Software-based analysis of 1-hour Holter ECG to select for prolonged ECG monitoring after stroke
Funding Information:
No funding information provided.
Abstract
Objective
Identification of ischemic stroke patients at high risk for paroxysmal atrial fibrillation (pAF) during 72 hours Holter ECG might be useful to individualize the allocation of prolonged ECG monitoring times, currently not routinely applied in clinical practice.
Methods
In a prospective multicenter study, the first analysable hour of raw ECG data from prolonged 72 hours Holter ECG monitoring in 1031 patients with acute ischemic stroke/TIA presenting in sinus rhythm was classified by an automated software (AA) into “no risk of AF” or “risk of AF” and compared to clinical variables to predict AF during 72 hours Holter-ECG.
Results
pAF was diagnosed in 54 patients (5.2%; mean age: 78 years; female 56%) and was more frequently detected after 72 hours in patients classified by AA as “risk of AF” (n = 21, 17.8%) compared to “no risk of AF” (n = 33, 3.6%). AA-based risk stratification as “risk of AF” remained in the prediction model for pAF detection during 72 hours Holter ECG (OR3.814, 95% CI 2.024-7.816, P < 0.001), in addition to age (OR1.052, 95% CI 1.021-1.084, P = 0.001), NIHSS (OR 1.087, 95% CI 1.023-1.154, P = 0.007) and prior treatment with thrombolysis (OR2.639, 95% CI 1.313-5.306, P = 0.006). Similarly, risk stratification by AA significantly increased the area under the receiver operating characteristic curve (AUC) for prediction of pAF detection compared to a purely clinical risk score (AS5F alone: AUC 0.751; 95% CI 0.724-0.778; AUC for the combination: 0.789, 95% CI 0.763-0.814; difference between the AUC P = 0.022).
Interpretation
Automated software-based ECG risk stratification selects patients with high risk of AF during 72 hours Holter ECG and adds predictive value to common clinical risk factors for AF prediction.
Introduction
Atrial fibrillation (AF) as a common cause of ischemic stroke is associated with increased mortality and disability.1 The incidence of AF rises with increasing age; thus, the burden of both AF and the incidence of AF-related stroke is expected to steeply increase in the next decades due to the anticipated ageing of the population.2, 3 Another factor contributing to the increased incidence of AF and AF-related stroke is the enhanced implementation of extended intervals of electrocardiogram (ECG) monitoring in clinical practice, as has been demonstrated by several studies.4 Nonetheless, the percentage of patients receiving prolonged ECG monitoring after ischemic stroke is still low5, 6 and its application needs further implementation in routine clinical practise in order to take advantage of the highly effective secondary preventive effect of oral anticoagulative medication in case of paroxysmal AF (pAF) detection.7 However, efforts to increase the detection rates of pAF after ischemic stroke are equally increasing the workload for ECG analysis and interpretation.8 In order to overcome these obstacles, selection criteria for patients undergoing prolonged monitoring times are needed, especially in the case of limited resources, working towards a more personalized medicine. Therefore, we recently proposed a scoring system (the Age and Stroke NIHSS>5 to Find AF (AS5F) score),9 based solely on clinical variables available at patients’ admission, calculating the risk for pAF detection during 72 hours of Holter ECG monitoring, which might contribute to motivate patients and physicians to tolerate and initiate prolonged Holter ECG monitoring.
Currently, atrial cardiopathy is discussed as a precursor condition of AF, which is characterized by endothelial dysfunction, fibrosis, impaired myocyte function and dilatation of the left atrium, and might also cause a thromboembolic state in the left atrium in the absence of AF.10 The morphological changes within the left atrium are accompanied by electrical changes,11, 12 premature beats13 and supraventricular runs,14, 15 the latter shown to be associated with increased risk of cerebral re-ischemia,14 supporting the clinical relevance of an intensified analysis of ECG data. In addition to clinical variables, automated software-based analysis might be capable of identifying the aforementioned ECG variations and could further individualize the allocation of prolonged ECG monitorings.
Within the current analysis, we hypothesized that patient stratification by an automated software method (AA) based on one hour of Holter ECG raw data increases the diagnostic yield of pAF during 72 hours of rhythm monitoring in stroke patients compared to assessment of clinical variables alone.
