Adeno-associated virus serotype 1-based gene therapy for FTD caused by GRN mutations
Abstract
Objective
Dominant loss-of-function mutations in the gene encoding the lysosomal protein, progranulin, cause 5-10% of frontotemporal dementia cases. As progranulin undergoes secretion and endocytosis, a small number of progranulin-expressing cells can potentially supply the protein to the entire central nervous system. Thus, gene therapy is a promising treatment approach.
Methods
We evaluated adeno-associated viral vector administration into the cerebrospinal fluid as a minimally invasive approach to deliver the granulin gene to the central nervous system in a murine disease model and nonhuman primates.
Results
In progranulin-deficient mice, vector delivery into the lateral cerebral ventricles increased progranulin levels in the cerebrospinal fluid and normalized histological and biochemical markers of progranulin deficiency. A single vector injection into the cisterna magna of nonhuman primates achieved CSF progranulin concentrations up to 40-fold higher than those of normal human subjects and exceeded CSF progranulin levels of successfully treated mice. Animals treated with an adeno-associated virus serotype 1 vector exhibited progranulin expression fivefold higher than those treated with an AAV5 vector or the AAV9 variant, AAVhu68, apparently due to remarkably efficient transduction of ependymal cells. Progranulin expression mediated by adeno-associated viral vectors was well tolerated in nonhuman primates with no evidence of dose-limiting toxicity, even at vector doses that induced supraphysiologic progranulin expression.
Interpretation
These findings support the development of AAV1-based gene therapy for frontotemporal dementia caused by progranulin deficiency.
Introduction
Up to 10% of all cases of frontotemporal dementia (FTD) are caused by loss-of-function mutations in the granulin (GRN) gene, which encodes the lysosomal protein progranulin (PGRN).1 GRN mutations are inherited in an autosomal dominant fashion with greater than 90% penetrance by age 70.1 GRN mutation carriers typically present with clinical phenotypes of behavioral variant FTD or primary progressive aphasia in the fifth or sixth decade of life, and universally exhibit a progressive course, with average survival of 6years from symptom onset.2 There are currently no disease-modifying therapies for FTD caused by GRN mutations (FTD-GRN).
Several lines of evidence suggest that the pathophysiology of FTD-GRN is related to lysosomal dysfunction secondary to decreased progranulin levels. While inheritance of a single GRN mutation causes FTD, patients with homozygous loss-of-function mutations present much earlier in life with neuronal ceroid lipofuscinosis (NCL, Batten disease), which is characterized by the accumulation of auto-fluorescent material (lipofuscin) in the lysosomes of neurons, rapid cognitive decline, and retinal degeneration.3 Although FTD patients with a single GRN mutation experience later symptom onset, they ultimately develop lysosomal storage lesions in the brain and retina identical to those of NCL patients prior to the first clinical manifestations of the disease.4 Recent studies show that PGRN plays a critical role in lysosomal function by promoting lysosome acidification and serving as a chaperone for lysosomal proteases, including cathepsin D (CTSD).5, 6 Mutations in the gene that encodes CTSD can also lead to an NCL phenotype, which supports a common pathophysiology related to deficient lysosomal protease activity.7
Like many lysosomal proteins, newly synthesized PGRN can either be transported directly from the trans-Golgi network to the lysosome or secreted. PGRN that is released into the extracellular fluid can be endocytosed and transported to the lysosomes of other cells.6 This uptake is mediated by direct binding of PGRN to sortilin on the cell surface, or via PGRN binding to prosaposin, a lysosomal protein that is taken up via mannose-6-phosphate receptors.8 An important consequence of the secretion and endocytosis of PGRN was demonstrated in transgenic mice that lack GRN selectively in neurons.9 In contrast to mice with global GRN deletion, ablating PGRN expression in neurons alone does not cause neuronal lipofuscin accumulation, which indicates that other cells can supply the protein to neurons.9 Overexpressing PGRN in a subset of cells may therefore be sufficient for widespread delivery of the protein to the brain, making gene therapy a potentially effective approach even with modest gene-transfer efficiency.
