Correlation between spinal cord stimulation analgesia and cortical dynamics in pain management
Li Chen and Zhen Zhang contributed equally to this study.
Abstract
Aim
Spinal cord stimulation (SCS) is an effective method to treat neuropathic pain. It is necessary to identify the responders of SCS analgesia before implantation. The aim of this study is to investigate the relationship between the cortical dynamics and SCS analgesia responders in pain management.
Methods
Resting-state EEG recording was performed in patients who underwent short-term implantation of spinal cord stimulation for pain therapy. We then did spectral analysis to capture the pattern of cortical oscillation between neuromodulation therapy analgesia responders and nonresponders.
Results
About 58.3% (14 out of 24) of participants were considered as analgesia responders, with average visual analogue scores reduction of 4.8 ± 1.0 after surgery, and 2.1 ± 0.7 for the nonresponder subgroup, respectively. The alpha oscillation was significantly enhanced in responder cohort compared with nonresponders. We also observed an increasing spectral power of gamma band in responders. Furthermore, the attenuation of pain severity was significantly correlated with the global alpha oscillation activity (r = 0.60, P = 0.002). Likely, positive and significant correlation was found between the pain relief and gamma activity (r = 0.58, P = 0.003).
Conclusions
Distinct pattern of neural oscillation is associated with the analgesic effect of spinal cord stimulation in pain management, enhancement of cortical alpha and gamma oscillation may be a predictor of analgesia responders.
Introduction
Neuropathic pain is one of the most common yet debilitating pathological conditions, caused by a primary lesion or disease of the peripheral or central nerve system.1 Patients may suffer intractable pain caused by postherpetic neuralgia, diabetic neuropathy, traumatic injury, postsurgical lesion, multiple sclerosis, and cancer.2-6 The estimated prevalence of neuropathic pain ranged between 7% and10%.7, 8 Recently, greater risk of neuropathic pain (14.6%) is reported among nursing home residents.9 As a result, the quality of life is significantly reduced in those with neuropathic pain.
The main goal of neuropathic pain management is to control the pain symptoms, characterized by allodynia, hyperalgesia, and paresthesia. In general, a great challenge is to control the spontaneous episodes as well as evoked painful perception.10 Pharmacotherapy remains the conventional option of neuropathic pain treatment. Currently, the first-line medications include gabapentinoids, tricyclic antidepressant, and selective serotonin–norepinephrine reuptake inhibitors according to the recommendation of The Special Interest Group on Neuropathic Pain.10 However, the therapeutic effect may be still unsatisfactory even combined with multiple analgesic agents (e.g., lidocaine patch, capsaicin, or opioids).
In addition to pharmacotherapy, invasive procedures can be considered when conservative methods fail to provide pain relief. Local or epidural nerve block is easy to perform to achieve immediate improvement of symptoms, but the long-term effect remains controversial.11 To achieve enduring relief of pain, one potential solution is to implant permanent intrathecal drug delivery system for administration of analgesic medications.12, 13 Despite intrathecal drug delivery system, spinal cord stimulation (SCS) may be an alternative option for long-term management of chronic neuropathic pain.
As one important outgrowth of the well-known gate control theory proposed by Melzack and Wall in 1965, SCS has been increasingly used to treat severe pain syndromes.14, 15 The indications of SCS cover multiple forms of neuropathic pain, such as failed back surgery syndrome (FBSS), complex regional pain syndrome, peripheral neuropathy, and phantom limb pain.16 Recently, emerging evidence supports its validation in pain management of herpes zoster, and acts as a potential tool to prevent the development of postherpetic neuralgia.17-20 It is generally accepted that SCS analgesia responder should report at least 50% reduction of pain. Thus, one necessary step of permanent implantation of SCS is to confirm its clinical efficacy during the trial period.21
However, the analgesic effect may still fade after initial pain relief.22 Recent evidence has revealed that about 12–20% of patients did not achieve satisfactory symptomatic improvement (≥50% pain relief from baseline) even with high-frequency SCS strategy.23, 24 It is significantly important for those suffering herpetic-related neuralgia, as adequate analgesic intervention may not only attenuate symptomatic pain, but also prevent the development of postherpetic neuralgia. Thus, potential biomarkers for identification of SCS responder is necessary and urgently needed. In addition, similar clinical data of SCS treatment for herpetic-related pain management remains less studied. In this study, we aim to investigate the response rate of SCS analgesia in patient with herpes zoster-related pain. Furthermore, our previous data has demonstrated the potential link between the neuromodulation therapy and cortical dynamics. Specifically, alpha rhythmic oscillatory activity was enhanced under activation of electoral nerve stimulation in patient with herpetic-related pain.20 Likewise, emerging data have indicated a potential role of electroencephalography (EEG) in comparison of different neuromodulation status during SCS treatment, such as tonic and burst stimulation strategy.25-27 In addition, cortical oscillation is altered in association with pain perception.28, 29 However, the cortical signature of SCS therapeutic responder remains less unknown. Thus, the other goal of this study is to assess the feasibility and clinical value of resting-state EEG in recognition of the SCS analgesia responder in pain management.