Methods
Study population
Data are available upon reasonable request from the corresponding author. The current analysis included individual patients from the prospective, multicenter observational IDEAS study,16 examining 72-hour Holter ECG monitoring in patients presenting with ischemic stroke or transient ischemic attack (TIA) with regard to detection of pAF. The detailed screening and exclusion numbers of patients are depicted in Figure 1 following the Standards for Reporting Diagnostic accuracy studies (STARD) guidelines.17 The following characteristics led to patient exclusion: age under 18 years, known AF or documented AF on admission ECG (12-lead ECG), life expectancy below one year or a pacemaker with an atrial lead. The full study protocol and the primary study results are accessible as original publication.16
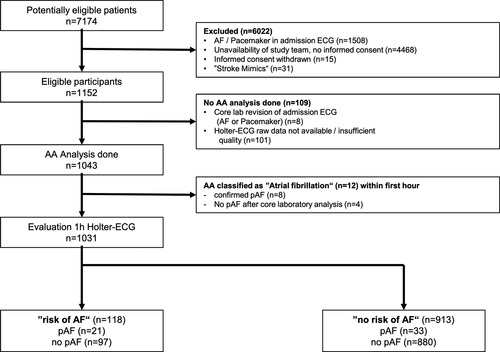
Here, we report the diagnostic accuracy of the risk stratification performed by an automated software algorithm for ECG analysis (SRA clinic®, Apoplex Medical Technologies, Pirmasens, Germany) with regard to detection of pAF during the subsequent 72 hours of Holter ECG and the ability to identify patients at high “risk for pAF”. The STARDS checklist was followed17; for details see online only supplementary material Table S1.
Standard protocol approval, patient consent
The study protocol was approved by the Ethics Committee of the Ärztekammer Westfalen-Lippe (AZ2009-363-fS) and by all local ethics boards. Either patients or their legal representatives gave written informed consent to the study. The study was conducted in accordance with the Declaration of Helsinki.
Data collection
Patient characteristics were obtained by a standardized questionnaire, including a detailed medical history and stroke severity as measured by the modified Rankin Scale (mRS) and the National Institute of Health Stroke Scale (NIHSS). All rating physicians underwent video training for assessment of clinical stroke scales (mRS, NIHSS).
ECG analysis
Automated analysis software (AA) used the first hour of Holter ECG monitoring for stratification into the groups “no risk of AF” and “risk of AF.” In short, the AA creates a report of whether pAF is detectable, mainly based on the regularity of preceding R-R intervals.8, 18 Patients classified as “AF” within the first hour of ECG recording (n = 12) were excluded from further analysis. We evaluated patients classified as “no risk of AF” in comparison to patients with “risk of AF” with regard to detection of AF within the following 72-hour Holter ECG monitoring, which was started within 12 hours after hospital admission. For recording, a commercially available 3-lead Holter monitoring device (Lifecard CF, Spacelabs, Healthcare, Issaquah, WA) was applied.
The ECG analysis with regard to detection of AF in 72-hour Holter ECG was performed as recently described by our group.19
Definition of atrial fibrillation
We followed the AF definition of current guidelines,20 that is, an absolute arrhythmia, without detectable p-waves, lasting at least 30 seconds, absolute irregular R-R intervals and no repetitive ECG pattern.
Statistical analysis
Data are presented as median (± interquartile range (IQR)) or numbers with percentages, if not indicated otherwise. For univariate analysis Student’s t test, Mann-Whitney U test, Chi-square test, or Fisher’s exact test were used where appropriate. Multiple binominal regression analyses were conducted including variables with P < 0.05. Receiver operating characteristic (ROC) curves were drawn and calculated for estimation of prognostic information on detection of pAF in 72-hour Holter ECG. Area under the ROC curve (AUC) analyses were compared using the MedCalc for Windows software (version 18.11.3, MedCalc software, Mariakerke, Belgium). A significant difference was considered for P < 0.05. Patients with missing ECG raw data were excluded from the current analysis, for details see Figure 1. Statistical analysis was performed using SPSS (version 23, IBM, Armonk, NY, USA).
Results
Between May 2010 and January 2011, 7174 patients were evaluated for eligibility. The diagnostic accuracy of risk stratification by AA, based on the first hour of Holter ECG recording, regarding detection of pAF in the following 72-hour Holter ECG monitoring, was assessed in 1043 patients. AA classified 12 patients as experiencing AF within the first analysable hour of ECG recording; after their exclusion 1031 patients remained for the final analysis. Figure 1 depicts the flow of patients through the study. The mean age of participants was 66.9 years (standard deviation ± 13.1), stroke severity according to NIHSS at hospital admission was 2 (IQR 0-4), and pAF was detected in 54 of 1031 (5.2%) patients.