A prior study demonstrated that adeno-associated viral (AAV) vector delivery to the brain parenchyma of GRN-knockout mice corrected lysosomal storage lesions, particularly in the regions surrounding the injection site.10 Translating an AAV-based gene therapy approach to patients with FTD-GRN will require identification of a vector capsid and method of administration that can safely achieve widespread PGRN delivery in the brain. Using a nonhuman primate model, we evaluated a minimally invasive intra-cisterna magna (ICM) approach to deliver PGRN-expressing AAV vectors into the cerebrospinal fluid.11-13 In addition, we compared PGRN expression in NHPs treated with diverse AAV capsid serotypes. Our findings highlight the critical role of capsid serotype in achieving high levels of PGRN expression following ICM administration, and demonstrate the safety and feasibility of this approach in NHPs, paving the way for human trials.
Materials and Methods
Animal procedures
We performed animal studies at the University of Pennsylvania, an AAALAC-accredited facility. The Institutional Animal Care and Use Committee of the University of Pennsylvania approved all animal protocols. We purchased breeding pairs of GRN-knockout mice from The Jackson Laboratory (stock #0131730) and maintained a colony at the University of Pennsylvania. Wild-type C57BL/6 mice (stock #000664) served as controls. In the first study, we anesthetized two-month-old mice (n = 10 per group) with isoflurane and injected 1 × 1011 vector genome copies (GC) of AAVhu68 expressing human PGRN or vehicle in a volume of 5 µL into the lateral cerebral ventricle (ICV). Sixty days post-injection, mice received ketamine/xylazine anesthesia and were euthanized by exsanguination; we confirmed death by cervical dislocation. In the second study, GRN-knockout mice (n = 8 per group) were treated at seven months of age and sacrificed at 11 months of age. At the time of necropsy, we collected blood by cardiac puncture into serum-separator tubes; we collected CSF via suboccipital puncture using a 32-gauge needle connected to polyethylene tubing. Serum and CSF samples were frozen on dry ice and stored at −80°C until analysis. We collected the frontal cortex for biochemistry and immediately froze the tissue on dry ice; the rest of the brain was fixed in 10% formalin for histology.
We purchased 3- to 4-year-old rhesus macaques from Envigo. Six animals were included in the GFP vector study (n = 2 per vector) and six animals were included in the PGRN vector study (n = 2 per vector). For intrathecal vector administration, we sedated animals with an intramuscular injection of dexmedetomidine and ketamine and administered a single ICM injection of 3 × 1013 GC of each AAV vector in 1 mL of artificial CSF. We verified needle placement via myelography using a fluoroscope (OEC9800 C-Arm, GE), as previously described.14 We administered the analgesic meloxicam to all animals post-injection. We collected CSF prior to injection and thereafter on a weekly basis via suboccipital puncture. Automated CSF cell counts with manual differential was performed by Antech Diagnostics. Animals were euthanized by barbiturate overdose. The collected tissues were immediately frozen on dry ice or fixed in 10% formalin for histology.
Vector production
Vector production is described in supplemental methods.
Statistics
We performed comparisons of hexosaminidase enzymatic activity, lipofuscin counts, and CD68+ area in wild-type, GRN-knockout and AAV-treated GRN-knockout mice using a one-way analysis of variance followed by a post-hoc Tukey’s multiple comparisons test.
Results
Proof-of-concept studies in a murine disease model
We utilized a murine disease model to assess AAV-based gene therapy for PGRN deficiency. Mice heterozygous for GRN mutations (GRN+/−) have been reported to exhibit subtle behavioral abnormalities but not the pathological hallmarks of GRN-related neurodegenerative disease, likely because the mouse lifespan does not allow for the development of the sequelae of GRN haploinsufficiency, which manifest after several decades in humans.15-18 In contrast, complete PGRN deficiency in GRN−/− mice recapitulates early hallmarks of GRN haploinsufficiency in humans, such as impaired lysosomal function, accumulation of auto-fluorescent lysosomal storage material (lipofuscin), and activation of microglia.16, 19 However, GRN−/− mice do not exhibit neuronal loss even up to two years of age.16, 19 Despite the absence of overt neurodegeneration or neurological signs, the biochemical and histological similarities to GRN haploinsufficiency in humans make GRN−/− mice a potentially informative model to evaluate novel therapies.
