At the time of the last follow-up, seizure frequency and severity of cortical tremors had a small, but nonsignificant, correlation (P = 0.055). Nearly all seizures occurred while patients were awake, except for one patient who experienced a BTCS during sleep.
Seizure triggers and prodromes
All patients with a history of seizures reported discrete triggers for the majority of their seizures. Seizure triggers included fatigue (70.0%, 68/97), emotional stress (41.2%, 40/97), sleep deprivation (37.1%, 36/97), photic stimulation (28.9%, 28/97), noise (25.8%, 25/97), and hunger (8.2%, 8/97) (Fig. 1C). The same triggers resulted in cortical tremors in each patient. Interestingly, 10 patients experienced their first BTCS during traditional Chinese funerals or weddings, upon exposure to a combination of multiple triggers including emotional stress, prolonged sleep deprivation, and noise. Unfortunately, three patients drowned in paddy fields as confirmed by witnesses. These three farmers experienced several BTCSs trigged by sunlight glare in paddy fields as well as fatigue. With increased experience of seizure triggers, some patients achieved seizure control later in life through avoidance.
Notably, over half (57.7%, 56/97) of the patients with seizures described a prolonged prodrome prior to the onset of an attack. These prodromes included palpitation, chest distress, shaking, dizziness, headache, blurred vision, and irritability, lasting minutes to hours, or sometimes up to a day. Some patients took anti-seizure medications (ASMs) once they felt the prodrome, which could halt the progression to seizures. The seizure triggers could also induce isolated prodromes without resulting in a seizure. Notably, compared with infrequent seizures, isolated prodromes occurred more frequently and resulted in greater distress in daily life. Overall, triggers and prodromes were reported as important and helpful approaches in prevention against seizure attacks. A time line of a typical seizure attack from trigger to BTCS in FCMTE patients is shown in Figure 1E.
Photosensitivity
Nearly a quarter of patients with FCMTE (n = 31, 24.8%), including 28 with seizures and three without seizures, reported photosensitivity characterized by an increased feeling of discomfort, myoclonic jerks or BTCSs in response to environmental photic stimulation (Table 1, Fig. 1D). All 31 patients were sensitive to strong light and/or flickering light, such as a glare reflected by snow or paddy fields, or flashing lights (e.g., in a nightclub). Additionally, five patients reported severe photosensitivity which impaired their daytime activities, and they therefore developed nocturnal lifestyles to avoid sunlight. Notably, seven patients exhibited strong “pattern sensitivity,” which was characterized by susceptibility to seizure occurrence or isolated prodromes viewing striped patterns (e.g., sunlight and shade under trees, a moving escalators in a shopping mall, or a zebra crossing the street). In response to photic stimulation, patients displayed discomfort, shaking, myoclonus, and in some cases, BTCSs. Three patients exhibited both pattern sensitivity and scotosensitivity. The scotosensitivity presented as serious discomfort, myoclonus, and even BTCSs in dark environments, leading to severe insomnia.
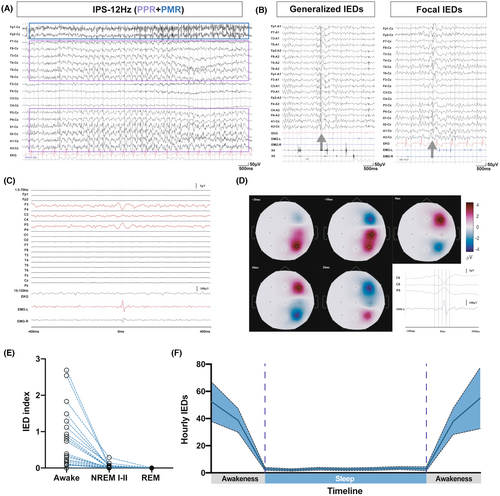
To further evaluate photosensitivity in FCMTE patients, intermittent photic simulation (IPS) was conducted throughout VEEG evaluation in 39 patients, including 35 with both cortical tremors and BTCSs, and four with cortical tremors only. Based on the classification described by Waltz et al.,20 31 patients (79.5%) were photoparoxysmal responses (PPR)-positive (Table 2, Fig. 2A), including 29 with a history of seizures and two without. Twelve patients were classified as PPR I–III and 19 patients were classified as PPR IV. Eighteen patients (46.2%) were positive for a photomyoclonic response (PMR) upon IPS, while in one patient (2.6%), this PMR was accompanied by fixation-off sensitivity.