Patients with a final diagnosis of pAF during 72 hours of Holter ECG monitoring were more frequently classified by AA analysis (performed within the first analysable hour of ECG monitoring) as “risk of AF” compared to patients without final diagnosis of pAF (pAF 21 (38.9%) vs. no AF 97 (9.9%); P < 0.001). Moreover patients with diagnosis of pAF were significantly older (pAF 78.0 years, IQR 72-81 vs. no AF 69 years, IQR 58-75, P < 0.001) and presented with more severe stroke symptoms at admission compared to patients without AF detection (NIHSS: pAF 4, IQR 1-9 vs. no AF 2 IQR 0-4, P = 0.002; mRS: pAF 2, IQR 1-4 vs. no AF 2, IQR 1-3, P = 0.045). Concomitant disease such as arterial hypertension, diabetes mellitus, coronary artery disease, congestive heart failure and dyslipidaemia were evenly distributed between the patients with and without detection of pAF. Nonetheless, patients with pAF detection more frequently received treatment with intravenous thrombolysis (pAF 17 (31.5%) vs. no AF 95 (9.9%), P < 0.001) and less frequently presented with transient symptoms (pAF 7 (13.0%) vs. no AF 243 (24.9%), P = 0.047). Transesophageal echocardiography was available in a subset of patients (n = 190); patients with pAF-detection showed increased left atrial diameter (LAD) compared to patients without pAF-detection (pAF 45.5 mm, IQR 42.8-47 vs. no AF 43 mm, IQR 41-45, P = 0.006). The AS5F score, which incorporates classical risk factors for AF diagnosis such as stroke severity and age, was higher in patients with AF diagnosis compared to patients without (pAF 69, IQR 62.8-78.8 vs. no AF 59.9, IQR 50.9-66.8, P < 0.001). For details, see Table 1.
| Variable | pAF | noAF | P value |
|---|---|---|---|
| N | 54 | 977 | |
| “risk of AF” 1h Holter ECG | 21(38.9%) | 97(9.9%) | <0.001 |
| Female | 30 (55.6%) | 431 (44.1%) | 0.100 |
| Age | 78 (72-81) | 69 (58-75) | <0.001 |
| BMI | 27.9 (24.5-31.6) | 26.5 (24.5-29.4) | 0.315 |
| History of stroke | 11 (20.4) | 167 (17.1) | 0.535 |
| History of TIA | 5 (9.3) | 81(8.3) | 0.802 |
| Arterial hypertension | 40 (74.1) | 598 (61.3) | 0.059 |
| Diabetes mellitus | 15 (28.8) | 187 (19.7) | 0.108 |
| Coronary artery disease | 10 (18.5) | 163 (16.7) | 0.725 |
| Congestive heart failure | 5 (9.3) | 56 (5.7) | 0.285 |
| Hyperlipidaemia | 21 (38.9) | 345(35.8) | 0.648 |
| Current/former tobacco use | 21 (38.9) | 491(51.0) | 0.082 |
| Stenosis of the internal carotid artery | 0.424 | ||
| None | 817/945 (86.5) | 46/53 (86.8) | |
| Symptomatic | 86/945 (9.1) | 3/53 (5.7) | |
| Asymptomatic | 42/945 (4.4) | 4/53 (7.5) | |
| ASPECT grouped (n = 1023) | 0.248 | ||
| ≤8 | 131/969 (13.5) | 11/54 (20.4) | |
| 9 | 238/969 (24.6) | 15/54 (27.8) | |
| 10 | 600/969 (61.9) | 28/54 (51.9) | |
| ASPECT total (n = 1023) | 9.30 ± 1.33 | 9.22 ± 0.98 | 0.655 |
| AS5F Score | 69.0 (62.8-78.8) | 59.9 (50.9-66.8) | <0.001 |
| NIHSS at admission | 4 (1-9) | 2 (0-4) | 0.002 |
| mRS at admission | 2 (1-4) | 2 (1-3) | 0.045 |
| LAD [mm] | 45.5 (42.8-47) | 43 (41-45) | 0.006 |
| i.v. thrombolysis | 17 (31.5) | 95 (9.9) | <0.001 |
| Qualifying event | |||
| Stroke (vs. TIA) | 47 (87.0) | 734 (75.1) | 0.047 |
- Data are presented as number (%) except for age, NIHSS, mRS, and LAD, which are presented as median (range). pAF, paroxysmal atrial fibrillation; TIA, transitory ischemic attack; AS5F, Age and Stroke NIHSS>5 to Find AF; NIHSS, National Institute of Health Stroke Scale; mRS, modified Rankin Scale; LAD, Left atrial diameter.
