Toward “off-the-shelf” allogeneic CAR T cells
Abstract
Chimeric antigen receptor (CAR) T cell therapy represents a major breakthrough in the field of immuno-oncology. Many potential issues are apparent for autologous CAR T cell therapy, such as time for manufacturing and need for interim therapies in progressing patients, wide variations in terms of quality and quantity of T cells, and difficulty to obtain enough cells for redosing. “Off-the-shelf” allogeneic CAR T cells premanufactured from third-party donors may theoretically provide solutions to these different problems. However, allogeneic T cells possess foreign immunological identities that can lead to histocompatibility considerations such as graft-versus-host disease and rejection of allogeneic cells. This review outlines the major recent advances for off-the-shelf T cell therapies currently in clinical trials or in preclinical development and describes strategies for reengineering or selecting specific T cell immune identities to create safe and efficient immunotherapies for patients.
1 INTRODUCTION
- Time to manufacturing: Manufacturing of autologous CAR T cells today is largely centralized to comply with quality control and reproducibility rules. On-site or point-of-care production schemes propose to reduce logistic complexity and overall vein-to-vein time, but this strategy of production has been limited to clinical trials thus far and still takes several days. CAR T cell manufacturing may thus create undesirable wait times for patients, especially for those with rapid progression of the disease. Furthermore, manufacturing failure may prevent patients from receiving CAR T cell therapy.3-5 Complexities of cross-departmental and cross-institutional coordination of manufacturing and treatment may delay cell infusion.6
- Need for interim therapies: Bridging chemotherapies may be necessary to control disease progression while the patient is waiting for infusion of CAR T cells, posing issues for refractory malignancies.3, 5
- Risk of malignant contamination: The leukapheresis product may contain malignant cells that become transduced with the CAR protein that binds cell surface antigen, protecting the contaminating cells from CAR T cell-mediated elimination.7 This risk may depend on the primary disease and timing of cell harvest.
- T cell variability: Patient-specific variation in T cell phenotype and prevalence, like the presence of more or less ‘early’ or ‘stem cell-like’ T cell subsets, may yield different expansion and persistence of cell products.8 Many factors may be involved in this T cell variability: age, disease, previous lines of treatment, explaining the interpatient heterogeneity observed with the use of autologous CAR T cells.8
- Risk of insufficient T cell expansion: Starting material is limited to T cells collected from leukapheresis and may not sufficiently expand to high numbers during manufacturing, especially in patients who are heavily pretreated and lymphopenic.3, 5
- T cell dysfunction: A cancer patient’s T cells may be dysfunctional as a result of disease burden or prior lines of therapy, and may therefore be a poor drug delivery vehicle for CAR T cell therapies.9-12 Collecting T cells during the first lines of cancer therapy may reduce the risk associated with the cancer treatments but not the effects of tumor-associated immunosuppression.
- Limited opportunity for redosing: CAR T cells are prepared in a single batch and may be of limited quantity, such that patients may not have the opportunity to quickly and easily receive a new infusion of CAR T cells.
Unlike autologous T cell therapies, “off-the-shelf” T cells from third-party donors would be expanded in high numbers prior to treatment and made quickly available to patients.13, 14 This strategy could overcome the different limitations discussed above. A manufacturing protocol performed on T cells from a single donor leukapheresis has the potential to supply enough cells to produce hundreds of doses, and a single donor may be able to support multiple rounds of manufacturing.15, 16 This high-volume manufacturing allows for an opportunity to quickly and easily re-dose patients with a new off-the-shelf cell therapy without delays due to manufacturing or scale of production. Of note, in the potential instance of an immunogenic response to an off-the-shelf therapy, the use of cells from another donor further facilitates re-dosing options. Furthermore, a pretreatment manufacturing protocol allows for featuring multiple edits, manipulations, or TCR selection strategies that otherwise would be difficult to accommodate in the autologous setting. As CAR T design gains in complexity, this may become even more important, with the weight and complexity of safety assays making testing on individual products prohibitive.
