Safety evaluation of shorter infusion for ocrelizumab in a substudy of the Phase IIIb CHORDS trial
Funding Information
This research was funded by Genentech, Inc., South San Francisco, CA, USA.
Abstract
The CHORDS trial evaluated ocrelizumab (OCR) in patients with relapsing-remitting multiple sclerosis who had a suboptimal response to previous disease-modifying treatment. The objective of the present study was to assess the safety of shorter OCR infusions in a substudy of CHORDS. After completing four doses of OCR per initial US prescribing recommendations in the main study, participants in the substudy (N = 129) received a fifth dose over a 2-h duration (vs. 3.5 h). Infusion-related reactions occurred in 12.4% of patients. None were severe, life-threatening or led to treatment discontinuation. Shorter infusion time did not change the safety profile of OCR. Clinicaltrials.gov (NCT0237856).
Introduction
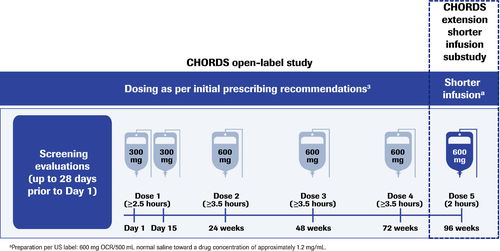
Ocrelizumab (OCR) is an anti-CD20 humanized monoclonal antibody approved as a disease-modifying therapy (DMT) for relapsing multiple sclerosis (RMS) and primary progressive multiple sclerosis (PPMS).1, 2 The initial recommended dosing schedule comprised two 300-mg intravenous infusions, each lasting ≥2.5 h and separated by 14 days, with subsequent single infusions of 600 mg, each lasting ≥3.5 h, administered every 6 months.3 A shorter, 2-h duration for 600-mg infusions was subsequently approved in Europe2 and the United States,1 offering patients improved convenience, limiting their time within medical facilities and reducing the load on medical resources.
Infusion-related reactions (IRRs) have been reported with OCR treatment, including in the pivotal trials of patients with RMS (OPERA I and OPERA II, 34.3%) and PPMS (ORATORIO, 39.9%). Most IRRs were mild to moderate in severity and decreased with subsequent dosing,4-6 supporting the investigation of shorter OCR infusions. Findings from the Phase IIIb ENSEMBLE PLUS (NCT03085810)7 and SaROD (NCT03606460)8 studies, in which patients received one or two doses of OCR as per the initial prescribing recommendations before receiving their next dose as a shorter infusion, demonstrated that a shorter infusion time was not associated with an increased rate or severity of IRRs.7, 8 This article describes outcomes from an extension substudy of CHORDS (NCT02637856), in which patients with relapsing remitting MS (RRMS) who completed four doses of OCR per the initial prescribing recommendations received the scheduled fifth dose over a shorter duration.
Patients and Methods
CHORDS is an open-label, single-arm, Phase IIIb study investigating OCR in patients with RRMS who had a suboptimal response after ≥6 months on another DMT. Patients who completed four doses of OCR per the initial prescribing recommendations and had no serious or life-threatening IRRs in the main study were eligible to receive a fifth dose of 600 mg at Week 96 over a reduced infusion time of ≈2 h (Fig. 1 and Table 1). The 2-h timeframe was chosen based on a Phase II dose-finding trial, in which patients receiving OCR 1000 mg received the final 600 mg in the last 1.5 to 2 h of a 4-h infusion; no relationship between dose or infusion rate with IRRs was observed.
| Initial prescribing recommendations3 | Shorter infusion | |||
|---|---|---|---|---|
| Dose 1 (Infusions 1 and 2) | Dose 2 and subsequent doses | CHORDS substudy | ||
| Time, min | Infusion rate, mL/h | Infusion rate, mL/h | Time, min | Infusion rate, mL/h |
| 0–30 | 30 | 40 | 0–15 | 100 |
| 15–30 | 200 | |||
| 30–60 | 60 | 80 | 30–60 | 250 |
| 60–90 | 90 | 120 | 60–90 | 300 |
| 90–120 | 120 | 160 | 90–120 | 300 |
| 120–150 | 150 | 200 | 120–150 | — |
| >150 | 180 | 200 | >150 | — |
| >180 | — | 200 | >180 | — |
| Total infusion time | ≥2.5 h | ≥3.5 h | 2 h | |
| Total cumulative dose | 300 mg | 600 mg | 600 mg | |
- OCR, ocrelizumab.
A follow-up visit was conducted 30 days after the shorter infusion. All adverse events (AEs) that occurred during the infusion and the 30-day follow-up (reported at scheduled or unscheduled visits) were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) version 22.0 and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Events reported during or within 24 h of the infusion that were judged by an investigator to be an IRR were managed according to severity (Table 2). The primary outcome of the substudy was the proportion of patients with severe (Grade 3) or life-threatening (Grade 4) IRRs; additional endpoints included Grade 1 and 2 IRRs and AEs overall.
| IRR grade | Action to be taken |
|---|---|
| Mild-to-moderate |
|
| Severe (or complex of flushing, fever and throat pain) |
|
| Life threatening (e.g. anaphylaxis) |
|
- IRR, infusion-related reaction.