Methods
Study design and participants
Twenty-four patients presented with moderate to severe pain after herpes zoster infection, affecting the cervical (n = 3), thoracic (n = 17), and lumbar (n = 4) dermatomes were prospectively enrolled in this study. All participants consented to undertake the SCS surgery and EEG recording during hospitalization. The medical data were systematically recorded for further analysis, including age, sex, duration of disease and SCS implantation, pain severity, etc. The prospective observational study was performed under the guidance of the Helsinki Declaration. The study was approved by the Ethics Committee of The Third Xiangya Hospital, Central South University, China (No. 22216).
Surgical technique of SCS implantation
Details of SCS procedure have been described previously.18, 20 Specifically, the preoperative fasting was required for administration of sedative agents, if necessary, in the procedure. The target of spinal segment was determined by the affected region of herpetic lesion preoperatively, and confirmed by the fluoroscopic imaging during operation.
Patient was placed in a prone position, and the local analgesia was applied before the epidural puncture with one Tuohy needle (14G). The needle stylet was removed after the needle entered the epidural space, and an eight-contact lead (No. 3873; Medtronic, Minneapolis, MN, USA) was then inserted through the cannula. The lead was advanced under the anterior–posterior view of fluoroscopy, and one sensory testing was conducted to ensure the electrical stimulation covered the painful regions reported by the patient. Patient was asked to stay in bed in the next 2 days to avoid potential lead migration. We excluded the participants with lead migration and dislocation in this study. The stimulation frequency was set at 50 Hz, with pulse width of 500 μsec. We adjusted the voltage of electrical stimulation according to the pain severity. The stimulation lead was removed within 2 weeks to avoid the potential of infection.
EEG recording
The protocol of EEG recording has been described previously.20 The EEG recording was scheduled 4–5 days after SCS implantation. The stimulation parameter was set prior to the EEG testing to ensure an optimal coverage of the painful region. To evaluate the cortical dynamics of SCS, the stimulator was inactivated at least 30 min before the first recording session, following by another session with activation of the SCS. The recording duration ranged between 5 and 7 min for each session.
During each visit, one resting state EEG recording was conducted in one quiet, temperature-controlled, and electrically shielded office. Participants were kept silent and awake during the experiment with eyes closed. We used a 16-channel biosensor (Cyton + Daisy, www.OpenBCI.com), which was connected to the OpenBCI electrode cap for the collection of EEG data. We chose the Cz channel as the reference electrode, and the Fpz for the ground electrode, respectively. To compare the cortical dynamics of different brain regions, we further divided the channels into five regions (frontal, central, occipital, parietal, and temporal sites), according to the cortical mapping. Definition of the EEG channels is given in the Table 1. The software for data visualization and acquisition was downloaded from the website (www.OpenBCI.com). The EEG data were recorded at a sampling rate of 128 Hz. We tested the impedances of each electrode with the OpenBCI GUI software prior to the onset of recording, and the impedance should be kept below 10 KΩ.
| ROI | Electrode location |
|---|---|
| Frontal site | FP1, FP2, F3, F4, F7, F8 |
| Central site | C3, C4 |
| Parietal site | P3, P4, P7, P8 |
| Occipital site | O1, O2 |
| Temporal site | T7, T8 |
- ROI, region of interest.