A T cell therapy sourced from healthy allogeneic donors, however, introduces potential risks for issues related to incomplete histocompatibility. Due to the highly polymorphic nature of human leukocyte antigen (HLA) genes, allogeneic donor lymphocyte infusions are difficult to achieve without including consideration for risk of graft-versus-host disease (GvHD)17, 18 and durability of donor cells.19 Complete donor-recipient matching of HLA haplotype is limited by low compatible donor availability, and healthcare providers must assess risk factors based on the extent of HLA matches for unmodified, unselected, or unmanipulated allogeneic T cells.20
In this Review, we will present the main approaches that are currently in development to deliver allogeneic T cell therapy and to address these potentials for GvHD and cell rejection. In this context, we will focus on TCR editing-based approaches and virus-specific memory T cells as examples of clinically advanced nonalloreactive T cells. We will discuss the challenge of persistence and the perspectives of optimization.
2 STRATEGIES TO AVOID GvHD
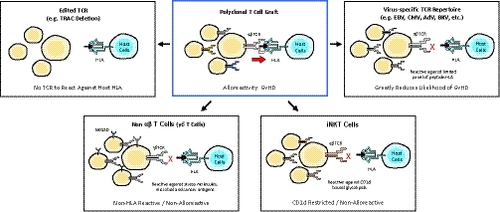
- Use of αβTCR negative cells: Editing cells to remove the TCR or using alternate cell types that lack αβTCR.
- Use of nonalloreactive T cells: Utilizing T cells with αβTCR specificity directed toward nonalloreactive targets.
3 USE OF αβTCR NEGATIVE CELLS
Cell therapy has broadly validated the use of αβ T cells as potent and efficient killers.21-23 Given their relative abundance in peripheral blood and their ability to proliferate rapidly upon stimulation, they are attractive for large scale manufacturing of allogeneic cells to treat a substantial number of patients per batch.24, 25
Several strategies have been used to remove or decrease expression or function of the TCR complexes on these cells. In one approach, a truncated dominant-negative CD3ζ protein (termed TCR inhibitor molecule, or TIM) was expressed in T cells, resulting in reduced TCR signaling and protection from xenogeneic GvHD.26 In another approach, an antibody-derived single-chain variable fragment specific for CD3ε was combined with amino acid sequences retaining it intracellularly. These protein expression blockers (PEBLs) colocalized intracellularly with CD3ε and inhibited surface expression of CD3 and αβTCR.27 Small hairpin RNA (shRNA) has also been proposed to decrease expression of TCR chains.28 Since the above approaches have the possibility to retain a low but functional amount of TCR, most entrants into the allogeneic space have opted for cellular gene editing approaches to ensure complete removal of the TCR. Several editing technologies are being used and all have in common their ability to cut DNA in a sequence-specific manner, creating a double strand break that then is patched using the cell’s natural DNA repair machinery via nonhomologous end-joining (NHEJ). This restores the integrity of the chromosome but creates insertions or deletions (INDELS), which lead to disruption of the locus (most often TRAC, the TCRα common chain). With no functional TRAC gene, the TCR complex cannot form.29
No approaches for αβTCR removal result in 100% of cells having αβTCR reduced or eliminated, necessitating a purification step to remove residual αβTCR positive cells. The efficiency of this process is critical to reduce the risk of any contaminating αβTCR positive cells that may induce GvHD.
