Biallelic mutations in ABCB1 display recurrent reversible encephalopathy
Funding Information:
This study was supported by grants from the National Research Foundation of Korea (2014M3C9A2064686, 2018M3C9A5064708 and 2018R1A6A3A01011946) and from the Korea Centers for Disease Control and Prevention (2018ER690101).
[Corrections added on 21 August 2020, after first online publication: an additional grant number ‘2018R1A6A3A01011946’ from the National Research Foundation of Korea has been inserted in Funding information field and in Acknowledgments section.]
Abstract
The clinical phenotype linked with mutations in ABCB1, encoding P-glycoprotein, has never been reported. Here, we describe twin sisters with biallelic mutations in ABCB1 who showed recurrent reversible encephalopathy accompanied by acute febrile or afebrile illness. Whole-exome sequencing was performed on one of the twin and her healthy parents, and revealed compound heterozygous loss-of-function variants in ABCB1. The patient brains displayed substantial loss of xenobiotic clearance ability, as demonstrated by [11C]verapamil positron emission tomography (PET) study, linking this phenotype with ABCB1 function. The endogenous cytokine clearance from the brain was also decreased in LPS-treated ABCB1 knockout mice compared to controls. The results provide insights into the physiological requirement of ABCB1 in maintaining homeostasis of various compounds for normal brain function.
Introduction
The ATP-binding cassette, subfamily B, member 1 (ABCB1) gene encodes a transmembrane transporter P-glycoprotein (or ABCB1) which pumps out a wide range of xenobiotic compounds from cells, often conferring multidrug resistance to anticancer and antiepileptic drugs.1-3 Many studies have evaluated the association of the ABCB1 variants and disease susceptibility, and its enhanced expression is one of the major causes of multidrug resistance.4-7 Although ABCB1 is known to be expressed in plasma membranes of various cells and organs, expression in the blood–brain barrier (BBB) endothelium and its related function restricting the permeability of drugs into brain has been extensively studied.8 Knocking out the gene generated viable and fertile mice with increased sensitivity to drugs such as ivermectin and vinblastine.9 However, no human phenotype resulting from a disease-causing variant in ABCB1 has been reported. Here, we identified biallelic loss of function (LoF) variants of ABCB1 in monozygotic twins presenting with recurrent reversible encephalopathy. We demonstrate that the variants substantially inhibited the ability to export drugs out of the brains of the patients, causing aberrant homeostasis in brain physiology. We further observe that LPS-treated Mdr1a/b (mouse ortholog of ABCB1) knockout mouse brains possess persisted cytokine elevation, possibly causing unexpected physiological burden.
Methods
The study was approved by the Seoul National University Hospital Internal Review Board (1502-085-649). Written consents were provided by the family involved in the study. Trio-based whole-exome sequencing (WES) analysis was performed for one of the twins and her healthy parents (ME1-1, ME1-3, and ME1-4) as described previously.10
To assess functional consequences of variants, deep sequencing of amplified RT-PCR fragments was used to quantify transcript variants. Barcoded PCR primers were used to distinguish amplified fragments from different samples.
To evaluate in vivo effect of xenobiotic transportation in the brains, positron emission tomography (PET)/computed tomography (CT) images were obtained using a dedicated PET/CT scanner after intravenous injection of [11C]verapamil (7.4 MBq/kg for children and 555 MBq for adults). The protocol of the PET study including the dose of [11C]verapamil was determined based on the previous studies.11, 12 The estimated dose of verapamil that was administered was approximately 5 ng, which would be 1/1,000,000 of the usual therapeutic dose.
To explore potential molecular triggers of their encephalopathy, 6- to 7-week-old male Mdr1a/b+/+ and Mdr1a/b-/- mice were injected with PBS or 5 mg/kg of LPS intraperitoneally and brain tissues were harvested for total RNA extraction and RT-PCR. The animal experiment was approved by Seoul National University (SNU-160617-5-6).
Detailed methods are described in the supplementary materials.