To aid in the design of pharmacology studies, we first evaluated the natural history of brain pathology in the GRN−/− mouse model. Progressive accumulation of lipofuscin deposits was apparent in the cortex, hippocampus, and thalamus (Fig. S1). Lipofuscin was apparent as early as 2 months of age, consistent with previous findings.20 To evaluate lysosomal function, we measured activity of the lysosomal enzyme, hexosaminidase, which is upregulated in the setting of lysosomal storage.15, 21, 22 In contrast to the progressive accumulation of lipofuscin, brain hexosaminidase activity was similarly elevated in GRN−/− mice of all ages (Fig. S1).
We performed our initial studies with an AAV vector based on the natural isolate, AAVhu68, which is closely related to the clade F isolate AAV9. We treated GRN−/− mice at two to three months of age with an intracerebroventicular (ICV) injection of either an AAVhu68 vector expressing human GRN or vehicle (phosphate-buffered saline/PBS; N = 10 per group). In addition, we injected a cohort of wild-type mice with vehicle (N = 10). Previous studies demonstrated that ICV administration of AAV vectors at similar doses results in transduction limited to brain regions near the injected ventricle.23 Thus, this is a useful system to evaluate whether global improvements in brain lesions can be achieved through the secretion of PGRN by a small population of cells.
Two months after vector administration, we euthanized the animals and collected brain, CSF, and serum. We were able to detect human PGRN in the brains and CSF of AAV-treated GRN−/− mice (Fig. 1). Expression of PGRN was accompanied by normalization of lysosomal enzyme expression, with hexosaminidase activity returning to near-normal levels in the brains of AAV-treated GRN−/− mice (Fig. 1). Compared to vehicle-treated GRN−/− mice, AAV-treated GRN−/− mice exhibited reduced lipofuscin deposits in all examined brain regions (Fig. 1).
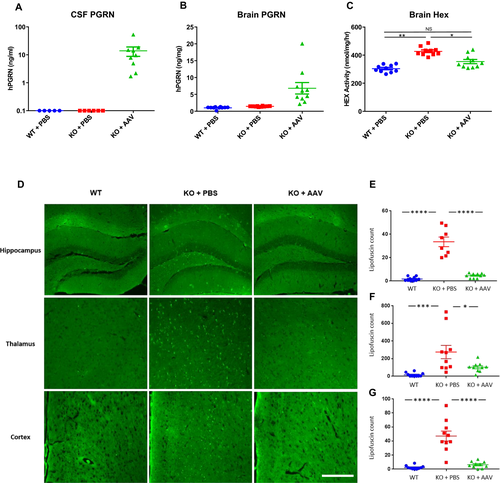
The initial proof-of-concept study demonstrated the therapeutic activity of AAV-mediated PGRN expression in mice treated at an early age, when storage material has just begun to appear in the brain. We subsequently evaluated the impact of gene transfer in older mice with more severe pre-existing pathology. We administered a single ICV injection of an AAVhu68 vector expressing human PGRN or vehicle to seven-month-old GRN−/− mice. At 11 months of age – in addition to extensive brain lipofuscin deposits (Fig. S2) – GRN−/− mice exhibited marked microgliosis, similar to patients with FTD caused by GRN mutations (Fig. 2).24 GRN gene transfer reduced brain hexosaminidase activity and lipofuscin deposits in aged mice similar to our findings in younger animals (Fig. S2). In addition, microgliosis was completely corrected in the brains of treated mice (Fig. 2).

Evaluating AAV-mediated GRN gene transfer in NHPs
Our findings in GRN−/− mice demonstrate that delivering an AAV vector into CSF can 1) achieve sufficient brain transduction to produce therapeutic levels of PGRN; and 2) prevent or reverse biochemical and histological findings associated with PGRN deficiency. In order to translate this approach to humans, we carried out studies in NHPs using a clinically relevant route of vector administration. ICM AAV delivery into the CSF is a minimally invasive approach that results in more extensive brain transduction than administration via lumbar puncture.13 In order to identify a vector capable of achieving optimal expression levels of human PGRN in CSF, we tested AAV serotypes 1, 5 and hu68, which represent distinct clades of natural AAV isolates. These vectors carried a human GRN transgene driven by a chicken beta-actin promoter with a cytomegalovirus immediate-early enhancer. We also tested an AAVhu68 vector carrying a human ubiquitin C promoter. We detected robust PGRN expression in the CSF of all NHPs following vector administration (Fig. 3). The CSF of the two animals treated with the AAVhu68 vector exhibited human PGRN levels that were up to tenfold greater than levels of healthy human controls. These PGRN levels were similar to those that reversed lysosomal abnormalities in the brains of GRN−/− mice. The CSF expression levels were roughly equivalent for both the AAV5- and the AAVhu68-treated groups. Expression of human PGRN was greatest in the animals treated with an AAV1 vector, reaching more than 40-fold normal human levels.