Transcription activator-like effector nucleases (TALEN) were the first technology to be used in patients for αβTCR removal,13 entering the clinic for compassionate use of a CD19 CAR for pediatric acute lymphoblastic leukemia (ALL) in 201530 and in two phase 1 trials for pediatric and adult ALL in 2016.31 Grade 2 skin GvHD was observed in one of the two compassionate use cases,30 and grade 1 skin GvHD in the 2 of 21 patients in the two UCART19 clinical trials. No higher grades of GvHD were observed. Other gene editing technologies currently being employed preclinically and in the clinic for allogeneic CARs are clustered regularly interspaced short palindromic repeat (CRISPR), ARC editing megaTALs and Zinc fingers.32
Gene edited allogeneic CAR T cells are classed as gene therapies by the FDA, necessitating long-term follow-up of patients to monitor for safety.33 Since gene editing may have a low frequency of off-target cutting, monitoring of predicted off-target cut sites and nonbiased approaches to identify off-target events are necessary. Assays to look for any consequences of off-target cutting (such as eg inactivation of tumor suppressors) are also required, which includes demonstrating lack of growth factor independence. If more than one edit is made simultaneously, consequences of translocation must also be investigated and translocation frequencies must be monitored.34 Although no instances of viral integration-related transformation have been reported with CAR T therapy to date, there is also a potential risk that this might occur given the random integration of both retroviral and lentiviral vectors. In addition to using gene editing to knock out αβTCR expression, it can also be used as a tool to add the CAR into the disrupted locus, thereby producing site specific integration.35, 36 Site specific integration may possess other advantages, for instance potentially lowering risk of translocations with two or more edits by favoring homology-directed repair rather than NHEJ.37
Although αβ T cells represent the ‘gold standard’ of cell sources for therapy, they are not the only option available. Several effector cell types exist which lack an αβTCR or express an invariant version and are not expected to cause GvHD.38 NK cells present in the peripheral blood for example can be expanded ex vivo to create an engineered product. CD19 CAR modified NK cells have been described,39 showing activity in patients in a published phase I/II.40 Eight 11 treated patients had a clinical response, including seven complete remissions (CRs) and one remission of a Richter’s transformation with persistent chronic lymphocytic leukemia. Other cell types such as iNKT or γδT cells may be as potent as αβ T cells and may have advantages in terms of their innate receptor activity, their ability to be expanded readily to large numbers using their natural ligands41 and ability to penetrate solid tumors.42. Their relative frequencies in peripheral blood are, however, very low and any contamination with alloreactive αβ T cells would require similar purification to avoid the risk of GvHD.
If a renewable cell source could be used to generate gene edited T cells or other cell types, clones with ideal characteristics and defined composition could be generated; for instance, absence of translocations, successful gene editing to result in a completely αβTCR negative cell source. Work has begun to derive T cells engineered from induced pluripotent stem cells (iPSCs).43-45 These cells can be engineered, then genetically screened clones can be expanded and differentiated. Proof of concept for this approach has been generated for NK and T cells,46 with at least one T cell product heading into the clinic (Table 1). Nonetheless, technical challenges still remain for alternatives to αβ T cells, including demonstrating functional equivalence to conventional T cells.