Results
The twin sisters were born after a spontaneous, uncomplicated twin pregnancy from the nonconsanguineous Korean parents (Fig. 1A). There were no reported diseases or medical problems in the older sister and both parents. The twin sisters underwent normal developmental milestones. They experienced several episodes of febrile or afebrile acute illness until 5 years of age. They had recovered from all events without any complication including seizure and hypersensitivity to drugs. Acute encephalopathy event occurred first in the second twin at the age of 5 years and 6 months. Then, similar events recurred multiple times in both sisters, sometimes on the same day (Fig. 1B). Every clinical episode began during acute illness, including fever, vomiting, and abdominal pain (Table S1). They initially showed irritability or seemed agitated. In some events, they complained visual hallucination. Then, consciousness gradually decreased into a drowsy or obtunded status with generalized spasticity and hyperreflexia. The durations of these encephalopathy episodes were variable from hours to one day. However, with hydration and symptomatic medications including antibiotics, anti-emetics, and antihistamines, they completely recovered from all episodes without any sequelae. Serum amino acid analysis, serum acylcarnitine profile, urine organic acid analysis, venous blood gas analysis, serum lactate, and serum creatinine kinases were tested at the age of 6 years and 8 months, when the sisters showed simultaneous episodes, and all were within the normal ranges. Cerebrospinal fluid examination did not reveal any evidence of infection or inflammation. Brain magnetic resonance imaging was also normal. Electroencephalography showed nonspecific background without evidence of electrical seizure. At the last follow-up, they were 14 years and 3 months old. They were completely normal in growth and cognition. They did not reveal any constitutive symptoms and signs between recurrent encephalopathies.
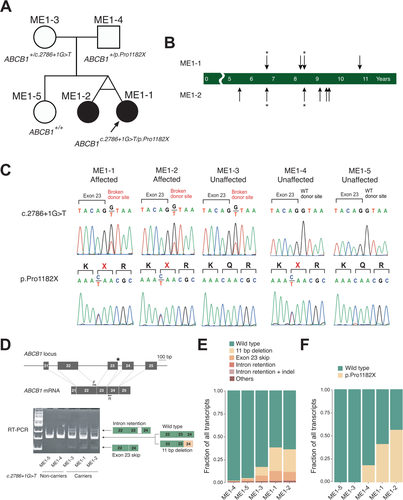
To identify the genetic cause of the observed symptoms, WES was performed on one of the twin and parents (Table S2). Among the list of variants that are potentially associated with the symptoms (Table S3), ABCB1 was selected as a strong candidate gene responsible for the symptoms for following reasons: (1) the two variants, one being nonsense (p.Pro1182X) and the other being a splicing regulating variant near exon 23 (c.2786 + 1G>T), were expected to pose damaging effect to protein synthesis, and (2) the gene encodes a xenobiotic exporting transporter expressed in multiple domains including BBB, providing a plausible connection to the phenotype that the twins displayed (Fig. S1). Segregation analysis confirmed that each variant is inherited from each parent and unaffected sister does not carry any of them (Fig. 1C). The variants were never seen in gnomAD or 1,020 Korean exome database.13, 14 gnomAD reports 47 heterozygous loss-of-function alleles evenly distributed throughout the protein, but these variants were not found in a homozygous status (Fig. S2). Simple estimation of the probability of carrying at least two of the damaging variants from the marriage of the carriers would be 2.8 x 10-8, making the occurrence of such carriers unlikely by chance.
To investigate the functional implication of the splicing variant, we monitored ABCB1 mRNA status from patient-derived B lymphocytes immortalized by Epstein–Barr virus. RT-PCR of ABCB1 mRNA using primers near the splice variant revealed multiple PCR products indicative of exon skipping or intron retention (Fig. 1D). Notably, the variant caused nearby bases to act as a proxy splicing donor, causing an 11-bp deletion in mRNA sequences (Fig. S3). Complete reading of all the variant PCR product fragments using Illumina amplicon sequencing (>5 million coverages) estimated 25.7% of the products being 11-bp deleted, 0.3% as intron retention, and 10.6% as exon skipping, all leading to frame shift or premature truncation of the protein (Table S4 and Fig. 1E). On the other hand, nonsense variant-carrying allele constituted 40.3% and 55.2% of the mRNA molecules (Table S4 and Fig. 1F). Taken the results together, it appears that even with the two loss-of-function variants, the sisters are likely to express a small portion of wild-type protein (~10%). Father’s nonsense allele constituted a much smaller portion (18.0%), implying more active nonsense-mediated decay compared to the affected sisters.