| EEG recording | n = 81 |
|---|---|
| Posterior dominant rhythm | 9.2 ± 1.2 Hz |
| IEDs | 56 (69.1%) |
| Generalized IEDs | 33 (58.9%) |
| Occipital/other regions | 24/9 |
| Focal IEDs | 23 (41.1%) |
| Occipital/other regions | 15/8 |
| Normal or nonspecific abnormalities | 25 (30.9%) |
| Correlation between IEDs and seizures | P < 0.01 |
| Overnight EEG | n = 34 |
| IEDs | 24 (70.6%) |
| Normal or nonspecific abnormalities | 10 (29.4%) |
| IPS | n = 39 |
| PPR positive | 31 (79.5%) |
| PPR I-III | 12 |
| PPR IV | 19 |
| PPR negative | 6 (15.4%) |
| PMR positive | 17 (43.6%) |
| PMR negative | 20 (51.3%) |
| IPS interrupted (incomplete) | 2 (5.1%) |
- EEG, electroneurophysiologcial testing; IEDs, interictal epileptiform discharges; IPS, intermittent photic stimulation; PPR, photoparoxysmal response; PMR, photomyoclonic response.
EEG
EEG examination was conducted in 81 patients, including 64 with both cortical tremors and seizures, and 17 with cortical tremors only. Jerk-locked averaging (JLA) with EEG monitoring during a cortical myoclonus demonstrated a premovement time-locked cortical activity, rather than concurrent epileptic spikes (Fig. 2C, D). No seizure attacks were captured throughout the duration of EEG recording. The frequency of the posterior dominant rhythm (PDR) was 9.2 ± 1.2 Hz, which did not significantly differ from that in age-matched controls from our center (10.3 ± 0.8 Hz). Interictal epileptiform discharges were recorded in 56 patients (56/81, 69.1%), including 50 with a history of seizures, and six without. Thirty-seven patients (56.6%, 37/56) presented with spike/polyspike waves, 12 had both spikes and sharp waves, and seven presented with sharp waves only. Thirty-three patients had generalized IEDs, including 24 (72.7%, 24/33) with occipital dominance and nine (27.3%, 9/33) with frontotemporal dominance (Fig. 2B). Twenty-three patients had focal IEDs, with 15 (65.2%, 15/23) having IEDs over the occipital lobe, and eight patients (34.7%, 8/23) over the frontal, temporal, or parietal lobe (Table 2). In addition, a high frequency of historical seizures was correlated with the presence of IEDs upon EEG (Table 2, P < 0.01).
Overnight EEG recording was performed in 34 of 81 patients, and IEDs were detected in 70.6% (24/34). The IED index during awake or sleep periods was calculated for the 24 patients with IEDs. The average IED index was 0.82 ± 0.88/min during awake periods and 0.04 ± 0.06/min during NREM I-II (Fig. 2E, F). No IEDs were recorded during REM sleep.
Response to anti-seizure medications
Sixty-four (64/97, 66.0%) patients with a history of seizures had treatment with ASMs, all of whom had a positive drug response. Forty-eight of the ASM regimens were monotherapies, 16 were multidrug regimens, while 33 patients were unmediated. As of the most recent follow-up, valproic acid was the most commonly administered ASM (n = 29), followed by benzodiazepines (clonazepam/diazepam/phenobarbital) (n = 19) and sodium channel blockers (phenytoin/oxcarbazepine/carbamazepine) (n = 16). Valproic acid and sodium channel blockers were the most frequently prescribed combination of ASM for individuals on multi-drug. Some patients described that taking benzodiazepines during prodromal phase might help prevent the prodrome from progressing to the seizures. It is worth noting that no patients experienced exacerbated cortical tremor or seizures when using sodium channel blockers.
Discussion
Familial cortical myoclonic tremor with epilepsy has been reported worldwide as BAFME or FCMTE in Asia, and FAME or ADCME in Europe, Oceania, and Africa.3-5, 22, 23 As the most common complaint in clinical visits, a thorough understanding of the key features of FCMTE-related seizures and a comprehensive electroneurophysiological examination is crucial for early and accurate diagnosis. In the current study, we characterized the clinical and electrophysiological features of seizures in FCMTE based on a large cohort of Chinese FCMTE patients. All patients were genetically diagnosed with pentanucleotide (TTTCA) repeat expansion in the SAMD12 gene.