| Sponsor | Therapy name | Therapy target | Development stage | Clinical Trial ID | Disease | Trial start date | Gene edits used | |
|---|---|---|---|---|---|---|---|---|
| Servier Allogene Therapeutics | UCART19 | CD19 | Phase 1 | NCT02808442 (PALL) | Pediatric R/R ALL | 6/3/16 | CD52KO TRACKO CD19 CAR (Lenti) | |
| Servier/Allogene Therapeutics | UCART19 | CD19 | Phase 1 | NCT02746952 (CALM) | R/R ALL | 8/1/16 | CD52KO TRACKO CD19 CAR (Lenti) | |
| Cellectis | UCART123 | CD123 | Phase 1 | NCT03190278 (AMELI-01) | R/R AML | 6/19/17 | TRACKO CD123 CAR (Lenti) | |
| Cellectis | UCART123 | CD123 | Phase 1a | NCT03203369 (ABC123) | BPDCN | 6/28/17 | TRACKO CD123 CAR (Lenti) | |
| Poseida | P-BCMA-101 | BCMA | Phase 1 | NCT03288493 | Multiple Myeloma | 9/20/17 | BCMA Centyrin (piggyBac) | |
| Celyad | CYAD-101 | NKG2D Ligands | Phase 1 | NCT03692429 (alloSHRINK) | CRC | 11/28/18 | TIM NKG2D CAR (Retro) | |
| Precision Biosciences | PBCAR0191 | CD19 | Phase 1/2 | NCT03666000 | R/R B-ALL and R/R NHL | 3/11/19 | CD19 CAR --> TRAC locus (ARCUS) | |
| Allogene Therapeutics | ALLO-501 | CD19 | Phase 1/2 | NCT03939026 (ALPHA) | R/R DLBCL and R/R FL | 5/1/19 | CD52KO TRACKO CD19 CAR (Lenti) | |
| CRISPR Therapeutics | CTX110 | CD19 | Phase 1 | NCT04035434 | NHL | 7/22/19 | MHC-I KO TRACKO CD19 CAR SSI | |
| Allogene Therapeutics | ALLO-715 | BCMA | Phase 1 | NCT04093596 (UNIVERSAL) | Multiple myeloma | 9/23/19 | CD52KO TRACKO BCMA CAR (Lenti) | |
| Cellectis | UCART123 | CD123 | Phase 1 | NCT04106076 | High risk AML | 7/11/19 | TRACKO CD123 CAR (Lenti) | |
| Cellectis | UCART22 | CD22 | Phase 1 | NCT04150497 (BALLI-01) | R/R B-ALL and R/R NHL | 10/17/19 | TRACKO CD22 CAR (Lenti) | |
| Cellectis | UCARTCS1 | CS1 | Phase 1 | NCT04142619 (MELANI-01) | Multiple Myeloma | 11/21/19 | CS1KO TRACKO CS1 CAR (Lenti) | |
| CRISPR Therapeutics | CTX120 | BCMA | Phase 1 | NCT04244656 | Multiple myeloma | 1/28/20 | MHC-I KO TRACKO BCMA CAR SSI | |
| Virus-specific T cells | ||||||||
| Atara Biotherapeutics | Tabelecleucel | EBV antigens Phase 3 | NCT03394365 (ALLELE) | R/R EBV+PTLD | After SOT or HCT | 12/29/17 | No Edits | |
| Atara Biotherapeutics | Tabelecleucel | EBV antigens | Phase 1/2 | NCT03769467 (EBV+ | NPC) EBV+ NPC | 11/28/18 | No edits | |
| Atara Biotherapeutics | Tabelecleucel | EBV antigens | Phase 1/2 | NCT00002663 | EBV-associated malignancies | 3/1/95 | No edits | |
| AlloVir | Viralym-M | Multiviral antigens | (N/A) | NCT02108522 (CHARMS) | Viral infections after SCT | 6/1/14 | No edits | |
| AlloVir | Viralym-M | Multiviral antigens | Phase 1/2 | NCT01570283 (ARMS) | Viral infections | 9/1/12 | No edits | |
| AlloVir | Viralym-C | Multiviral antigens | Phase 1 | NCT02313857 | CMV infections | 9/1/15 | No edits | |
| MSKCC | [none] | EBV antigens and CD19 | Phase 1 | NCT01430390 | B cell malignancies | 9/1/11 | + CD19 CAR | |
| Fred-Hutch | [none] | EBV antigens and WT1 | Phase 1/2 | NCT01640301 | AML | 7/12/12 | + Affinity-enhanced WT1 TCR | |
| Atara Biotherapeutics | ATA188 | EBV antigens | Phase 1 | NCT03283826 | Multiple sclerosis | 10/19/17 | No edits | |
| Atara Biotherapeutics | ATA520 | EBV antigens and WT1 | Phase 1 | NCT00620633 | Leukemia | 2/1/08 | No edits | |
| Atara Biotherapeutics | ATA320 | CMV antigens | Phase 2 | NCT02136797 | CMV infection after HSCT | 5/1/14 | No edits | |
| Baylor College of Medicine | [none] | EBV antigens (EBNA1, LMP, BARF1) | Phase 1 | NCT02287311 (MABEL) | EBV+lymphoma | 2/1/15 | No edits | |
- Start dates as reported in Clinical trials.gov
- Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; BPDCN, blastic plasmacytoid dendritic cell neoplasm; CMV, cytomegalovirus; DLBCL, diffuse large B-cell lymphoma; EBV, Epstein Barr virus; FL, follicular lymphoma; HCT, allogeneic hematopoietic cell transplant; NHL, non-Hodgkin lymphoma; NPC, nasopharyngeal carcinoma; PTLD, post-transplant lymphoproliferative disease; R/R, relapsed/refractory; SOT, solid organ transplant.