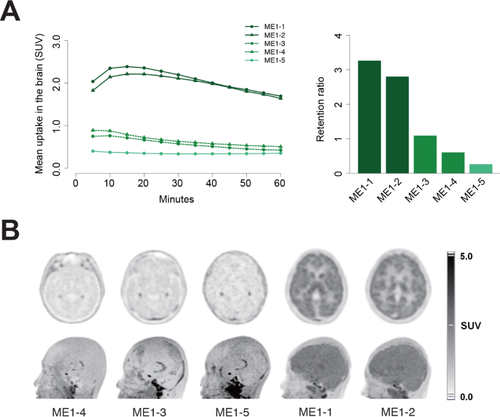
Under the hypothesis that the ABCB1 variants indeed cause xenobiotic transport problems in patient cells, we tested if the loss of ABCB1 causes delayed clearance of xenobiotic in the patient brains. Thus, dynamic PET was performed using [11C]verapamil, a ABCB1-targeting agent. The twin patients showed markedly increased uptake in the whole cortex of the brain, whereas the parents and sister showed low uptake. In the dynamic images of the twin patients, the brain uptake reached peak activities at 15 min after injection, and gradually decreased (Fig. 2A). In the parents and sister, the uptake never showed visible peaks and gradually decreased after initial injection. When the brain uptake was compared between the brain and a region of nonspecific uptake, the target-to-reference ratios of the twins were as high as 3.27 and 2.81, whereas they were 1.10 and 0.61 in parents. The older sister, representing a wild-type, exhibited the lowest value of 0.27 (Fig. 2A). On the static images taken 30–60 minutes after the injection, both the twin patients showed significantly increased uptake and retention of [11C]verapamil in their brains (Fig. 2B). Uptake was observed in the whole cerebral and cerebellar cortex including the deep gray matters of the thalamus and basal ganglia. In contrast, their parents and sister did not exhibit increased uptake except physiologic uptake in the choroid plexus area.
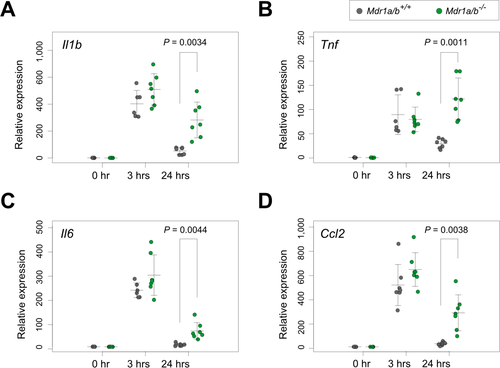
Given the broad ranges of ABCB1 target molecules,8, 15 it would be unrealistic to screen a target molecule that might cause encephalopathy from these patients. Therefore, we reviewed the patients’ medical records focusing on medication history. However, no consistent relationship between specific drug and encephalopathy episode was identified. Instead, we tried to test if exogenous LPS clearance was also hampered by the absence of ABCB1. Based on the clinical observation that the twins experienced the episodes after acute illnesses, we hypothesized that a sudden increase in immune signals might perform some role. To test this, we treated wild-type and Mdr1a/b knockout mice with LPS and examined their brains for the persistent presence of immune response signatures. RT-PCR of selected cytokine genes revealed upregulation of Il1b, Tnf, Il6, and Ccl2 after 3 hours of LPS injection both in wild-type and mutant animals. However, after 24 hours, whereas wild-type brains showed cytokines clearance, mutant brains still harbored some of the cytokine expressions from the 3-hour peaks (Fig. 3). This result demonstrates that the absence of Mdr1a/b caused delayed clearance of cytokines in the mouse brains, suggesting one of the physiological consequences possibly responsible for the encephalopathy seen in our patients.
Discussion
Our clinical, genetic, and functional validation strongly suggests that the two twin sisters represent phenotype due to the reduction in ABCB1. ABCB1 is located in the luminal membrane of BBB endothelium and is expected to function as an active efflux pump, to keep the brain from neurotoxic endogenous or xenobiotic molecules.16, 17 Dysfunction of ABCB11, a bile salt export pump, leads to recurrent episodes of cholestasis which are typically triggered during acute illness.18 Thus, it is also reasonable to postulate that acute illness in our patients could contribute as a triggering factor perhaps through transiently impairing overall transporter functions which are highly dependent on ATP. It would be interesting to further identify the molecular trigger and molecular mechanism that gave rise to such an interesting clinical response in the patients with ABCB1 defect.
Several considerations for therapeutic planning of the patients should be addressed. For example, unexpected side effects of drugs involving the central nervous system could emerge in certain medical conditions, such as severe infection, pregnancy, or surgery.
Here, we identified and functionally studied the twins who are almost devoid of ABCB1, providing evidence that the patients display reduced xenobiotics clearance abilities. Although we failed to identify additional patients with same genotype and phenotype through local communication efforts and gene-matching servers such as GeneMatcher,19 our observation that the twin sisters exhibited identical genotype and phenotype serves as evidence that ABCB1 variants are the cause of the symptoms. We further demonstrate that cytokine clearance is reduced in the Mdr1a/b mutant brains after LPS challenge, offering a possible cause for the unique clinical features that we observed. Therefore, this study suggests a human model of ABCB1 deficiency.
Acknowledgments
We thank the patients and family for the involvement of the study. This study was supported by grants from the National Research Foundation of Korea (2014M3C9A2064686, 2018M3C9A5064708 and 2018R1A6A3A01011946) and from the Korea Centers for Disease Control and Prevention (2018ER690101).
Conflicts of Interest
No significant conflicts of interest.