Familial cortical myoclonic tremor with epilepsy is an autosomal dominant inherited disease with high morbidity and penetrant.24 Cortical tremors had full penetrance in our cohort at 53 years of age. While they were >95% penetrant at 62 years, seizures were not fully penetrant in our cohort. Similarly, non-penetrance of seizures was reported in Japanese FCMTE patients, which may be correlated with the configuration and/or size of the pentanucleotide (TTTCA) repeat expansion in the SAMD12 gene.7, 25-27 Notably, >95% of FCMTE patients experienced seizures after 24 years of age. This onset age is much later than is seen in other myoclonic epilepsies such as JME28 and PMEs.29 Therefore, a seizure onset of 24 years or earlier constitutes a red flag for diagnosis of an FCMTE alternative.
Seizures in FCMTE are notable due to the presence of triggers and prodromes. Interestingly, FCMTE patients frequently self-reported triggers and prodromes unique to each individual. This allows patients to actively avoid the triggers and take measures (e.g., rest, meditation, or take ASMs temporarily) to prevent a seizure attack. This accumulating experience enables better self-management of seizures in FCMTE patients and gives rise to effective nondrug strategies for seizure prevention. This may explain the seemingly nonprogressive course of seizures in FCMTE patients.
Previously, seizures in FCMTE were considered to have generalized onset, given the BTCS semiology and the presence of generalized spike/polyspike and wave complexes in small cases or pedigree series EEG findings.30, 31 However, similar to findings from a Japanese cohort,16 both generalized and focal IEDs were recorded in our cohort. An occipitally dominant feature of distribution was notable for either generalized or focal IEDs. Considering photosensitivity and the high rate of IPS-induced PPRs in FCMTE, we propose FCMTE as a photosensitive epilepsy. This hypothesis was further corroborated by our recent imaging studies. Structural and functional alterations within occipital regions (sagittal stratum and bilateral calcarine sulcus) were detected32, 33 and correlated with photosensitivity (i.e., photophobia32) and seizure frequency.33 Altogether, clinical, electrophysiological, and radiological evidence support occipital lobe hyperexcitability and the visual-related network in the pathophysiology of FCMTE.
Notably, a clear sleep–wakefulness rhythmicity was observed in FCMTE. Both seizures and IEDs clustered during awaking hours and were minimal during sleep. During the sleep–wakefulness transition, consisting of a mix of the two states, the gradual change in IED frequency suggests a primary influence of the sleep–wake cycle rather than circadian or environmental factors (e.g., light–dark cycles) on cortical excitability. Our findings align with a previous report by Hitomi et al.,34 which also revealed a reduction in epileptiform discharges during sleep. The sleep–wakefulness rhythmicity in FCMTE was different from most focal and generalized epilepsies, in which IEDs are prominently active during sleep.35, 36 Previous studies reported a strong wakefulness preference in occipital lobe seizures,37, 38 while seizures beginning in the frontal lobe or parietal lobe tend to occur during sleep.38 The sleep–wakefulness modulation of slow waves, which is more prominent in posterior quadrant areas, may contribute to topological differences.39
Familial cortical myoclonic tremor with epilepsy may be understandably misdiagnosed as JME or PMEs, given the overlap in seizure features such as manifestations of myoclonic jerks and/or BTCSs, as well as being triggered by photic stimulation.40, 41 The cortical myoclonus in JME occurs spontaneously and is highly associated with EEG polyspikes. In FCMTE, however, cortical myoclonus is non-epileptic, typically elicited by motor activation and correlated cortical activity on the contralateral sensorimotor area.40 Progressive myoclonus epilepsies are comprised of a group of heterogeneous genetic disorders characterized by epileptic myoclonus and epileptic seizures, some of which are invariably fatal (e.g., Lafora disease, the neuronal ceroid lipofuscinoses). Other PME disorders, such as Unverricht–Lundborg disease (ULD) progress more slowly, and may mimic FCMTE, especially in its early stages.29 However, seizures in PMEs mostly occur upon awakening or during sleep. These are always drug-resistant and accompanied by ataxia and progressive neurocognitive impairment, differing from the more benign course in FCMTE-related seizures. In our cohort, valproic acid was the most commonly used ASM followed by benzodiazepine agents. Furthermore, sodium channel blockers, either in monotherapy or combined with other ASMs, were also effective in 16 of our patients. In addition, most PMEs are inherited via an autosomal recessive pathway, with characteristic horizontal transmission, whereas FCMTE is autosomal dominant, with vertical transmission. Specific differences in diagnostic features are summarized in Table 3.