Processing of EEG data
After EEG recording, raw data were extracted with c. The open-source EEGLAB toolbox was introduced for offline processing and analysis.30 All data were manually checked by one independent researcher (L.C.) to rule out the artifacts and malfunctioning channels. The bandpass filter of 1–45 Hz was used, and the filtered continuous data were segmented into consecutive 2 sec epochs. The threshold amplitude of epochs for the exclusion was set beyond ±80 μV. The independent component analysis was performed for the identification and correction of eye movement artifacts. Thirty artifact-free segments were used to generate the dataset during each session for the statistical calculation. Spectral power analysis was performed using the fast Fourier transform algorithm with the “spectopo.m” function script in the EEGLAB. The EEG data were sub-banded into five ranges: δ (delta, 0.5–4.0 Hz), θ (theta, 4.0–8.0 Hz), α (alpha, 8.0–13.0 Hz), β (beta, 13.0–30.0 Hz), and γ (gamma, 30.0–45.0 Hz). We calculated the spectra of individual channel by averaging the data across epochs for each patient.
Pain assessment and follow-up
Pain severity was assessed by the visual analog scale (VAS), which ranged between 0 (“pain free”) and 10 (“worst pain imaginable”). The therapeutic effect was calculated by the reduction of pain scores between the baseline and postoperative visit. The SCS analgesia responder was defined as pain relief over 50% at discharge.31 Routine telephone interview was conducted at 1-, 3-, 6-, and 12-month after treatment by one independent researcher (L.C.).
Statistical analysis
Descriptive data were used to capture the clinical features of participants. Variables are presented as means ± SDs. EEG raw data were processed under the circumstances of EEGLAB software (MathWorks, Inc., Natick, MA, USA). The extracted data were analyzed with Prism Version 8.0 (GraphPad, San Diego, CA, USA). We compared the pain scores and spectral power with the two-way repeated-measures analysis of variance, with post hoc multiple pairwise Bonferroni correction. The measurement of normality was calculated with the Shapiro–Wilk testing for each variable. Spearman's correlation analysis was then used for the variable with abnormal distribution. The two-tailed value of P < 0.05 was considered statistically significant.
Results
Clinical features of enrolled patients
Fourteen patients reported pain relief over 50% compared with baseline at discharge, and ten were considered as nonresponders respectively. About 42% of the participants were female in this study, with mean age of 66.7 ± 9.3 years old. Most patients (16 out of 24) were at sub-acute phase of herpetic lesion, with duration of disease ranging between 1 and 3 months. The percentage of postherpetic neuralgia was 33% that moderate to severe pain condition lasted over 3 months. We found that the nonresponder subgroup had significantly increasing implantation time compared with SCS responders (12.4 ± 2.0 vs. 10.4 ± 2.0, P = 0.02). Both cohorts reported moderate to severe neuropathic pain before SCS treatment, with mean VAS of 7.5 ± 1.1 at baseline.
To evaluate the clinical outcome of SCS treatment, we recorded the pain severity before discharge, and did three routine telephone follow-ups at 1-, 3-, and 6-months after surgery. Both cohorts represented with significant improvement of symptoms at each visit compared with baseline (Table 2). However, the immediate therapeutic effect was significantly improved in the SCS-responder subgroup, as demonstrated by the lower VAS at discharge. In contrast, the short-term analgesic effect was better but not statistically significant in the responder at 1-month follow-up. Likely, the medium- (3 months), and long-term (6 months) SCS functioning was neither significantly improved in the responders.
