Expansion of the genetic landscape of ERLIN2-related disorders
Funding Information
This research was funded by Spastic Paraplegia Foundation (SPF) Inc., the Thrasher Foundation and CureSPG47 Inc.
Abstract
ERLIN2-related disorders are rare conditions of the motor system and clinical details are limited to a small number of prior descriptions. We here presented clinical and genetic details in five individuals (four different families) where three subjects carried a common homozygous p.Asn292ArgfsX26, associated also with sensorineural hearing loss in one child. One further subject had a de novo p.Gln63Lys and one harbors the homozygous p.Val136Gly because of maternal isodisomy of chromosome 8. Overall, we expanded the clinical and genetic spectrum of ERLIN2-related disorders and we reiterate that autosomal-dominant transmission is a potential mode of inheritance. Future research will elucidate disease mechanisms.
Introduction
ERLIN2 (ER lipid raft-associated 2) is an endoplasmic reticulum protein that mediates intracellular calcium signaling through its action on inositol 1,4,5-trisphosphate receptors, making it an important regulator of several cellular processes important for neurodevelopment and neurotransmission.1-3
Defects in ERLIN2 are a known cause of several neurodegenerative conditions. Biallelic pathogenic variants in ERLIN2 are responsible for autosomal-recessive hereditary spastic paraplegia (HSP) 18 (SPG18; MIM 611225), a progressive disorder characterized by intellectual disability (ID), multiple joint contractures, and gradual development of spasticity and weakness primarily affecting the lower extremities. Affected individuals often present during infancy or childhood with delayed motor milestones and loss of motor abilities.4 Recently, an autosomal-dominant form was described in two unrelated families with affected members carrying the same heterozygous missense variant in ERLIN2 (c.386G> C; p.Ser129Thr).5 Finally, a homozygous splice junction variant causing reduced ERLIN2 transcript was the cause of juvenile primary lateral sclerosis in a consanguineous family with several members affected by language regression, pseudobulbar palsy, reduced muscle bulk, distal greater than proximal weakness, and kyphosis/scoliosis.6
In this report, we expand the clinical and genetic spectra of ERLIN2-related disorders by presenting five individuals from four different families with varying presentations of developmental regression, spasticity, motor impairment, and in one case, epileptic seizures. Three families (four affected individuals) demonstrate autosomal-recessive inheritance with biallelic variants, whereas the fifth affected individual has a heterozygous de novo variant consistent with an autosomal-dominant form of ERLIN2-related disorder.
Patients and Methods
Participants in this study underwent clinical evaluations at Boston Children’s Hospital, Kennedy Krieger Institute, IRCCS Fondazione Stella Maris, and IRCCS Istituto delle Scienze Neurologiche di Bologna. Two participants underwent clinical whole exome sequencing through GeneDx, and three participants had multigene panel testing using methodologies and a bioinformatic pipelines already reported elsewhere.7 No other pathogenic or likely pathogenic variants were identified that would explain the clinical presentation.