Multivariable regression analysis including variables which were imbalanced on the univariate level (P < 0.05) revealed the following independent risk factors for pAF detection during 72 hours of Holter ECG monitoring: “risk of AF” by AA (odds ratio (OR) 3.814, 95% confidence interval (CI) 2.024-7.186, P < 0.001), age (OR 1.052, 95% CI 1.021-1.084, P = 0.001), NIHSS (OR 1.087, 95% CI 1.023-1.154, P = 0.007), and treatment with intravenous thrombolysis (OR 2.639, 95% CI 1.313-5.306, P = 0.006). LAD was not considered for multivariable analysis, due to limited availability (n = 190 patients). Moreover we did not consider mRS for multivariable analysis, as NIHSS was already included as a variable rating clinical stroke severity, or the AS5F score, due to the overlap with age, stroke severity, and stroke classification into persisting compared to transient symptoms. For details, see Table 2.
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| “risk of AF” | 3.814 | 2.024-7.186 | <0.001 |
| Age | 1.052 | 1.021-1.084 | 0.001 |
| NIHSS | 1.087 | 1.023-1.154 | 0.007 |
| IV thrombolysis | 2.639 | 1.313-5.306 | 0.006 |
- Corrected for “risk of AF”, Age, NIHSS, Stroke compared to TIA, IV thrombolysis.
- Abbreviations: OD, odds ratio; CI, confidence interval; AF, atrial fibrillation; NIHSS, National Institute of Health Stroke Scale; IV thrombolysis, intravenous thrombolysis; TIA, transient ischemic attack.
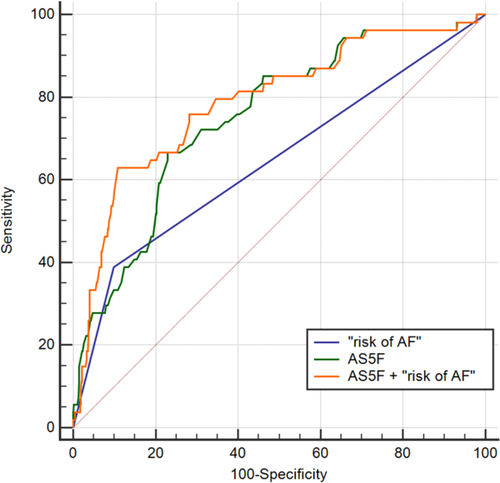
In comparison to the AS5F score as an established clinical composite risk score for pAF detection during 72 hours of Holter ECG monitoring, AA exhibited additional predictive value: the AUC was 0.751 (95% CI 0.724-0.778, P < 0.001) for AS5F and 0.645 (95% CI 0.615-0.674, P < 0.001) for AA risk stratification (Figure 2). A combination of AS5F and AA significantly improved the AUC to 0.789 (95% CI 0.763-0.814, P < 0.001, p for difference between the AUC 0.022). Moreover risk stratification by AA reduced the number needed to screen (NNS), that is, the number of patients receiving 72-hour Holter ECG monitoring necessary to detect one patient with pAF, from 9 (high-risk group classified by AS5F score) to 7; the NNS can be further reduced to 5 by combining the clinical score and AA-based risk stratification.