| Variables | Responder | Nonresponder | P value |
|---|---|---|---|
| Number of patients | 14 | 10 | |
| Age (years) | 66.1 ± 9.6 | 68.5 ± 9.1 | 0.87 |
| Sex (female, %) | 7 (50) | 3 (30) | 0.42 |
| Duration of disease (%) | |||
| 1–3 months | 9 (64) | 7 (70) | |
| Over 3 months | 5 (36) | 3 (30) | 0.99 |
| Painful region (%) | |||
| Cervical | 2 (14) | 1 (10) | |
| Thoracic | 10 (72) | 7 (70) | |
| Lumbar | 2 (14) | 2 (20) | 0.90 |
| Comorbidities (n, %) | |||
| Hypertension | 6 (43) | 4 (40) | |
| Diabetes mellitus | 4 (29) | 2 (20) | 1.0 |
| Duration of implantation (days) | 10.4 ± 2.0 | 12.4 ± 2.0 | 0.02* |
| Duration of hospitalization (days) | 15.3 ± 3.1 | 17.8 ± 4.8 | 0.13 |
| Pain severity (VAS) | |||
| Baseline | 7.7 ± 1.0 | 7.3 ± 1.3 | 1.0 |
| Discharge | 2.9 ± 0.7 | 5.2 ± 1.0 | 0.00*** |
| One month | 3.5 ± 1.9 | 4.9 ± 1.4 | 0.22 |
| Three months | 2.9 ± 2.5 | 4.8 ± 1.4 | 0.20 |
| Six months | 2.7 ± 2.6 | 4.6 ± 1.9 | 0.28 |
- Variables are presented as means ± SDs.
- * P < 0.05.
- *** P < 0.0001.
Comparison of cortical dynamics between SCS analgesia responder and nonresponder
Next, we investigated the cortical effects associated with the neuromodulation therapy, by recording the 16-channel EEG at resting state in the responders (Fig. 1A, B) and nonresponders (Fig. 1C, D), respectively. To conduct further analysis quantitatively, we calculated the grand average spectral power by averaging across all channels in two subgroups (Fig. 1E). By dividing the neural oscillations into five physiological sub-bands (Table 1), we found that the general spectral density of SCS responders was significantly increasing at the alpha band, with or without activation of SCS (Fig. 1F). Besides, the gamma oscillatory activity was also enhanced in the SCS responders under baseline condition (SCS off), as shown in the Figure 1G.
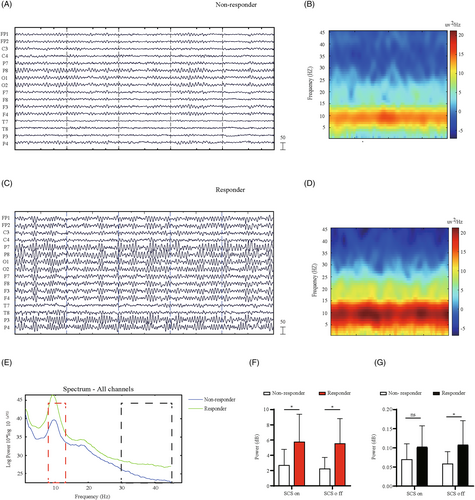
Enhancement of oscillations in the responder to SCS treatment
In Figure 2, we compared the distinct pattern of cortical responses to SCS intervention by filtering the alpha sub-band (8.0–13.0 Hz) (Fig. 2A, B). The spectral power was lower in the nonresponders compared with responders. Furthermore, we found that the source of enhanced alpha activities may originate from the frontal and temporal region, as shown in the Figure 2C.
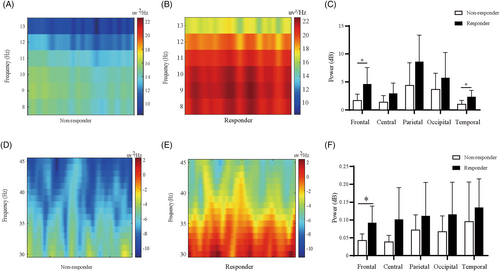
Next, we evaluated the impact of gamma oscillations on the therapeutic effect of SCS. Consistent with alpha rhythm, we also observed an increasing spectral power at gamma band in SCS analgesia responders, as shown in the representative spectrogram (Fig. 2D, E). We then compared the spectral density of gamma activity between two cohorts at different cortical regions. In Figure 2F, we can find that the gamma activity was statistically more significant in SCS responders at the frontal regions.
Correlation between the pain relief and neural oscillations
In Figure 3, we show the relationship of pain reduction between the alpha, and gamma oscillations. Specifically, significant and positive correlation was found between the pain relief, assessed by the percentage of pain reduction to baseline, and the alpha spectral power (Fig. 3A, r = 0.60, P = 0.002). This phenomenon was also significant in the frontal region, as shown in the FP1 channel (Fig. 3B). Likely, the overall gamma activity was positively associated with the pain reduction (Fig. 3C, r = 0.58, P = 0.003) in all participants. Also, strong and positive relationship was found between the gamma oscillations at the frontal (F3 channel) site, and the percentage of pain relief (Fig. 3D, r = 0.63, P = 0.001).