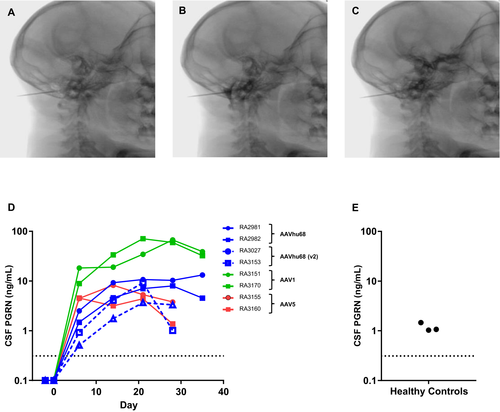
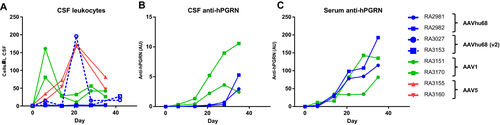
ICM AAV delivery was well tolerated in all treatment groups. We did not identify any treatment-related abnormalities on daily observations, physical exams, complete blood counts, or serum chemistry panels. We tested the CSF and plasma samples from NHPs treated with AAVhu68 and AAV1 vectors carrying the beta-actin promoter for antibodies to human PGRN. All four animals developed antibodies to the human transgene product (Fig. 4). The kinetics of the anti-human PGRN antibody response in CSF correlated with transgene expression levels, peaking earlier in the AAV1 group. Similar to other ICM AAV studies that utilize a xenogenic transgene,25 CSF analysis revealed an asymptomatic lymphocytic pleocytosis beginning 7–21 days after injection for all vector serotypes, mirroring the antibody response to the transgene product (Fig. 4). CSF cell counts declined from peak levels but remained elevated at the time of necropsy for most animals.
After 35 days following injection, we sacrificed the animals treated with the two highest expressing vectors—AAV1 and AAVhu68 carrying the beta-actin promoter—to evaluate the histopathology of the brain and spinal cord. Our findings were similar to previous ICM AAV studies.25, 26 We observed occasional, minimal lymphocytic infiltrates in the meninges and choroid plexus as well as degeneration of sensory neurons and their associated axons in some dorsal root ganglia and spinal cord sections (Fig. S3). The sensory neuron findings in dorsal root ganglia and axonal degeneration of dorsal white-matter tracts in the spinal cord were generally minimal to mild in severity for AAV1 and AAVhu68-treated animals. These findings were consistent with previous ICM AAV studies and were not associated with clinical signs.21, 25, 26 No vector-related abnormalities were noted in the brain parenchyma of any of the AAV1- or AAVhu68-treated animals.
Differing patterns of CNS transduction following ICM administration of AAV1 and AAVhu68 vectors to NHPs
The markedly higher PGRN levels in the CSF of NHPs treated with an AAV1 vector led us to explore the differences in the transduction patterns of AAV1, AAV5, and AAVhu68 in the primate CNS. We administered a single ICM injection of an AAV1, AAV5, or AAVhu68 vector (3 × 1013 GC) expressing a green fluorescent protein (GFP) reporter gene to adult NHPs (n = 2 per vector).
Twenty-eight days after injection, immunohistochemistry revealed diffuse, patchy transduction throughout the brains of NHPs treated with AAV1 or AAVhu68 vectors (Fig. 5). Minimal transduction was evident in the brains of animals that received the AAV5 vector. In order to precisely characterize the differences in transduction between AAV1 and AAVhu68, we developed a semi-automated method to quantify transduced cells in sections collected from multiple brain regions. Using sections stained with fluorescently labeled antibodies against GFP and markers of specific cell types, we quantified the total numbers of neurons, oligodendrocytes, microglia and astrocytes using NeuN, Olig2, IBA1, and GFAP staining, respectively, and then quantified GFP-expressing cells of each type (Fig. 5, Table S1). AAV1 and AAVhu68 each transduced less than 1% of each cell type in all examined regions. Transduction of neurons was nearly equivalent between the two vectors. However, AAVhu68 appeared to transduce modestly greater numbers of astrocytes and oligodendrocytes. We did not observe transduction of microglia in any sections examined.