This study was conducted according to the International Council for Harmonisation E6 Guideline for Good Clinical Practice. Approval was provided by local Institutional Review Boards and all patients provided informed consent.
Statistical methods
All patients who received any amount of OCR in the substudy were included in the analyses. All AEs were summarized and tabulated by body system and preferred term. The number and proportion of patients who experienced Grade 3 or 4 IRRs and the number of patients who required IRR-related treatment interruption or infusion rate reduction were calculated with corresponding two-sided 95% exact Clopper-Pearson confidence intervals of the proportion.
Results
Patient characteristics and disposition
All patients in the CHORDS main study (n = 608) were given the option to enter into the study extension, which included 129 patients (21.2%). Substudy patients had a mean (SD) age of 36.7 (8.1) years, baseline Expanded Disability Status Scale score of 2.5 (1.4) and time since initial MS symptom of 5.5 (3.6) years. Most patients received prior treatment with one (67 [51.9%]) or two (54 [41.9%]) DMTs, most commonly glatiramer acetate (67 [51.9%]), dimethyl fumarate (44 [34.1%]) and fingolimod (30 [23.3%]). All 129 patients received the shorter ocrelizumab infusion, and most (121 [93.8%]) completed the infusion within 2.5 h, with an overall mean (SD) infusion time of 2.2 (0.3) h. The majority of patients (125 [96.9%]) completed the 30-day substudy; three patients (2.3%) were lost to follow-up, and one (0.8%) discontinued for a non-safety-related reason.
Safety outcomes
No severe or life-threatening IRRs were observed during the substudy (Table 3). Grade 1 or 2 IRRs were reported in 16 patients (12.4%), consistent with observations in the main study for other 600-mg infusions (Dose 3, 11.9%; Dose 4, 9.5%). Twelve patients who reported IRRs in the substudy previously reported IRRs in the main study, including nine who reported IRRs with multiple OCR infusions. Treatment interruption or infusion rate reduction were required for nine patients (7.0%; vs. 9.2% and 5.5% with Doses 3 and 4 in the main study). In the four who required treatment interruption, IRRs resolved without further medical intervention, and patients went on to complete treatment. Serious IRRs and IRR-related treatment discontinuations were not observed.
| IRRs | |
|---|---|
| Total number of events | 16 |
| Patients with ≥1 IRR, n (%) | 16 (12.4) |
| 95% CI | 7.3–19.4 |
| Patients with ≥1 local IRR, n (%) | 3 (2.3) |
| 95% CI | 0.5–6.6 |
| Patients with ≥1 systemic IRR, n (%) | 13 (10.1) |
| 95% CI | 5.5–16.6 |
| Patients with ≥1 Grade 3 or 4 IRR, n (%)1 | 0 |
| 95% CI1 | 0–2.8 |
| Patients with IRRs by Grade, n (%) | |
| Grade 1 | 10 (7.8) |
| Grade 2 | 6 (4.7) |
| Grade 3 | 0 |
| Grade 4 | 0 |
| Patients with ≥1 IRR leading to dose interruption, n (%) | 4 (3.1) |
| 95% CI | 0.9–7.7 |
| Patients with ≥1 IRR leading to slowed infusion, n (%) | 5 (3.9) |
| 95% CI | 1.3–8.8 |
| Patients with ≥1 serious IRR, n (%) | 0 |
| Patients with IRR-related treatment discontinuation | 0 |
- IRR, infusion-related reaction.
Additional safety data are presented in Table 4. Overall, findings in the CHORDS substudy were consistent with findings from the main study, and no new safety signals were observed.
| AEs | ||
|---|---|---|
| During or within 24 h of infusion | Through 30-day follow-up1 | |
| N | 129 | 129 |
| Patients with ≥1 AE, n (%) | 20 (15.5) | 39 (30.2) |
| Total number of events | 22 | 51 |
| Total number of deaths | 0 | 0 |
| Type of event by MedDRA PT, n (%) | ||
| IRR | 16 (12.4) | 16 (12.4) |
| Headache | 0 | 3 (2.3) |
| UTI | 1 (0.8) | 2 (1.6) |
| Fatigue | 1 (0.8) | 2 (1.6) |
| Muscle spasms | 1 (0.8) | 2 (1.6) |
| Dizziness | 1 (0.8) | 1 (0.8) |
| Infusion site extravasation | 1 (0.8) | 1 (0.8) |
| Patients with ≥1 SAE, n (%) | 1 (0.8) | 2 (1.6) |
| Muscle spasms | 0 | 1 (0.8) |
| Neoplasm2 | 1 (0.8) | 1 (0.8) |
- AE, adverse event; MedDRA, Medical Dictionary for Regulatory Activities; PT, preferred term; SAE, serious adverse event; UTI, urinary tract infection.