- a Study terminated.
Most of the work described above has been carried out in the context of CD19 CARs since CD19 still represents the most clinically validated cell therapy target. Allogeneic cell products are now starting to be created against targets for other hematologic malignancies, such as BCMA,47, 48 and for some solid tumor targets. Although more challenges are expected for CAR therapies moving into solid tumor indications, these may be the areas where allogeneic approaches give the most benefit. Multiple gene edits to overcome solid tumor challenges may be possible, and the larger patient populations requiring treatment will be much more feasible with an allogeneic cell source.
3.1 Selection of nonalloreactive T cells: A nongene edited approach
Alternative approaches to editing of αβTCR have also demonstrated significant clinical progress for off-the-shelf cell therapies. One example of this has been the use of virus-specific T (VST) cells derived from healthy donors. Exposure of lymphocyte collections to viral antigens ex vivo expand T cells expressing virus-specific αβTCR. Virally-infected antigen presenting cells are commonly used for this stimulation and expansion of T cells expressing virus-specific αβTCR49, 50 and may be genetically engineered51 or be directly loaded with viral peptides.14 Similarly, virus-specific αβTCR can be directly isolated using MHC-bound antigen peptide.52, 53 The resulting virus-specific αβTCR repertoire functions via formation of a classical immune synapse and is thus activated via specific recognition of virus and not host peptide in complex with a specific HLA. As a primary therapeutic composition, VST cells can be manufactured from multiple donors, each associated with different HLA alleles, and banked in large numbers based on HLA restriction.14, 53
One immediate application for these off-the-shelf banks of VST cells is in the treatment of diseases originating from viral infection. For instance, Epstein-Barr virus (EBV) is a known driver of cell transformation and progression of several diseases including lymphoproliferative disorders,50, 54-56 nasopharyngeal carcinoma,49 leiomyosarcoma,57 and multiple sclerosis.58 The first evidence of successful use of off-the-shelf VST therapy was performed with minimally HLA matched EBV-specific T cells to treat patients with EBV-positive lymphoma following solid organ transplant.56 In this multicenter phase II trial, 14 of 33 patients achieved CR and seven patients achieved partial remissions. Similarly, Prockop et al has described the treatment of 46 patients with rituximab relapsed/refractory EBV-positive lymphoma using a bank of partially HLA matched off-the-shelf EBV-specific T cells.59 In this experience, response rates in patients developing lymphoma following a previous stem cell transplant or solid organ transplant were 68% and 54% respectively. Of the 46 patients treated, only one developed a de novo low grade GvHD, which resolved with topical therapy. Despite partial HLA-matching, allogeneic EBV-specific T cells expanded in patients without lymphodepletion pretreatment and were detected in circulation for up to 27.3 months after the date of infusion.60, 61
Beyond EBV-specific T cells, other off-the-shelf VST therapies have been deployed similarly in the clinic, including those specific for adenovirus, BK virus, and human herpesvirus 6 (HHV-6).62-64 In these examples, low incidence of GvHD is attributed to enrichment of virus-specific αβTCR repertoire and the concurrent depletion of cells expressing functionally alloreactive αβTCRs. These studies demonstrate that a low degree of HLA matching provides sufficient persistence and patient exposure to facilitate complete and durable clinical response. Together, examples of allogeneic VST cell therapies have clinical history establishing benchmarks for efficacy and safety in the context of partial HLA allele match, including with as few as one HLA allele match.54 This limited requirement for HLA matching for this class of allogeneic cell therapy significantly decreases the required size of an off-the-shelf bank containing suitable HLA coverage to address a general population.