Detailed patient characteristics stratified by “risk/no risk of AF” are presented in Table 3. Patients classified as “risk of AF” were more frequently diagnosed with pAF compared to patients classified as “no risk of AF” (21 (17.8%) vs. 33 (3.6%), P < 0.001). Moreover, patients classified as “risk of AF” were older (76 years (71-82) vs. 68 years (58-78), P < 0.001) and had a higher probability of previous history of stroke (28 (23.7%) vs. 150 (16.4%), P = 0.048). Concerning concomitant diseases, patients classified as “risk of AF” by AA more frequently suffered from coronary artery diseases (32 (27.1%) vs. 141 (15.4%), P = 0.001) and congestive heart failure (15 (12.7%) vs 56 (5.0%), P = 0.001) compared to patients classified as “no risk of AF”. On the contrary, patients with “risk of AF” as opposed to “no risk of AF” as classified by AA were less frequently current or former tobacco users (44 (37.9%) vs. 468 (52%), P = 0.004). The AS5F score as a composite risk score for pAF detection during 72 hours of Holter ECG was higher in patients classified as “risk of AF” compared to patients classified as “no risk of AF” (66.8 IQR 61.6-72.3 vs. 59.2 IQR 50.8-66.0, P < 0.001). In addition, patients with “risk of AF” presented with more severe and disabling stroke symptoms compared to patients classified as “no risk of AF” by AA (NIHSS: 3, IQR 1-5 vs. 2, IQR 0-4, P = 0.021; mRS: 2, IQR 1-3 vs. 2, IQR 1-3, P = 0.026). For details, see Table 3. Multiple logistic regression analysis with variables which were unbalanced (P < 0.05) on the univariate level revealed the following patient characteristics to be associated with the patient group “risk of AF” as classified by AA within the first analysable hour of ECG recording: age (OR 1.073, 95% CI 1.048-1.097, P < 0.001) and pAF detection during 72-hour Holter ECG (OR 3.739, 95% CI 1.989-7.026, P < 0.001). Again, due to an overlap with previously included variables, the AS5F score and mRS were not considered for additional multivariable analysis. For details see Table 4.
| Variable | ‘risk of AF” | ‘no risk of AF’ | P value |
|---|---|---|---|
| N | 118 | 913 | |
| pAF | 21 (17.8) | 33 (3.6) | <0.001 |
| female | 50 (42.4) | 411 (45.0) | 0.587 |
| Age | 76 (71-82) | 68 (58-75) | <0.001 |
| BMI | 26.4 (24.3-28.5) | 26.6 (24.5-29.7) | 0.237 |
| History of stroke | 28 (23.7) | 150 (16.4) | 0.048 |
| History of TIA | 12 (10.2) | 74 (8.1) | 0.445 |
| Arterial hypertension | 78 (66.1) | 560 (61.4) | 0.323 |
| Diabetes mellitus | 27 (23.1) | 181 (19.9) | 0.416 |
| Coronary artery disease | 32 (27.1) | 141 (15.4) | 0.001 |
| Congestive heart failure | 15 (12.7) | 46 (5.0) | 0.001 |
| Hyperlipidaemia | 45 (38.8) | 321 (35.6) | 0.504 |
| Current/former tobacco use | 44 (37.9) | 468 (52.0) | 0.004 |
| Stenosis of the internal carotid artery | 0.553 | ||
| None | 762/905 (86.4) | 101/118 (87.1) | |
| Symptomatic | 81/905 (9.2) | 8/53 (6.9) | |
| Asymptomatic | 39/905 (4.4) | 7/53 (6.0) | |
| ASPECT grouped (n = 1023) | 0.655 | ||
| ≤8 | 129/905 (14.3) | 13/118 (11.0) | |
| 9 | 227/905 (25.1) | 26/118 (22.0) | |
| 10 | 549/905 (60.7) | 79/118 (66.9) | |
| ASPECT total (n = 1023) | 9.29 ± 1.31 | 9.36 ± 1.34 | 0.573 |
| AS5F-Score | 66.8 (61.6-72.3) | 59.2 (50.8-66) | <0.001 |
| NIH-SS at admission | 3 (1-5) | 2 (0-4) | 0.021 |
| mRS at admission | 2 (1-3) | 2 (1-3) | 0.026 |
| LAD [mm] | 44 (43-46) | 43 (41-45) | 0.052 |
| i.v. thrombolysis | 17 (14.4) | 95 (10.6) | 0.210 |
| Qualifying event | |||
| Stroke (vs. TIA) | 92 (78.0) | 689 (75.5) | 0.551 |
- Data are presented as number (%) except for age: median (range), NIH-SS: median (range), mRS: median (range), LAD: median (range). pAF, paroxysmal atrial fibrillation; TIA, transitory ischemic attack; AS5F, Age and Stroke NIHSS>5 to Find AF; NIH-SS, National Institute of Health Stroke Scale; mRS, modified Rankin Scale; LAD, Left atrial diameter.
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| pAF | 3.739 | 1.989-7.026 | <0.001 |
| Age | 1.073 | 1.048-1.097 | <0.001 |
- Corrected for pAF, age, history of stroke, coronary artery disease, congestive heart failure, smoking status and NIH-SS.