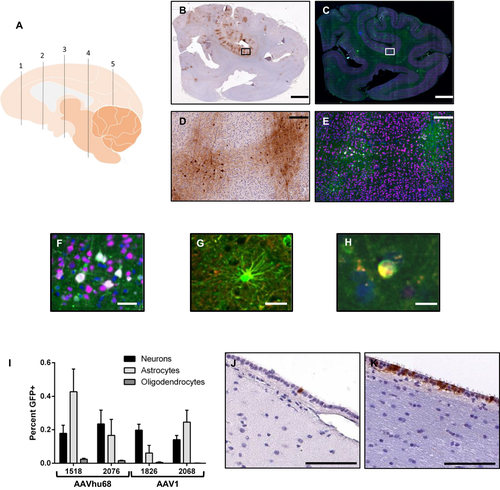
The roughly equivalent brain transduction observed with AAV1 and AAVhu68 vectors was unexpected given the dramatically higher CSF PGRN levels achieved with AAV1. Interestingly, multiple brain sections from an AAV1-treated animal (RA1826) that contained portions of the ventricular system demonstrated extensive transduction of the ependymal cells that line the ventricles, which we did not observe in either AAVhu68-treated animal (Fig. 5). An average of 48% of ependymal cells were transduced across all sampled regions, including the frontal, temporal, and occipital horn of the lateral ventricle as well as the fourth ventricle. In contrast, only 1-2% of ependymal cells were transduced in the same brain regions of the animals that were given the AAVhu68 vector. We were only able to evaluate small segments of one lateral ventricle in the second AAV1-treated animal, which showed approximately 1% ependymal cell transduction, although the analysis was limited to the small sampled region. These findings suggest that highly transduced ependymal cells in AAV1-treated animals could be the primary source of PGRN in the CSF given that the transduction of other cells types appeared to be similar across the two serotypes.
Discussion
ICM AAV delivery offers a potential minimally invasive approach to achieve durable and widespread PGRN delivery to the CNS of GRN mutation carriers.13, 27 In the absence of an authentic animal model of FTD-GRN, we evaluated the impact of AAV-mediated PGRN expression on lysosomal abnormalities in GRN−/− mice. Recent studies revealed that PGRN is a downstream target for a lysosomal master-regulator transcription factor EB.28, 29 PGRN is also a promoter of lysosome acidification and a chaperone for lysosomal proteases.5, 6 Transcriptomic and proteomic analysis of GRN−/− mice showed upregulation of lysosome-related genes,16, 20 further establishing the importance of PGRN for proper lysosomal function. These findings are consistent with the prominent lysosomal storage lesions observed in the brains of patients with NCL and FTD caused by GRN mutations.3, 4 Therefore, we used markers of lysosomal function as the primary readout for proof-of-concept studies. ICV delivery of an AAVhu68 vector expressing human PGRN reduced lysosomal lipofuscin deposits and normalized lysosomal enzymatic activity in the brains of GRN−/− mice. Microgliosis, a prominent finding in brains of FTD-GRN patients, was also reversed by vector administration in the murine disease model. These findings demonstrate that GRN gene delivery can effectively correct key aspects of the underlying pathophysiology of GRN-related neurodegenerative disease.
Recently, another group raised a potential safety concern for AAV-mediated PGRN expression, reporting that ICV administration of an AAV9 vector expressing human PGRN in mice caused T-cell infiltration and extensive hippocampal degeneration.30 Similar T-cell responses to a xenogenic transgene product following AAV delivery to the brain have been extensively described in the literature,27, 31-33 particularly when the vector is administered into the lateral ventricle or the brain parenchyma, which can provoke a local inflammatory response. In our mouse study using AAVhu68, a variant of AAV9, we did not observe any lymphocyte infiltration or gross morphological abnormalities in the hippocampus two or four months after injection. Another group that delivered AAV1-expressing human PGRN to the mouse brain reported only a minor, localized cellular infiltrate at the injection site two months after injection.10 These apparently contradictory results could stem from differences in vector production or formulation, technical aspects of injections, study duration, or differences in vector serotype. Regardless, the finding of a cytotoxic T-cell response to a foreign transgene in a knockout mouse is likely of no relevance to gene therapy for heterozygous GRN mutation carriers, which express normal PGRN protein, albeit at reduced levels, and are expected to be immunologically tolerant to the protein. In addition, we previously found that vector administration via ICM injection, rather than injection into the lateral ventricle, can dramatically reduce the risk of a T-cell response to even a highly immunogenic transgene.27
To assess the safety and feasibility of GRN gene transfer in a more clinically relevant system, we performed a study in NHPs. The size and anatomic similarity of NHPs to humans allowed us to replicate the ICM route of administration that would be employed in human trials. ICM vector injection in rhesus macaques was well tolerated. All animals eventually developed antibodies to the human PGRN protein in CSF, which was accompanied by a lymphocytic pleocytosis. As with immune responses to human PGRN in mouse models, the antibody response to the human transgene product observed in NHPs is not expected to be relevant to FTD-GRN patients, who are immunologically tolerant to the protein due to residual endogenous expression.