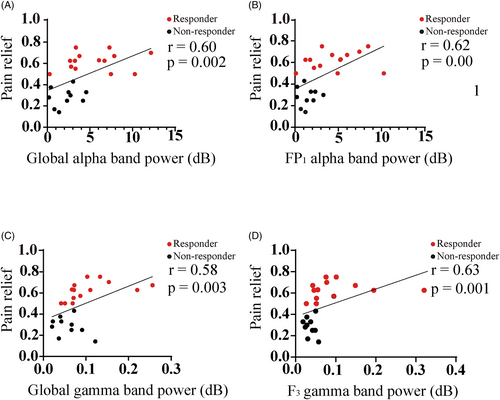
Discussion
Spinal cord stimulation has been increasingly applied to treat intractable neuropathic pain. Recently, its validation in short-term implantation therapy for postherpetic neuralgia management has been generally accepted in the Chinese population.32 However, the response rates of this neuromodulation for herpetic-related pain remains less known, and it is essential to identify the proper candidates for this invasive treatment. In this study, we examined the role of the EEG in recognition of SCS analgesia responder in the postherpetic neuralgia population. We found that, consistent with our previous data that the therapeutic effect may be partially associated with the alpha oscillatory activities,20 which can be promisingly used as one biomarker for the classification of responder to the neuromodulation therapy.
Compared with trigeminal postherpetic neuralgia,31 we noticed the response rate was relatively lower in those who were treated by SCS (58%), and 83% for peripheral nerve stimulation respectively. We assume that several reasons may contribute to the divergence of responsive forms to neuromodulation therapy, including herpetic and implantation site, stimulation parameter, tolerance threshold, and neuropathic processing at distinct level (peripheral vs. central sensitization). Consistent with pain relief, the implantation duration was significantly prolonged in the nonresponder group, about 2 days longer compared with the responder cohort. Thus, we think it necessary to identify the truly responder to neuromodulation procedure to achieve a better clinical outcome.
Despite the methodological and clinical discrepancy, we have previously demonstrated one common supra-spinal dynamic phenotype of electrical nerve stimulation treatment,20 that the analgesic effect may partially be mediated by the alpha oscillatory activity. Thus, we speculate that the cortical oscillations may be one promising biomarker of SCS analgesia responder. EEG is commonly used to detect the neurological processing, and considered as one potential biomarker of pain perception in health state.33 One obvious advantage of EEG recording is the noninvasive setup with a high temporal resolution.
To capture the cortical patterns of neuromodulation, we conducted EEG recording with eye closed at resting state as described in the previous protocol,20 with or without SCS activation. Inconsistent with previous data, we did not observe dramatically spectral power enhancement with activation of SCS compared with baseline. One possible explanation is the relatively small sample size of previous pilot study. In addition, the combination of peripheral nerve and spinal cord bias may also contribute the cortical alternations of neuromodulation.
Meanwhile, we found that the cortical oscillations at alpha and gamma band were significantly enhanced in SCS-responders. Majority of the EEG study reported an increasing activity of gamma power during painful stimuli, yet, more evidence supports the decreasing trend in alpha range.33-36 In our cases, synchronous gaining at alpha and gamma rhythm of the SCS analgesia responder may reveal the mechanism of pain regulation with involvement of top-down and button-up system.37 For instance, enhanced gamma power may not be associated with more severe pain in the SCS analgesia responder, instead, increasing gamma activity may indicate cortical control of pain endogenously.38, 39
Likewise, the enhancement of alpha oscillations in the SCS responders may indicate the reduced input of nociception to the brain network. However, it remains controversial about the actual changes of alpha oscillations during pain perception, as demonstrated by an opposite change in the frontal region.40, 41 Thus, we think it is more important to investigate the whole brain network rather than isolating specific cortical activity.30 In addition to alpha and gamma oscillatory enhancement, the generalized increasing trend of global spectral density across other physiological range, may potentially reveal the active status of brain in the SCS analgesia responder cohort.