- Abbreviations: AA, automated analysis; pAF, paroxysmal atrial fibrillation.
The test characteristics of the AA-based risk stratification into “risk of AF” and a combination of a clinical score (AS5F) with the AA-based risk stratification are demonstrated in Table 5. The AA-based risk stratification into “risk of AF” exhibits a higher specificity (90.1%, 95% CI 88.0-91.9%) than sensitivity (38.9%, 95% CI 25.9-53.1%). This is in line with a higher negative predictive value (NPV 96.4%, 95% CI 95.6-97.1%) than positive predictive value (PPV 17.8%, 95% CI 12.9-24.1%) of the AA-based classification into “risk of AF” with regard to pAF detection. The negative predictive value and the sensitivity for pAF detection during 72-hour Holter ECG could be further increased by using a combination of the AA-based classification into “risk of AF” and the AS5F score (sensitivity 63.0%, 95% CI 48.7-75.7%; NPV 97.8%, 95% CI 96.9-98.4%), whereas the specificity remained almost unchanged for the combination (89.1%, 95% CI 86.9-90.9%).
| Risk of AF | 95% CI | Risk of AF + AS5F | 95% CI | |
|---|---|---|---|---|
| Sensitivity | 38.9% | 25.9-53.1% | 63.0% | 48.7-75.7% |
| Specificity | 90.1% | 88.0-91.9% | 89.1% | 86.9-90.9% |
| PPV | 17.8% | 12.9-24.1% | 24.1% | 19.5-29.4% |
| NPV | 96.4% | 95.6-97.1% | 97.8% | 96.9-98.4% |
| AUC | 0.645 | 0.615-0.674 | 0.789 | 0.763-0.814 |
| Accuracy | 87.4% | 85.2-89.4% | 87.7% | 85.5-89.6% |
- Abbreviations: AA, automated analysis; 95% CI, 95% confidence interval; AUC, area under the receiver operating characteristic curve; NPV, negative predictive value; PPV, positive predictive value.
Discussion
Automated software-based risk stratification of the first analysable hour of Holter ECG monitoring was able to select patients with a high probability of pAF detection during the 72 hours of Holter ECG monitoring. The addition of ECG-based automated risk stratification showed additive value to clinical variables and thereby even improved the patient selection beyond common clinical risk factors such as age and stroke severity. The automated risk stratification was able to reduce the number needed to screen to detect one patient with pAF by 72 hours of Holter ECG from 9 to 7 patients. In clinical routine practice, this might be used to identify “high risk” patients for pAF detection to personalize prolonged ECG monitoring if there are limited resources.
Moreover the high NPV for pAF detection (96.4%) of AA-based classification into “risk of AF” suggests that this approach excludes patients with a low probability of pAF-diagnosis within the next 71 hours of Holter ECG monitoring. Nonetheless, the AA-based classification is not without restrictions and randomized trials showed an increase in pAF detection rates with prolonged monitoring times4; thus, the ultimate goal should be the application of guideline-recommended ECG monitoring for at least 72 hours as part of the stroke-unit work-up in all patients after ischemic stroke.
Due to the high secondary preventive effect of cerebral re-ischemia by means of oral anticoagulation in patients with pAF, recent studies aimed to increase the diagnostic yield of AF after ischemic stroke. Higher detection rates of pAF were achieved in observational studies16, 21-23 and randomized trials4, 24 by merely prolonging monitoring times. However, only a minority of patients are actually receiving prolonged ECG monitoring in clinical practice.5, 6, 25 The reasons for underutilization of prolonged ECG monitoring are manifold. The cohesive time- and resource-consuming analysis of increasing ECG data might detract from implementation of prolonged ECG monitoring into clinical practice. With this in mind, the ECG-based automated risk stratification offers a more resource-effective approach to patient selection and thereby potentially overcomes the staff- or cost-barrier to implement prolonged monitoring times into clinical practice. Moreover, it is currently under debate whether stroke patients should undergo prolonged monitoring irrespective of the assumed stroke aetiology or only in case of certain strokes, especially those considered cryptogenic/ESUS (embolic stroke of unknown source). Therefore, risk stratification systems including clinical features,9 biomarkers26 and ECG parameters14 are needed to identify patients with a high probability of AF detection within prolonged monitoring periods. In this regard, the concept of atrial cardiopathy,27 as a precursor of pAF, might be able to select patients at high risk of developing AF. Biomarkers of atrial stretch,26 atrial enlargement28 and electrical changes within cardiomyocytes, detectable on surface-29 and Holter ECG recordings,14 are associated with atrial myopathy. Indeed, risk stratification as “risk of AF” by the AA was associated with coronary artery disease and congestive heart failure, both common clinical risk factors of atrial cardiopathy.27, 28 This suggests that the AA-based risk stratification used here could potentially aid in detecting electrical changes within the left atrium associated with atrial cardiopathy.