Detailed quantification of transduced cells in the primate brain following ICM AAV delivery revealed that while transduction is widespread, only a small fraction of neurons, astrocytes, and oligodendrocytes are transduced. Both AAV1 and AAVhu68—a vector closely related to AAV9 with similar transduction characteristics—transduced less than 1% of neurons at a moderate vector dose. Transduction efficiency of AAV5 was even lower, precluding analysis by the same methods used for AAV1 and AAVhu68-treated animals. Any assessment of transduction efficiency must be interpreted with caution due to potential biases introduced by the sensitivity of the method used to detect the transgene product or the brain regions sampled. However, our findings clearly suggest that the current approach of AAV delivery into CSF will be inadequate to address diseases of the CNS in which the transgene product must be expressed in a significant fraction of neurons or glia. This is a critical observation for other genetic forms of FTD, such as those caused by C9ORF72 or MAPT mutations, where gene therapy approaches will be far more challenging because of the cell-autonomous toxicity caused by these mutations. In contrast, the bystander effect mediated by secreted PGRN makes FTD caused by GRN mutations uniquely amenable to AAV gene therapy.
As extracellular PGRN can be taken up by neurons, the remarkably high CSF PGRN levels achieved with the AAV1 vector—apparently mediated by robust ependymal cell transduction—could make AAV1 an ideal choice for GRN gene therapy. Others have reported that AAV1 transduces ependymal cells after delivery into the lateral cerebral ventricles.34 We found that AAV1 also appears to effectively target ependymal cells after ICM delivery. However, ependymal cell transduction could only be adequately assessed in one animal, and future studies should further evaluate this finding. The ependymal cells of the brain are believed to be post-mitotic and may be an effective depot for long-term production of therapeutic proteins in the CSF.35, 36 Although the minimum therapeutic concentration of PGRN in CSF is unknown, our studies in GRN−/− mice demonstrate that expression of PGRN in CSF at tenfold normal human levels is sufficient to normalize histological and biochemical markers of GRN deficiency throughout the brain. NHPs treated with an AAV1 vector expressed up to 40-fold normal human PGRN levels in CSF, indicating that therapeutic expression levels of PGRN may be achievable with ICM AAV1 delivery.
Acknowledgments
The authors thank Tamara Goode, Erin Bote, Amber Hamilton, Kristin Gardiner, Brenden Nosratbakhsh, and Victoria Kehm for technical assistance. The authors thank the support of the Program for Comparative Medicine and the Pathology Core of the Gene Therapy Program at the Perelman School of Medicine. This research was funded by a sponsored research agreement with Passage Bio.
Author Contributions
C.H., R.M., C.D., J.J., P.B., and E.B. designed and performed experiments. J.M.W. designed experiments. C.H., R.M., and J.M.W. wrote the manuscript.
Conflict of Interest
J.M. Wilson is a paid advisor to and holds equity in Scout Bio and Passage Bio; he holds equity in Surmount Bio; he also has a sponsored research agreement with Ultragenyx, Biogen, Janssen, Precision Biosciences, Moderna Inc., Scout Bio, Passage Bio, Amicus Therapeutics, and Surmount Bio which are licensees of Penn technology. JMW is an inventor on patents that have been licensed to various biopharmaceutical companies and for which he may receive payments. C. Hinderer is an inventor on patents licensed to biopharmaceutical companies and holds equity in Scout Bio.