Results
Demographic and genetic features
The five individuals (two males and three females) in this cohort had heterogenous demographic, clinical, and genetic characteristics (Table 1, Table S1). The age at last follow-up ranged from 3 to 51 years, and two were male. Three individuals were of South Asian descent (Pakistan and Sri Lanka), one case was from Italy, and one case was of Ghanese descent.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 (sister of Patient 3) | Patient 5 | |
|---|---|---|---|---|---|
| Family 1 | Family 2 | Family 3 | Family 4 | ||
| Sex/Age at last exam | Male/12 years | Female/10 years | Male/9 years | Female/3 years | Female/51 years |
| Genetics | |||||
| Inheritance/Consanguinity | Recessive (parents are carriers)/Yes (parents are first cousins) | Mother heterozygous/No | Recessive (parents are carriers)/Yes (parents are second cousins) | Recessive (parents are carriers)/No | de novo/No |
| Accession number | NM_007175.6 | NM_007175.6 | NM_007175.6 | NM_007175.6 | NM_007175.6 |
| cDNA change | c.861_874dup14 | c.407T> G | c.861_874dup14 | c.861_874dup14 | c.187C> A |
| Protein change | p.Asn292ArgfsX26 | p.Val136Gly | p.Asn292ArgfsX26 | p.Asn292ArgfsX26 | p.Gln63Lys |
| Zygosity | Homozygous | Homozygous due to maternal uniparental isodisomy of chromosome 8 | Homozygous | Homozygous | Heterozygous |
| Found in gnomAD | No | No | No | No | No |
| Variant detected by | Clinical exome sequencing | Clinical exome sequencing | Targeted gene panel | Targeted gene panel | Targeted gene panel |
| Previously reported? | No | Yes (previously reported with limited clinical information1) | Yes (previously reported with no clinical information2) | Yes (previously reported with no clinical information2) | No |
| Growth parameters at last exam | |||||
| Head circumference | Normal | Normal | Normal | Normal | Normal |
| Weight | z-score −0.27 | z-score −1.09 | not available | not available | not available |
| Height | z-score −1.46 | z-score −0.87 | not available | not available | not available |
| Symptom Onset | |||||
| Age of onset | 8 months | 2 years | 2 years | 10 months | 32 years |
| Initial symptoms | Motor delay, spastic diplegia | Motor delay, spastic diplegia | Motor delay | Motor delay | Spastic diplegia, wide base gait |
| Developmental regression (age of onset) | Yes (5 years) | Yes (unknown) | Yes (2 years) | Yes (1 year) | No |
| Nature of regression | Lost ability to crawl and climb steps | Lost ability to independent walking, worsening dysarthria | Lost ability to walk independently | Loss of standing position | No |
| Current motor abilities | Able to pull to stand, cruise, walk with posterior walker | Requires wheelchair | Able to stand, crawl, and walk with bilateral assistance | Unable to walk | Requires cane to walk |
| Language and cognition | |||||
| Current language abilities | 200 + words, phrases | Age appropriate comprehension but difficulty speaking, hypophonia, difficulty opening mouth | Limited speech | Absent speech | Dysarthric speech |
| Intellectual disability | Yes | No | Yes (mild-moderate) | Yes | No |
| Behavioral dysregulation | No | No | No | No | No |
| Motor | |||||
| HSP phenotype | Complex | Pure | Complex | Complex | Pure |
| Axial hypotonia | Yes | No | Yes | Yes | No |
| Appendicular spasticity | Yes | Yes (spastic quadriplegia) | Yes | Yes | Yes |
| Motor impairment | Lower> upper limbs | Lower> upper limbs | Lower>> upper limbs | Lower>>upper limbs | Lower limbs |
| Dystonia | Yes (upper limbs) | Yes (hands and lower limbs) | Yes (hands, fluctuating) | No | No |
| Pyramidal tract signs | All limbs | All limbs | All limbs | All limbs | Lower> upper limbs |
| Tremor | No | No | Yes (occasional, hands) | No | No |
| Dysmetria | No | No | Yes (mild) | No | Yes |
| Gait pattern | Spastic gait (scissoring gait) | Spastic gait (scissoring gait, able to take few steps) | Spastic gait (scissoring gait with support) | Crawling | Spastic, ataxic gait |
The types of ERLIN2 alterations represented in this cohort included a common homozygous frameshift variant found in two unrelated consanguineous families (p.Asn292ArgfsX26) and a de novo heterozygous missense variant (p.Gln63Lys) (Figure 1). The remaining individual was homozygous for a c.407T> G variant (p.Val136Gly) due to maternal uniparental isodisomy (UPD) of chromosome 8, with the unaffected mother heterozygous for this variant. Evidence for pathogenicity of the missense variants derives from scores in multiple in silico prediction tools and a high evolutionary conservation of mutated residues across species (see Figure 1).