The question remains whether pAF detected during prolonged ECG monitoring is clinically relevant. Does it simply increase the proportion of patients diagnosed with pAF? Or does the resulting oral anticoagulation in these patients reduce recurrent stroke? A recent meta-analysis demonstrated a reduction of recurrent cerebral ischemic events by ECG monitoring after stroke,30 arguing to anticoagulate stroke patients with pAF detected by prolonged monitoring. Nonetheless, a randomized trial demonstrating a reduction in ischemic stroke rates by means of prolonged ECG monitoring as primary outcome is needed to establish the necessity of prolonged monitoring as effective stroke prevention in clinical practice. The AA utilized in our study could be used for both evaluation of ECG telemetry recorded during Stroke Unit monitoring23, 31, 32 and Holter ECG data.8 Recently, the same AA was also used in patients with cryptogenic stroke to select those who may benefit from prolonged cardiac monitoring afforded by an implantable device.32 In a comparable approach, a recent study demonstrated that application of an artificial intelligence algorithm on 12-lead ECG in sinus rhythm was able to select patients at high risk for developing AF and might thereby not only decrease the diagnostic workload of ECG analysis but also increase the diagnostic yield of AF.33
Moreover our data support a more intensified screening for pAF in patients with increased age, previous administration of intravenous thrombolysis or more severe stroke symptoms at admission, as measured by the NIHSS. These observations are in line with the literature14, 21, 34, 35 and are underlined by the AS5F score, which calculates the risk of pAF detection during 72 hours of Holter ECG monitoring based solely on clinical parameters readily available at the patient’s admission such as age, transient in contrast to persisting symptoms and clinical deficits.9 Our study suggests that the integration of clinical risk factors into ECG-based risk stratification could further increase its diagnostic accuracy. Nonetheless, further studies are needed to confirm our observation. Moreover all ECG recordings underwent core-laboratory analysis; however, there is the possibility that AF might have been overlooked, which represents a potential bias in our results.
Although this study benefits from the large sample size and data collection within a prospective multicenter study, it should be noted that the software analysis in our study was performed on Holter ECG data; the value of this type of automated analysis within telemetry data may lead to different results due to reduced ECG quality. Prior to broad applicability of the algorithm in clinical practice, further studies need to address its utility in analysing Stroke Unit telemetry ECG recordings.
Conclusions
Our study suggests that ECG-based risk stratification by AA might be a tool - in addition to established clinical scores (e.g., AS5F) – to identify patients at high risk for pAF and thereby contribute to a more personalized approach to medicine aiming for prolonged monitoring times preferably within patients at higher risk in order to overcome obstacles of limited resources.
Acknowledgments
This work was supported by the Else Kröner-Fresenius Stiftung (2018_EKMS.21) to T.U. The automated analysis software (SRAclinic®) was kindly provided by Apoplex Medical Technologies GmbH (Pirmasens, Germany). The authors thank Dr. L. Rosin for his efforts within the IDEAS trial. The authors also thank Dr. Cheryl Ernest for proofreading and editing the manuscript.
Conflict of Interest
TU reports personal fees from Merck Serono and Pfizer, grants from Else Kröner-Fresenius Stiftung. RW reports having been an investigator or consultant for, or received fees from Bayer, Berlin Chemie, Bristol-Myers- Squibb, Boehringer Ingelheim, CVRx, Daiichi Sankyo, Gilead, Johnson & Johnson, Medtronic, Novartis, Pfizer, Sanofi, Servier since 2003. He received research grants from Boehringer Ingelheim, European Union and Bundesministerium für Bildung und Forschung (BMBF). KG reports personal fees and/or non-financial support from Bayer, Boehringer Ingelheim, Bristol-Meyers Squibb, Daiichi Sankyo and Pfizer. SG, BL, KW and MH report no disclosures.