Similar to previously described engineered T cell platforms, VSTs can also be transduced to additionally express a CAR, functionally redirecting their effector function to new, nonviral tumor targets through CAR-specific and HLA-independent mechanisms.65-67 EBV antigens may also be present along with other B-cell surface markers, such as CD19, commonly expressed in B cell malignancies.68 In this case, EBV-specific T cells transduced to express a CD19-targeting CAR can recognize and kill lymphoma cells via either the endogenous virus-specific TCR, which targets EBV antigen through HLA-dependent mechanisms, or via engagement of the CAR against CD19-antigen in an HLA-independent mechanism. Preclinical studies with off-the-shelf allogeneic EBV-specific CD19-CAR T cells demonstrate this capability with CARs containing either CD28 or 4-1BB co-stimulatory domains, while maintaining the central memory VST phenotype when stimulated with antigen.66, 67 Preliminary results from a phase 1 evaluation of allogeneic EBV-specific CD19-CAR T cells recently described 83% response rate and no reported incidence of confirmed GvHD or CRS or ICANS above grade 2 across six patients with relapsed/refractory B cell malignancies with 26.9 months median follow-up.69
Similar strategies have also been extended to solid tumor targets, as demonstrated with autologous EBV-specific VST expressing CAR targeting disialoganglioside (GD2) for treatment of neuroblastoma.70, 71 In this phase I trial, investigators reported 3 of 11 patients with active disease achieved CR with EBV-specific GD2-targeted CAR T cells. In this study, the GD2 CAR-VST demonstrated superior cell persistence (up to 192 weeks), twice as long as standard αβT cells bearing CAR alone (96 weeks). This comparable persistence benefit may provide further support for transitioning these approaches to the allogeneic setting.
Beyond CAR, allogeneic VSTs have also been combined with engineered TCR approaches. Similar to redirection of VST function via a CAR, redirection may also be achieved via transduction with engineered TCRs directed toward nonviral tumor-specific antigens. In this way, allogeneic donor-derived EBV-specific VSTs further transduced with an engineered TCR specific to Wilm’s tumor 1 antigen (WT1) have also been explored clinically.72-74 This therapy was recently tested in patients with acute myeloid leukemia (AML) who received an allogeneic hematopoietic cell transplantation (HCT). These WT1 TCR-redirected EBV-specific T cells were associated with increased persistence of greater than 10 months, while similarly WT1-TCR transduced CMV-specific T cells persistence was sporadic and declined after 2 months.72, 74 This differential persistence was associated with an enriched central memory T cell profile commonly seen with the EBV subset of VSTs. At 44 months after infusion, 100% relapse-free survival was reported for AML patients receiving EBV-specific WT1-targeting T cells, and incidence of GvHD remained low and attributable to prior HCT.73
As a specific example of a nongene edited allogenic αβT cell, VSTs may provide advantages and feasible alternatives for creating off-the-shelf cell therapies that do not require elimination of either endogenous αβTCR expression or the expression of HLA. These advantages potentially impact both the ease of GMP manufacturing and risk of gene edited T cells inducing extraneous effects, such as p53-mediated DNA damage response.75 EBV-specific T cells may also provide additional potential for approaching other solid tumor targets, as EBV-specific T cells have been shown to traffic to and penetrate solid tumor tissues, where they may facilitate broad antitumor immune response upon stimulation.76
4 EXPANSION AND PERSISTENCE OF ALLOGENEIC CAR T CELLS
The clinical efficacy of CAR T cells is associated with their initial expansion to achieve a sufficient effector-to-target ratio. Post-infusion CAR T cell expansion has been reported to be a major factor for clinical response in the case of autologous CD19 CAR T cells in B-ALL.77 This expansion is enhanced by homeostatic cytokines such as IL-7 and IL-15, whose levels are elevated after lymphodepletion.78, 79 CAR T cell persistence can result in long-term immunosurveillance. The persistence required to achieve a prolonged remission may vary according to the disease. One school of thought proposes that prolonged persistence may be required for a long-term control of diseases such as B-ALL.5 In contrast, most of DLBCL patients achieving a CR at 6 months remain disease-free.4 This difference probably reflects the biology of the disease and the persistence or not of a tumor reservoir after a first step of tumor killing.