- 1 Additional events occurring in one patient (0.8%) each during the 30-day follow-up included skin lacerations, bronchitis, conjunctivitis, ear infection, gastroenteritis, hordeolum, nasopharyngitis, sinusitis, staphylococcal infection, upper respiratory tract infection, band sensation, hypoesthesia, trigeminal neuralgia, visual field defect, influenza-like illness, back pain, abdominal discomfort, dry mouth, cough, upper respiratory tract congestion, ear pain, depression and nephrolithiasis.
- 2 Patient had a brain lesion visible on the MRI conducted on the same day of (but prior to) the shorter infusion.
Discussion
Shortening the infusion time of intravenous therapies can improve convenience and treatment satisfaction, streamline medical resource use and reduce costs without compromising efficacy and safety.9-11 In the CHORDS substudy, patients with RRMS completed their fifth dose of OCR 600 mg over a mean infusion time of 2.2 h, a reduction of ≈37% compared with the initial prescribing recommendation of 3.5 h, and a reduction of ≈42% and ≈41% versus the infusion duration of Doses 3 and 4 in the main study. Reductions may be even greater compared with real-world data, with some anecdotal evidence suggesting 600-mg infusions could last as long as 5 to 6 h.12, 13 Importantly, the incidence and severity of IRRs in this substudy were consistent with observations in the pivotal studies,6 and the overall safety profile was consistent with that seen in ENSEMBLE PLUS7 and SaROD.8 Overall, findings demonstrate that the safety profile of OCR remains unchanged with a shorter infusion across different patient populations.
Acknowledgments
The authors thank all the patients, their families and the investigators who participated in this trial. Writing and editorial assistance was provided by Aleksandra Erac-Zganec of Meditech Media, Sydney, Australia, and Health Interactions, Inc. (US), and funded by Genentech, Inc., South San Francisco, CA, USA. All authors were involved in reviewing the manuscript critically for important intellectual content, had full editorial control of the manuscript, and provided final approval of the submitted version.
Conflict of Interest
R Bermel has received consulting fees from Biogen, EMD Serono, F. Hoffmann-La Roche Ltd, Genentech, Inc., Genzyme, Novartis and Viela Bio. E Waubant is a site primary investigator for ongoing trials with Genentech and Biogen. She has received research funding from the National Institutes of Health, Patient-Centered Outcomes. Research Institute, National Multiple Sclerosis Society and Race to Erase MS. She has received honoraria for lectures from Medscape, The Corpus and the American Association of Neurology, and for consulting work from Jazz Pharmaceuticals, Emerald and DBV. She is co-chief editor for Multiple Sclerosis and Related Disorders. G Pardo has received consulting fees from and/or serves on the speaker’s bureau for Alexion, Biogen, Celgene/BMS, EMD Serono, Genentech, Inc., Novartis and Sanofi Genzyme. A Bass has served on advisory boards and/or speaker bureaus and received research funding from Actelion, Biogen, EMD Serono, F. Hoffmann-La Roche Ltd, Genentech, Inc., Mallinckrodt, Novartis, Sanofi-Genzyme and TG Therapeutics. P Repovic has received consulting or speaking honoraria from Alexion, Biogen, Celgene, EMD Serono, Genentech, Inc, Genzyme, Novartis, Teva, and Viela. S Newsome has participated in scientific advisory boards for Biogen, Genentech, Celgene, Novartis, and EMD Serono, and he is an advisor for the Gerson Lehrman Group, BioIncept, and Autobahn Therapeutics, and a clinical adjudication committee member for a MedDay Pharmaceuticals clinical trial. He has received grant/research funding (paid directly to institution) from Biogen, Genentech, Department of Defense, National MS Society, and the Patient Centered Outcomes Research Institute. J Lindsey has received personal compensation for speaking or consulting for EMD Serono, Celgene and Genzyme; is participating in clinical trials funded by Genentech, Inc., Biogen, Atara, EMD Serono and AbbVie; and has received research funding from the National Multiple Sclerosis Society and Genentech. D Kile is a contractor for Genentech, Inc. A Pradhan is an employee of Genentech, Inc., and a shareholder of F. Hoffmann-La Roche Ltd. B Musch is an employee of Genentech, Inc., and a shareholder of F. Hoffmann-La Roche Ltd. A Zabeti received honoraria for speakers’ bureaus or advisory councils from Acorda, Biogen, Celgene, Genentech, Inc., Genzyme/Sanofi, Novartis and Serono.
Author Contributions
RA Bermel, E Waubant, G Pardo, A Bass, P Repovic, S Newsome, JW Lindsey, and A Zabeti contributed to investigation, writing (reviewing and editing), and final approval. D Kile contributed to formal data analysis and validation, writing (reviewing and editing), and final approval. A Pradhan contributed to conceptualization, methodology, writing (reviewing and editing), and final approval. B Musch contributed to supervision, conceptualization, writing (reviewing and editing), and final approval.
Data Sharing
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).