| FCMTE | PMEs-ULD | JME | |
|---|---|---|---|
| Family History | Familial clustering | Sporadic | Sporadic or familial clustering |
| Genetics basis | Autosomal dominant | Autosomal recessive29, 42 | Heterogeneous43 |
| Onset age | After 24 years | Usually 6–16 years42 | Usually 10–25 years27, 28 |
| Core phenotype | Non-epileptic cortical myoclonus/tremor; BTCSs | Epileptic-myoclonus; BTCSs; complicated by dysmetria or ataxia44 | Epileptic-myoclonus; BTCSs; absence |
| BTCSs features | Infrequent; mostly during wakefulness | Infrequent in the early stages and later may cascade42; upon awakening or during sleep29, 44 | Infrequent; mostly upon awakening28 |
| EEG features | |||
| PDR | Matched with age or mild slow45 | Matched with age or mild slow42, 46 | Matched with age |
| IEDs | GSWDs or focal SWs (prevalent over the occipital); decrease during sleep | GSWD or focal SWs (prevalent over the parietal and occipital); increase during sleep44 | 3–6 Hz GSWDs or PSWs; increase at sleep onset or awakening43 |
| IPS | PPR up to 80% | PPR up to 80% and tends to abate with age46 | PPR up to 30%47 |
| ASMs | Good response | Drug resistant | Good response; high relapse rate upon withdrawal |
| Cognitive | Normal | Mild-to-moderate decline | Normal |
| Structural MRI | Normal | Cortical and/or subcortical atrophy42 | Normal |
| Prognosis | Remains good with appropriate treatment | From a lethal entity toward a bearable disability44 | Remains good with appropriate treatment |
- FCMTE, familial cortical myoclonic tremor with epilepsy; PMEs, progressive myoclonic epilepsies; ULD, Unverricht-Lundborg disease; JME, juvenile myoclonic epilepsy; BTCSs, bilateral tonic–clonic seizure; EEG, electroencephalogram; PDR, posterior dominant rhythm; IEDs, interictal epileptiform discharges; IPS, intermittent photic simulations; REM, rapid eye movement; GSWs, generalized spike-waves; SWs, spike-waves; PSWs, polyspike-waves; ASM, anti-seizure medication; PPR, photoparoxysmal responses.
Our study has several limitations. First, ictal events during EEG recordings were not recorded, largely due to the low frequency of seizures in FCMTE. The interictal EEG data, along with our previous multimodal MRI findings, partially address this limitation through the contribution of valuable information on the resting-state network in FCMTE and support the involvement of the occipital lobe and the visual-related network. Second, the cohort was limited to genetically-diagnosed FCMTE type 1 patients. However, FCMTE type 1 remains the most common and most representative subtype of FCMTE. Future studies on different cohorts with other FCMTE subtypes are required to confirm our findings.
Conclusions
We propose FCMTE as a photosensitive epilepsy syndrome and occipital lobe hyperexcitability. This proposal considers its photosensitivity, occipital dominant IEDs, and the high PPR positivity in IPS observed in FCMTE. The adult onset of epilepsy, characterized by prolonged prodromes and presence of specific triggers, clustering of seizures in wakefulness, and good response to ASMs may differentiate seizures in FCMTE from those in other photosensitive myoclonic epilepsy syndromes such as JME and PMEs. Early identification and appropriate management of seizures in FCMTE may avoid escalating use of anti-seizure medications and improve quality of life.
Acknowledgements
We thank the individuals and families who participated in the collection of clinical data for this project and for enrolling in our research studies. We thank our colleagues who referred families to our center and our clinical colleagues who evaluated patients.
Author Contributions
W.L., S.W., and M.P.D. contributed to conception and study design. X.Q., Y.M.Y., B.W., Z.J.W., H.M.Y., W.J.M., Y.G., F.F., D.H.Y., Z.Y.O., H.T.W., and S.W. contributed to acquisition and analysis of data. Y.D., Z.D.C., Y.Z., X.H.C., L.L.H., and C.H.S. contributed to preparing the figures. Y.D., Z.D.C., and Y.Z. contributed to drafting the manuscript.
Conflict of Interest
The authors declare no conflicts of interest.