Costimulatory domains significantly affect CAR T cell expansion and persistence. It has been shown that CD19.28.z-CAR T cells expand more rapidly and to higher levels than CD19.BB.z-CAR T cells.80, 81 This accelerated and higher expansion of T cells transduced with a CAR with CD28 versus 4-1BB costimulatory domain may be explained by a greater signal strength downstream of CD28 versus 4-1BB.82 However, autologous T cells expressing a CAR with a CD28 costimulatory domain rarely persist more than 1 or 2 months, whereas those with 4-1BB can persist for months or years.5, 77 Because the half-life of allogeneic CAR T cells will be reduced in most of cases due to rejection by the host immune system, it may be suggested in this context to use preferentially CD28 as a costimulatory domain, in order to obtain a rapid expansion of the CAR T cells and thus optimize the antitumor effect on a limited period of time. The use of CD28 may be, however, associated with a higher risk of cytokine release syndrome compared to 4-1BB.83 Of note, there is no data available to date in the clinic to compare allogeneic CAR T cells using CD28 versus 4-1BB as a costimulatory domain.
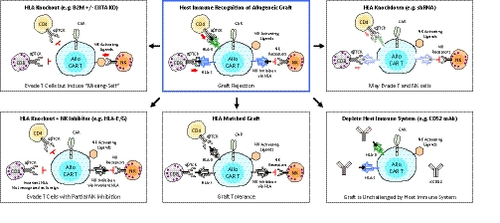
Once the risk of GvHD has been controlled, a potential issue remaining for allogeneic CAR T cell therapy is their limited persistence in the recipient. Different approaches can be proposed to allow initial expansion and improve persistence of allogeneic CAR T cells: HLA-matching, reinforcement of the lymphodepletion, HLA class I and II deletion, and use of immunomodulatory factors.
To increase persistence, a bank of cells could be prepared from donors with different HLA alleles, providing partial HLA coverage for the majority of the targeted patient population (as performed for VST cells derived from third party donors). Considering that the most important alleles to match for are HLA-A, -B, -C and potentially -DR, the size of the bank could be relatively limited. However, the persistence of the injected cells will be still reduced in absence of full HLA matching compared to autologous T cells. Indeed, the results obtained with third party donor-derived VST establish a persistence of the cells of 14-90 days and in the case of partially matched EBV specific T cells persistence for up to 27.3 months after the date of infusion has been reported60, 61 whereas VST from HCT donors were particularly long (up to 10 years).84
The first clinical trials evaluating gene-edited allogeneic CAR T cells have used a reinforced lymphodepletion. An anti-CD52 antibody was added to the classical fludarabine and cyclophosphamide combination, with the aim of inducing transient tolerance to the transplant by increasing the depth and duration of the lymphopenia. CAR T cells are in this case protected by deletion of CD52.18 This approach has been shown to lead to expansion of the allogeneic CAR T cells associated with efficacy. Two compassionate use patients first treated with UCART19 showed durable CR30 and 82% of patients in the two phase 1 trials who were treated with the anti-CD52-containing lymphodepletion regimen achieved CR.31 Furthermore, in two of three patients who failed to show benefit from the initial infusion, reinfusion gave minimal residual disease negative-CR. In a similar way, deletion of the dCK protein may provide resistance against purine nucleotide analogue chemotherapies such as fludarabine used for lymphodepletion.85 These approaches have the advantage of suppressing all immune cell classes which may mediate rejection such as T, B, NK and potentially monocytes. However, the flip side of this benefit is that a prolonged immunosuppression is associated with a higher risk of opportunistic infections and viral reactivation.