It is also essential to identify the biomarker of SCS treatment responders with feasible and easy read-out index. Here, we have shown that both alpha and gamma oscillations had significant and positive correlation with the pain relief. Specifically, the characteristic features of cortical responder were obviously detected in the frontal region. This finding is consistent with previous data reporting the gamma activity in the prefrontal and frontocentral region.34, 40 Thus, we think it promising to develop an algorithm for the detection of SCS analgesia responder with the information of neural oscillation at the frontal sites.
Despite EEG, quantitative sensory test may contribute to the recognition of responder in pain management.42, 43 One predictive model combined with the quantitative sensory test and EEG has been demonstrated to be useful to distinguish the postoperative opioid analgesia responders.44 For those who suffer postherpetic neuralgia, the patterns of sensory dysfunction may vary between different subjects despite the common etiology.45 This distinct phenotype of sensory impairment may indicate the underlying responsive effect to analgesic agents,46 similarly, that different responsive patterns of SCS may be also associated with the forms of sensory deficiency in our study.
In addition to EEG, multiple clinically relevant factors can be used to predict the response of high frequency SCS in patients with FBSS, including the trial pain relief, predominant pain location, and the number of previous surgeries.46 However, it is not available to apply all these parameters in herpetic population. Likewise, risk factor of postherpetic neuralgia development may be considered to be as predictors for SCS responder (e.g., elderly, female gender, severe acute pain).
There are several limitations in our study. First, we only compared postoperative analysis between SCS analgesia responders and nonresponders. It will be worth to identify the potential responders preoperatively in the future application of neuromodulation therapy. In addition, we only examined the relationship between SCS treatment responders and spectral power density, further analysis of EEG data may be of help to detect the analgesia responders. Also, it is promising to combine other clinical, experimental, and imaging data to develop an optimal algorithm of responder recognition. Furthermore, we only investigate the cortical dynamics by isolating the brain networks, it is equally important to confirm the functional connectivity of the whole brain in the future study.33 Finally, one common challenge of EEG study in the field of pain research, remains the reproducibility of the findings across different subjects or sessions.47 Thus, it is necessary to set standard procedures and well-designed experimental control to enhance the reproducibility of data.48
Conclusions
In this prospectively study, we found the distinct pattern of cortical oscillations between SCS analgesia responders and nonresponders. The alpha and gamma spectral density was significantly enhanced in the responder subgroup. Furthermore, we identified that both alpha and gamma oscillations were strongly and positively correlated with the reduction of pain. Thus, we propose that the noninvasive and commonly used experimental device, namely the EEG may be one promising tool to identify the analgesia responders of SCS treatment for those suffered with postherpetic neuralgia.
Acknowledgements
We would like to thank all the participants who were enrolled in this study.
Author contributions
Conceptualization, Haocheng Zhou and Dong Huang; methodology, Li Chen Rui Han, and Zhen Zhang; software, Haocheng Zhou, Li Chen, Rui Han, and Zhen Zhang; validation, Li Chen and Zhen Zhang; formal analysis, Li Chen; investigation, Li Chen and Haocheng Zhou; resources, Haocheng Zhou and Dong Huang; data curation, Li Chen; writing—original draft preparation, Haocheng Zhou; writing—review and editing, Haocheng Zhou; visualization, Haocheng Zhou; supervision, Haocheng Zhou; project administration, Haocheng Zhou; funding acquisition, Haocheng Zhou All authors have read and agreed to the published version of the manuscript.
Funding Information
This research was funded by National Natural Science Foundation of China, (81901146 and 82271259 to H.Z.), Excellent Youth Foundation of Hunan Scientific Committee, Key Laboratory of Hunan Province grants (2018TP1009 to H.Z. and D.H.), and Huizhiyucai Project of the Third Xiangya Hospital, Central South University.
Conflict of interest
The authors declare no conflict of interest.
Ethic statement
The prospective observational study was performed under the guidance of the Helsinki Declaration. The study was approved by the Ethics Committee of The Third Xiangya Hospital, Central South University, China. Informed consent was obtained from all subjects involved in the study.
Open Research
Data availability statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical reasons.