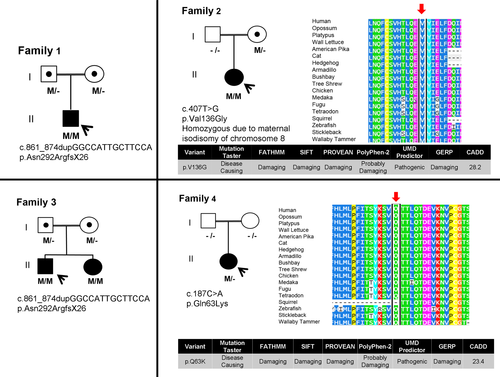
The three different ERLIN2 variants found in the five participants in our cohort are likely pathogenic changes. Patient 1, patient 3, and patient 4 harbored the same homozygous frameshift variants expected to cause loss of function, a known mechanism of disease for ERLIN2-related disorders, although this was not proved at the cDNA level. The missense variants identified in patient 2 and patient 5 were novel but several lines of evidence support that they are disease-causing. The variants are absent in large population databases (ExAC, 1000 Genomes) and predicted to be deleterious by in silico tools (CADD scores> 23).
Symptom onset
Age of onset of motor delay and/or spasticity spanned infancy/early childhood in the four individuals with homozygous ERLIN2 variants to adulthood in one individual with heterozygous de novo missense. Developmental regression was notable in three patients (patient 1, patient 2, patient 3), characterized by loss of ambulatory abilities. In patient 1, regression occurred at age 5 years, when he lost the ability to crawl and climb steps. Patient 2 had motor delay and spastic diplegia noted around age 2 years, after which she developed progressive motor deterioration, losing the ability to walk with a walker and requiring a wheelchair for mobility. Patient 3 had loss of ambulation after age 2 years.
Language, cognition, and epilepsy
ID was present in the three participants with the homozygous ERLIN2 frameshift variants and absent in the two participants with the ERLIN2 missense variants. Among those without ID, dysarthria and hypophonia were the noted speech difficulties. Patient 1 has sensorineural hearing loss, which has not been previously reported in association with ERLIN2-related disorders. One patient presented with generalized myoclonic absence seizures at the age of 2 years, which stopped after treatment with valproate.
Motor phenotype
The primary motor phenotype of the participants was axial hypotonia and appendicular spasticity. In four patients, spasticity affected lower extremities greater than upper extremities, and in the remaining individual, spasticity was limited to the lower extremities. More than half (3/5) of the cohort had dystonia. Patient 1 had intermittent dystonia affecting his arms. Patient 2 also had intermittent dystonic posturing affecting her arms and legs. Patient 1 and 2 did not undergo empiric carbidopa/levodopa trial. Hyperreflexia and predominantly lower extremity weakness were present in all five individuals. Patient 4 could crawl but not walk; the other four individuals had a spastic gait, with superimposed cerebellar features (tremor or dysmetria) in two individuals.
Neuroimaging features
Neuroimaging findings were variable. Thinning of the corpus callosum and other white matter changes were the primary abnormalities in two cases. Two individuals had a normal brain MRI. Patient 1 had arachnoid cyst that was fenestrated without any clinical improvement.
Discussion
The patients in our cohort constitute a wide spectrum of phenotypes consistent with previous reports of ERLIN2-related disorders (Table S2). There appear to be three clinical phenotypes associated with ERLIN2 defects: pure HSP, complex HSP, and juvenile primary lateral sclerosis. The first two phenotypes are equally represented in the five participants from this study, who demonstrated progressive spasticity, weakness, and gait disturbance, with age of onset ranging from infancy to adulthood.
One of the novel clinical features seen in association with ERLIN2-related disorders is sensorineural hearing loss (seen in patient 1). This patient did not have any pathogenic variants detected in mtDNA sequencing, and no other variants were reported on clinical exome sequencing to suggest an alternative cause for the hearing loss. Thus, hearing loss may be an expansion of the clinical spectrum of ERLIN2-related disorders, and individuals with a new diagnosis should consider undergoing audiological evaluation.