Another approach consists in modifying allogeneic cells in order to obtain “universal cells” that will evade host immune detection. In the case of CD8 T cell rejection, the priority is to eliminate the expression of HLA class I molecules on CAR T cells. Deletion of the conserved gene beta 2-microglobulin completely removes surface expression of HLA class I.86 However, while immunogenic recognition by CD8 T cells will be reduced by this approach, the complete loss of HLA class I molecules will increase the risk of recognition of the allogeneic CAR T cells by NK cells, the so called ‘missing self’ mechanism. NK cell mediated destruction of HLA-edited T cells has been proposed to be prevented by expression on CAR T cells of nonpolymorphic HLA molecules such as HLA-E that will bind inhibitory receptors on NK cells.87 In addition to CD8 T cell-mediated rejection, CD4 T cells can contribute to rejection of allogeneic T cells through recognition of HLA class II molecules. For this reason, limiting class II expression by deleting one of the transcription factors required for all HLA class II genes, such as CIITA or RFXANK, has been proposed.88
Finally, expression of immunomodulatory molecules, such as PD-L1 or FASL, may suppress or kill the recipient’s activated T lymphocytes respectively.88 This approach may be, however, limited by the expression of PD-1 and FAS molecules on the activated CAR T cells themselves, inducing thus their inhibition for PD-L1/PD-1 and fratricide killing for FASL/FAS interaction. How inhibition of phagocytosis by monocytes or macrophages may increase the persistence of allogeneic T cells is less clear. Adding molecules such as CD47 and CD200 may allow modified allogeneic T cells to avoid macrophage-mediated clearance.88, 89 Currently it is unclear if some of the above will be dominant, or if all modifications will be needed to avoid rejection.
The different possibilities of T cell engineering open new perspectives in the field of immune cell therapy. However, it remains to be elucidated whether the production of universal cells that completely evade the host immune system may also induce safety issues, since the immune system will not be able to recognize transformed cells in case of an oncogenic event. The initial screening of the administered allogeneic T cells for oncogenic mutations would therefore be of crucial importance in the context of extensive editing of the T cell genome. Given that late oncogenic events can still occur, and because rare oncogenic cells may have not been detected during the process, an optimized suicide system may be advantageous in modified T cells to allow their efficient elimination.90 Linking the suicide gene to a cell-division gene, as described by Liang et al,91 may be particularly useful in this context.
5 CONCLUSIONS
Engineered adoptive cell immunotherapies are quickly showing promise for patients. As logistical and scientific insights into the manufacturing of these therapies evolve, patients could greatly benefit from readily available off-the-shelf cell therapies tailored to the patient and their disease. By engineering T cell immune identities, off-the-shelf allogeneic T cell therapies currently in clinical development (Table 1) could provide broader immunotherapeutic options for patients. Current studies with nonengineered allogeneic T cells, such as the phase 3 ALLELE (NCT03394365) trial for patients with relapsed/refractory EBV+ post-transplant lymphoproliferative disease, and engineered allogeneic CAR T cell studies like UCART19 for ALL (NCT02746952), could begin to lay the groundwork for off-the-shelf therapies as standard-of-care treatments for patients.
CONFLICT OF INTEREST
SD has been employee of Cellectis. He is the founder of ErVaccine Technologies and member of the management team of Swiss Rockets. Consulting or scientific advisory boards: Servier, Celyad, PDC*line Pharma, Erytech, AstraZeneca, Elsalys, Netris Pharma, Vaximm. BTA and JK are employees of Atara Biotherapeutics. BS and EG are employees of Allogene Therapeutics
ETHICAL STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to. No ethical approval was required as this is a review article with no original research data.