The variants discovered in our cohort expand the genetic landscape of ERLIN2-related disorders. Four out of the five patients had alterations in ERLIN2 that are previously unreported in association with the disorders (patient 2 is part of a prior report, but detailed clinical information about her in that report is limited8; similar is the case for patients 3 and 47). In addition, patient 2 has a novel missense variant that is homozygous due to maternal UPD of chromosome 8, with her mother being a heterozygous carrier.
Four of the five patients in our study had homozygous variants, whereas patient 5 carried a de novo variant, confirming the recent report of an autosomal-dominant form of ERLIN2-related disorder. The vast majority of previous studies of ERLIN2-related disorders have identified biallelic variants (Table S2).4, 6, 9-12 One report, however, identified a recurrent heterozygous missense variant (c.386G> C; p.Ser129Thr) in multiple affected individuals from two large unrelated families.5 Overall, these data lend support to the notion that there are autosomal-recessive and autosomal-dominant forms of ERLIN2-related disorders, similar to a small number of other genetic causes of HSP, such as defects in ATL1 (SPG3A),13 KIF1A (SPG30),14 KIF1C (SPG58),15 REEP1 (SPG31),16 and SPG7 (SPG7).17
Certain genotype-phenotype correlations emerged within our cohort. The patient with the de novo heterozygous ERLIN2 variant (patient 5) appeared less severely affected compared to the other participants with homozygous ERLIN2 variants, as was shown by a later age of symptom onset, absence of developmental regression, and absence of ID. In fact, among prior reports of ERLIN2-related disorders (Table 2), ID was absent in 5/6 unrelated individuals who had a missense ERLIN2 variant on at least one allele (three families with affected individuals);5, 11, 12, 18, 19 conversely, ID was present in all individuals with a homozygous truncating variant.4, 9, 20, 21 This observation suggests that ERLIN2-related disorders can present dosage-dependent clinical features likely due to reduced protein function. It is worth noting that our data lend further support to the notion that zygosity is no longer a barrier to defining a molecular diagnosis in HSP, suggesting that even more HSP genes might be associated with different modes of inheritance. If this is truly the case, it would have implications for the estimated diagnostic yield and could imply differences in disease-associated phenotypes. The question of why parental carriers of a single variant in ERLIN2 are healthy, though harboring a heterozygous predictably pathogenic variant, remains unclear. It is possible that the ERLIN2 missense variants causing disease in heterozygous state have different effect on protein, such as dominant-negative or gain-of-function effects, whereas the variants that cause disease only when biallelic cause loss/reduction of protein function 5. This would need to be investigated with functional studies. A final comment worth mentioning is the maternal UPD seen in our report. This unusual modality of transmission should alert clinicians to carefully evaluate results of next-generation gene sequencing when counseling families, especially on risks of recurrence.
In sum, we define new clinical presentations and gene variants in ERLIN2-related pathology; we report a common pathogenic variant in families of South Asian ancestry; and we affirm that autosomal-dominant transmission can be a mode of inheritance associated with ERLIN2-related disorders. Further research is needed to elucidate disease mechanisms of ERLIN2-related disorders.
Acknowledgments
We thank the families for their involvement. D.E.F. acknowledges funding from the Spastic Paraplegia Foundation (SPF) Inc., the Thrasher Foundation and CureSPG47 Inc.
Author contribution
Conception and design of the study: S.S., A.D., D.E-F., F.M.S. Acquisition and analysis of data: S.S., A.D., J.S.C, L.S., I.R., A.P., A.F., D.E-F., F.M.S. Drafting a significant portion of the manuscript or figures: S.S., A.D., D.E-F., F.M.S., J.S.C.
Conflict of Interest
JSC is a consultant to Invitae. SS received consulting fees from Guidepoint. The other authors declare no conflict of interest related to this study.




